With increases in obesity and metabolic syndrome because of lifestyle-related factors, the prevalence of non-alcoholic fatty liver disease (NAFLD) also is increasing worldwide. In a subset of patients with NAFLD, an inflammatory process arises in the steatotic liver, known as non-alcoholic steatohepatitis, that leads to liver fibrosis and liver cirrhosis. In selected patients with obesity, bariatric surgery, and bariatric endoscopy are important therapeutic options.
Materials and MethodsThis prospective interventional pilot study was conducted to investigate two types of intragastric balloons (IGB). The IGBs were the Orbera and the Spatz3. Liver fibrosis changes were monitored non-invasively using point and 2D shear wave ultrasound elastography (SWE) and transient elastography that allowed for quantification of liver steatosis using the controlled attenuation parameter (CAP). Patients were followed for 12 months.
ResultsOf 34 patients implanted with an IGB, 30 completed follow-up at month 12; results for one patient were excluded because of initiation of obesity pharmacotherapy. Fifteen patients received the Orbera IGB, and nineteen patients received the Spatz3 type. In month 12, total and excess weight loss was 7.88 % and 30.13 %. Elastography values decreased from baseline (3.88 kPa) to 3.61 kPa at month 12 (p 0.024). 2D SWE values decreased from baseline (5.42 kPa) to a value of 4.91 kPa at month twelve (p 0.135). Transient elastography values decreased from baseline (5.62 kPa) to a value of 4.17 kPa at month twelve (p 0.009).
ConclusionsBariatric endoscopy in the form of IGB implantation leads to weight reduction and improvement of liver fibrosis and steatosis.
ClinicalTrials.gov registrationNCT04895943
Liver disease has a significant impact on morbidity and mortality worldwide, with liver cirrhosis and liver cancer listed as the 11th and 16th most common causes of death, respectively [1]. Globally, non-alcoholic fatty liver disease (NAFLD), in particular, is expected to increase. The disease is characterised histologically as >5 % representation of steatotic hepatocytes in liver tissue, in two categories: simple steatosis, known as non-alcoholic fatty liver (NAFL) and associated only with fat accumulation in liver tissue, and non-alcoholic steatohepatitis (NASH), in which an inflammatory process accompanies fat accumulation [2]. NAFLD is closely associated with metabolic syndrome and obesity, and like these conditions, its incidence and prevalence are increasing in all regions of the world [3]. A global NAFLD prevalence of around 25 %, reported in a 2016 meta-analysis [4], has increased to an estimated 32 %, according to a 2022 meta-analysis by Riazi et al. [5].
The relationship between NAFLD and metabolic syndrome is captured in the new terms [6] metabolic dysfunction–associated steatotic liver disease (MASLD) and metabolic dysfunction–associated steatohepatitis (MASH). Diagnosis requires the presence of cardiometabolic risk factors in addition to hepatic steatosis without the need to rule out other liver diseases. With regard to the different criteria for diagnosing NAFLD or MASLD, where they are not exactly the same cohorts of patients [7], and since we used older criteria in this publication, we continue to refer to the disease as NAFLD/NASH.
NAFLD shares aetiopathogenesis with metabolic syndrome, such as lack of exercise and excessive dietary energy intake, especially involving a high-calorie diet [8]. The increasing prevalence of NAFLD/MASLD and especially the NASH/MASH subcategory, is already beginning to influence hepatologic care worldwide, as NASH cirrhosis is the second most common indication for liver transplantation in the United States and Europe [9,10]. NAFLD also entails many extrahepatic complications; the presence of simple steatosis alone is associated with a higher incidence of the components of metabolic syndrome, and NAFLD is an independent risk factor for cardiovascular disease and is associated with chronic renal disease, hypothyroidism, and other diseases [11,12]. Treatment of NAFLD consists mainly of lifestyle modification with restriction of food intake and adequate physical activity [2], but these interventions often fail and efforts are ongoing to identify pharmaceutical candidates that lead to regression of steatosis and fibrosis. Few known drugs have shown a clear effect, and bariatric surgery and bariatric endoscopy are options selected as interventions; the intragastric balloon (IGB) is one of the endoscopic procedures [13].
The aim of our study was to assess the prevalence of hepatic steatosis (NAFLD) among a cohort of patients undergoing bariatric endoscopy in the form of IGB implantation and to evaluate the effect of this intervention on ultrasound liver elastography and fat quantification by CAP and other metabolic and anthropometric parameters.
2Material and Methods2.1PatientsPatients were recruited those interested in bariatric endoscopy, specifically in the form of IGB, and the examination was performed at the Department of Internal Medicine and Cardiology, Division of Gastroenterology, Hepatology and Pancreatology, University Hospital Ostrava, Ostrava, Czech Republic. Initial examination and screening for other hepatopathies took place in December 2020, and actual IGB implantation took place between February 2021 and February 2022, with follow-up until February 2023. The entry criteria were age 18 to 75 years, BMI <27 kg/m2, and the failure of standard weight loss methods and cognitive behavioral approaches to weight reduction. Exclusion criteria for the study was excessive alcohol intake and use of addictive substances, history of surgery on stomach and bariatric surgery, severe disorder of upper gastrointestinal tract (e.g., achalasia, severe esophagitis, strictures, ulcers), coeliac disease and other malabsorption disorders, idiopathic bowel disease or other severe gastrointestinal tract disorder, type 1 diabetes mellitus or type 2 diabetes mellitus on insulin therapy, specific genetic diseases associated with obesity, uncontrolled thyroid disorders or other serious endocrinological disorders, history of malignant disease, haematopoietic disorders, serious cardiovascular diseases, autoimmune diseases, on immunosuppressant or glucocorticoid therapy, serious psychiatric diseases, advanced renal insufficiency, patients with BMI >50 or the inability to adequately measure elastography, pregnant patients. All patients were followed by a multidisciplinary team, including an experienced internal medicine physician and a board-certified gastroenterologist with many years of practice.
Comprehensive clinical and laboratory examinations and bioimpedance anthropometric measurements were performed on all patients before the intervention.
2.2Basic clinical investigationsIn bariatric endoscopy candidates, we initially performed a comprehensive clinical examination, a questionnaire survey, and blood draws for a comprehensive biochemical analysis after fasting overnight. Serum aliquots were frozen for later analysis of adipokines or other special parameters. Patients underwent abdominal sonography with a focus on parenchymal organs, including liver elastography. Initial blood counts, coagulation, and routine biochemistry were evaluated along with lipid metabolism and a panel focused on liver screening. Using these investigations, we excluded other hepatopathies (infectious hepatitis, chronic cholestatic liver disease, autoimmune hepatitis, Wilson's disease, hereditary hemochromatosis, alpha1 antitrypsin deficiency, known liver cirrhosis and its complications, elevation of alanine transaminase [ALT] or aspartate transaminase [AST] >5× the upper limit of normal, and excessive alcohol intake according upon of regular alcohol consumption of >20 g/d in females and >30 g/d in males).
Non-invasive assessment of liver fibrosis by liver elastography was performed on a Samsung RS85 Prestige ultrasound device in two modes (point shear wave elastography [SWE] and 2D SWE) and on a Fibroscan 630 Expert device, which, in addition to transient elastography (TE), could quantify liver fat content using the controlled attenuation parameter (CAP). Candidate patients without contraindications were then sent for diagnostic gastroscopy followed by IGB implantation. After implantation, patients were under the care of a nutritionist.
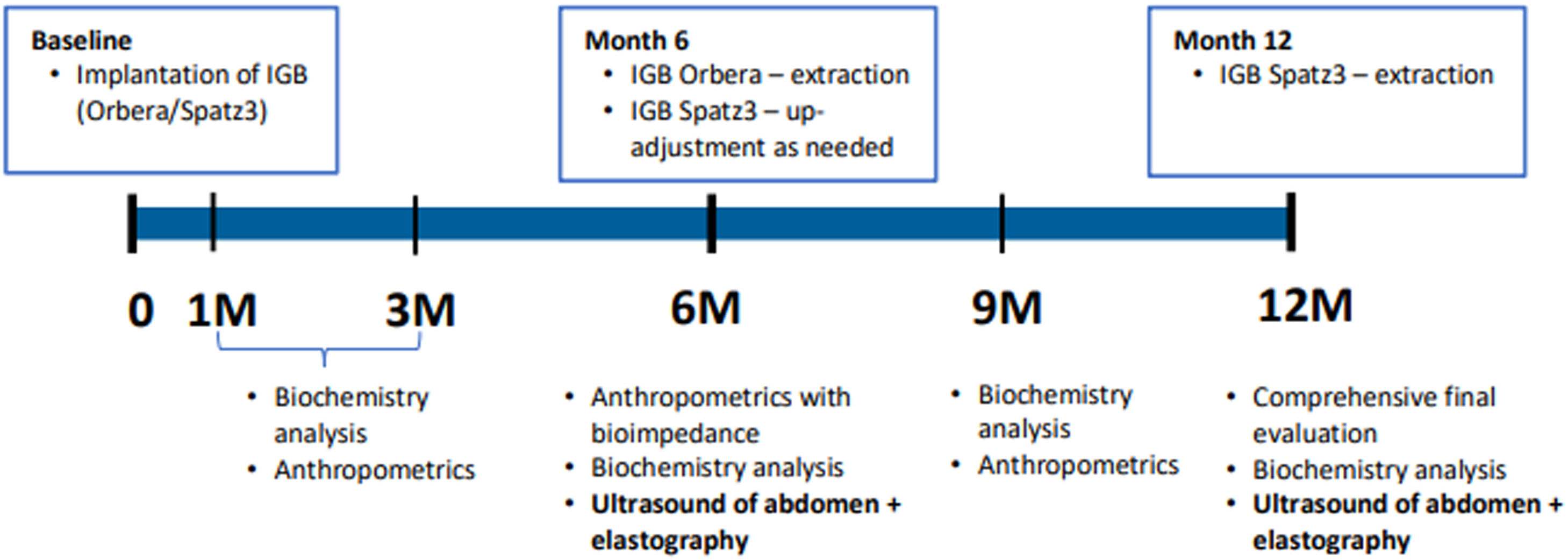
2.3Endoscopic interventionsWe used two types of IGB, the Orbera®(Apollo Endosurgery), designed to be inserted for 6 months, and the Spatz3®, designed to be inserted for 12 months. For the second type, in case of an insufficient effect on weight loss at 6 months, down-adjustment or up-adjustment could be used (i.e., partial release or filling of the balloon). The type of IGB used in each patient was randomised and chosen according to a randomisation list. This study was not blinded. Further follow-ups with necessary investigations were performed according to the scheme shown in Fig. 1. The main investigations were carried out generally at 6 and 12 months when follow-up examinations were performed with abdominal ultrasound and liver elastography in addition to extensive serum collection and serum aliquot freezing.
2.4Laboratory examinationsBlood samples for analysis were obtained by venepuncture after an overnight fast and subsequently processed within 20 min. Blood counts were conducted in a Sysmex XN-9000 (Sysmex Corporation, Japan). The routine biochemical examination included serum concentrations of ALT, AST, alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), bilirubin, glucose, triglycerides (TGs), total and high- and low-density lipoprotein cholesterol (HDL, LDL), and albumin (Atellica CH 930 analyser, Siemens Healthcare Diagnostics Inc., USA). Glycated haemoglobin A1C (HbA1c) was measured on an ARKRAY HA-8180 V ADAMS A1c analyser (Arkray, Inc., Japan). In all patients, as part of the initial laboratory to exclude another liver disease, we examined hepatology screening; we assessed iron metabolism (ferritin, transferrin, transferrin, and serum iron saturation), vitamin B12, vitamin D and folate, ceruloplasmin, alpha-1-antitrypsin, total immunoglobulins G, A, and M, and thyroid-stimulating hormone; excluded hepatitis A, B, and C infection by serology; and performed liver and celiac autoantibody screening according to convention.
2.5Imaging, ultrasound, and elastography of the liverStandard abdominal sonography focusing on the parenchymal organs was performed in patients after a minimum of 6 h of fasting on a Samsung Prestige RS85 machine using a conventional convex probe. Subsequently, we non-invasively measured liver elastography on the same device according to the standard recommendations, focusing on the most homogeneous regions of the liver between segments S5 and S8 and avoiding subcapsular areas. During the actual elastography measurement, the patient always performed a brief breath hold without taking a deep breath, and the measurement was taken using point SWE (S-Shearwave™, per the manufacturer) and 2D SWE (S-Shearwave Imaging™). In each mode, 10 elastographic measurements were taken each time, and the machine selected the median of the individual measurements, with the result given in kPa. A table of cut-off values supplied by the manufacturer was used to assess the degrees of fibrosis. We also measured the distance between the skin and the liver capsule during the elastography, the so-called skin-to-liver distance (SLD).
Liver elastography and fat content measurements using a Fibroscan 630 Expert were performed at the University Hospital Olomouc, II. Internal Clinic according to normal standards after a fasting period of 6 h Liver elastography was measured by TE by obtaining the elastic parameter in kPa and quantifying liver fat by CAP in dB/m. The machine used 10 valid measurements to calculate median values, and the degrees of fibrosis and liver steatosis were assessed according to the cut-off values determined from a table supplied by the manufacturer.
2.6Statistical analysisData were stored and processed in the central data warehouse of the University Hospital Ostrava and evaluated using descriptive standard statistics (means, standard deviations, and medians). We used R software (The R Foundation for Statistical Computing, Vienna, Austria). To determine statistical significance, corresponding to p < 0.05, we used the t-test, analysis of variance, Fisher's exact test, or the Kruskal–Wallis test.
2.7Ethical statementThis prospective interventional study (ClinicalTrials.gov registration: NCT04895943) was approved by the local ethics committee of the University Hospital Ostrava (ref. number 351/2021). The ethical principles were followed according to standards corresponding to the Declaration of Helsinki of 1975, revised in 2019.
3Results3.1Characteristics of the patients and interventionOf 43 screened patients, 34 were included in the study; the rest of the patients did not wish to participate in the study after the initial interview or did not meet the inclusion criteria. Our patients had undergone IGB placement via endoscopic bariatric surgery, with 15 patients receiving Orbera® and 19 receiving Spatz3®. At 6 months, 32 patients had completed follow-up, and at 12 months, 30 patients had done so. Results for one patient were excluded because of the initiation of obesity pharmacotherapy before the end of the study. The flowchart of our work is in the Fig. 2. At the time of entry, there were six men (18.2 %) and 27 women (82.8 %) with a mean age of 42.4 ± 8.1 years (minimum-maximum, 24 and 60 years) and a mean BMI of 36.4 ± 4.48 (minimum–maximum 28 and 50.1). Regarding risk factors, three patients (9.1 %) were active smokers, two patients (6.1 %) had a known diagnosis of type 2 diabetes mellitus (T2DM), 10 patients (30.3 %) had been or were being treated for arterial hypertension, and three patients (9.1 %) had known or treated dyslipidaemia.
3.2Adverse events (AEs)No serious adverse events (SAEs) related to IGB Spatz or Orbera were reported. The severity rating for AEs was mild – awareness of signs or symptoms that do not interfere with usual activity; moderate – symptoms that interfere with usual activity; and severe – inability to do work or perform usual activities. Overview of AEs (Table 1)
Adverse events.
| Adverse event | Total (N) and proportion (%) Spatz/Orbera | ||
|---|---|---|---|
| Ballon deflation, migration into small intestine, gastrointestinal obstruction, death | 0 | 0 | |
| AEs regardless of their attribution to the balloons | Eructation | 15 (79 %) | 11 (73 %) |
| Dyspepsia | 15 (79 %) | 9 (60 %) | |
| Abdominal distension | 12 (63 %) | 19 (53 %) | |
| Nausea | 13 (68 %) | 7 (47 %) | |
| Severe AEs related to the baloon | Nausea | 3 (16 %) | 2 (13 %) |
| Dyspepsia | 3 (16 %) | 3 (20 %) | |
| Vomiting | 2 (11 %) | 3 (20 %) | |
| Diarrhea | 2 (11 %) | 0 | |
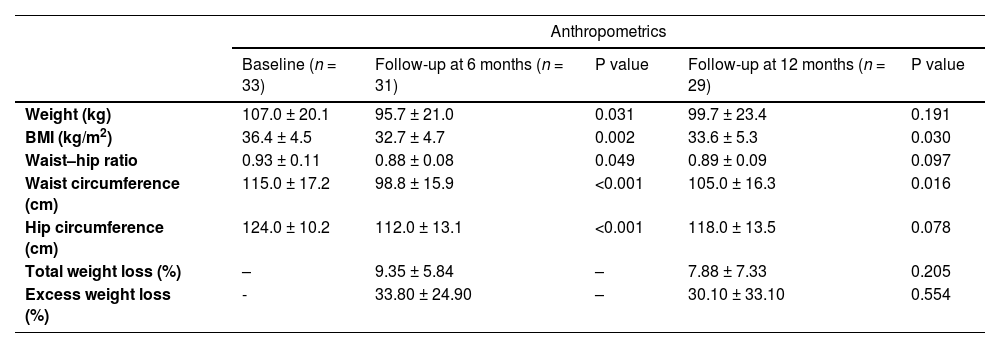
Among anthropometric parameters, weight decreased significantly from 107.0 ± 20.1 kg at baseline to 95.7 ± 21.0 kg at 6 months (p = 0.031), corresponding to a mean weight loss of 11.3 kg. Weight had decreased to 99.7 ± 23.4 kg at month 12 (p = 0.191), for a mean weight loss of 7.3 kg (Table 2).
Changes in anthropometric parameters before and after intervention.
| Anthropometrics | |||||
|---|---|---|---|---|---|
| Baseline (n = 33) | Follow-up at 6 months (n = 31) | P value | Follow-up at 12 months (n = 29) | P value | |
| Weight (kg) | 107.0 ± 20.1 | 95.7 ± 21.0 | 0.031 | 99.7 ± 23.4 | 0.191 |
| BMI (kg/m2) | 36.4 ± 4.5 | 32.7 ± 4.7 | 0.002 | 33.6 ± 5.3 | 0.030 |
| Waist–hip ratio | 0.93 ± 0.11 | 0.88 ± 0.08 | 0.049 | 0.89 ± 0.09 | 0.097 |
| Waist circumference (cm) | 115.0 ± 17.2 | 98.8 ± 15.9 | <0.001 | 105.0 ± 16.3 | 0.016 |
| Hip circumference (cm) | 124.0 ± 10.2 | 112.0 ± 13.1 | <0.001 | 118.0 ± 13.5 | 0.078 |
| Total weight loss (%) | – | 9.35 ± 5.84 | – | 7.88 ± 7.33 | 0.205 |
| Excess weight loss (%) | - | 33.80 ± 24.90 | – | 30.10 ± 33.10 | 0.554 |
To probe the cause of the less steep decreases in weight loss by month 12 versus month 6, we conducted a sub-analysis of changes in weight, BMI, and waist and hip circumference according to the type of IGB introduced. As the results presented in Table 3 show, this subgroup of patients had an Orbera IGB, which was intended to be inserted for 6 months, so that at month 12 after implantation, they had been IGB-free for half a year. As many of these patients had regained weight, this factor negatively affected the results.
Changes in anthropometric parameters and liver parameters before and after intervention according to IGB type.
| IGB | Sub-analysis of anthropometrics by IGB type | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 19)/(n = 15) | Follow-up at 6 months (n = 19)/(n = 13) | P value | Follow-up at 12 months (n = 17)/(n = 13) | P value | ||
| Weight (kg) | Spatz3 | 106.0 ± 15.8 | 94.6 ± 16.2 | 0.041 | 95.1 ± 22.0 | 0.105 |
| Orbera | 108.0 ± 24.9 | 97.3 ± 26.9 | 0.268 | 106.0 ± 24.8 | 0.821 | |
| BMI (kg/m2) | Spatz3 | 35.7 ± 3.7 | 32.1 ± 3.47 | 0.005 | 32.0 ± 5.1 | 0.023 |
| Orbera | 37.3 ± 5.3 | 33.6 ± 6.0 | 0.098 | 35.8 ± 5.0 | 0.459 | |
| Waist circumference (cm) | Spatz3 | 117.0 ± 18.8 | 101.0 ± 12.1 | 0.005 | 101.0 ± 15.1 | 0.009 |
| Orbera | 113.0 ± 15.2 | 95.8 ± 20.0 | 0.018 | 110.0 ± 17.3 | 0.656 | |
| Hip circumference (cm) | Spatz3 | 123.0 ± 7.9 | 113.0 ± 9.7 | 0.002 | 115.0 ± 12.7 | 0.037 |
| Orbera | 125.0 ± 12.6 | 111.0 ± 17.0 | 0.024 | 123.0 ± 13.7 | 0.752 | |
| point SWE (kPa) | Spatz3 | 3.57 ± 0.61 | 3.22 ± 0.74 | 0.130 | 3.46 ± 0.48 | 0.563 |
| Orbera | 4.26 ± 1.16 | 3.57 ± 0.64 | 0.060 | 3.83 ± 1.07 | 0.323 | |
| 2D SWE (kPa) | Spatz3 | 5.18 ± 2.28 | 4.72 ± 0.58 | 0.158 | 4.68 ± 0,69 | 0.137 |
| Orbera | 5.72 ± 2.29 | 4.94 ± 0.95 | 0.242 | 5.23 ± 0.81 | 0.454 | |
| Transient elastography (kPa) | Spatz3 | 6.26 ± 2.66 | 4.52 ± 1.07 | 0.021 | 4.44 ± 1.35 | 0.023 |
| Orbera | 4.89 ± 2.02 | 4.60 ± 1.38 | 0.671 | 3.62 ± 1.08 | 0.080 | |
| CAP (dB/m) | Spatz3 | 278 ± 54.8 | 241 ± 45,1 | 0.048 | 236 ± 53.1 | 0.052 |
| Orbera | 298 ± 56.9 | 278 ± 54.6 | 0.373 | 285 ± 28.1 | 0.473 | |
Point SWE – point shear wave elastography, 2D SWE – two-dimensional shear wave elastography, CAP - controlled attenuation parameter, BMI – body mass index.
Liver fibrosis and steatosis were evaluated non-invasively by liver elastography using a Samsung Prestige RS85 ultrasound device in two SWE modes and by TE on a Fibroscan 630 Expert device, along with quantification of fat in the liver by CAP. The results are shown in Table 4. Liver stiffness decreased significantly when point SWE was measured at month 6, whereas values at month 12 were no longer significant compared with baseline. With TE, liver stiffness was significantly lower at months 6 and 12 compared with baseline after the intervention. Fat content, as measured by CAP, significantly decreased in both months 6 and 12 compared with the baseline after the intervention. When focusing on the quality and reliability of elastography, there was an improvement in the IQR/Med parameter at months 6 and 12, statistically significant in IQR/Med for point SWE. SLD parameter was significantly smaller at months 6 and 12.
Liver elastography values, liver fat quantification, change in biochemical parameters before and after intervention.
| Elastography and biochemical parameters (mean ± SD) | |||||
|---|---|---|---|---|---|
| Baseline (n = 33) | Follow-up at 6 months (n = 31) | P value | Follow-up at 12 months (n = 29) | P value | |
| Point SWE (kPa) | 3.88 ± 0.95 | 3.37 ± 0.71 | 0.016 | 3.61 ± 0.78 | 0.224 |
| 2D SWE (kPa) | 5.42 ± 1.77 | 4.81 ± 0.75 | 0.076 | 4.91 ± 0.78 | 0.135 |
| Transient elastography (kPa) | 5.62 ± 2.45 | 4.55 ± 1.18 | 0.035 | 4.17 ± 1.30 | 0.009 |
| SLD (mm) | 23.48 ± 4.85 | 19.58 ± 4.24 | <0.001 | 19.92 ± 4.92 | <0.001 |
| IQR/Med point SWE (%) | 31.3 ± 14.80 | 20.7 ± 6.94 | 0.001 | 18.7 ± 7.22 | <0.001 |
| IQR/Med 2D SWE (%) | 25.5 ± 14.23 | 20.97 ± 8.67 | 0.119 | 20.49 ± 9.35 | 0.128 |
| CAP (dB/m) | 287.0 ± 55.9 | 257.0 ± 51.8 | 0.036 | 252.0 ± 51.1 | 0.031 |
| ALT (µkat/L) | 0.53 ± 0.30 | 0.49 ± 0.29 | 0.621 | 0.51 ± 0.30 | 0.780 |
| AST (µkat/L) | 0.40 ± 0.13 | 0.39 ± 0.13 | 0.703 | 0.42 ± 0.14 | 0.616 |
| ALP (µkat/L) | 1.31 ± 0.33 | 1.26 ± 0.31 | 0.497 | 1.16 ± 0.28 | 0.059 |
| GGT (µkat/L) | 0.50 ± 0.33 | 0.34 ± 0.18 | 0.017 | 0.39 ± 0.29 | 0.168 |
| Glycaemia (mmol/L) | 5.72 ± 0.97 | 5.29 ± 0.46 | 0.027 | 5.39 ± 0.79 | 0.155 |
| HbA1C (mmol/mol) | 40.1 ± 6.3 | 36.7 ± 3.3 | 0.009 | 37.1 ± 5.4 | 0.052 |
| Total cholesterol (mmol/L) | 5.05 ± 0.96 | 4.51 ± 0.89 | 0.021 | 4.58 ± 0.68 | 0.029 |
| HDL (mmol/L) | 1.33 ± 0.32 | 1.28 ± 0.32 | 0.532 | 1.38 ± 0.34 | 0.517 |
| LDL (mmol/L) | 3.43 ± 0.97 | 3.09 ± 0.97 | 0.170 | 3.19 ± 0.79 | 0.277 |
| TG (mmol/L) | 1.58 ± 0.58 | 1.34 ± 0.57 | 0.099 | 1.40 ± 0.64 | 0.246 |
Point SWE – point shear wave elastography, 2D SWE – two-dimensional shear wave elastography, SLD – skin-to-liver distance, IQR/Med – inter quartile range/median, CAP - controlled attenuation parameter, ALT – alanine transaminase, AST – aspartate transaminase, ALP – alkaline phosphatase, GGT – Gamma-glutamyl transferase.
HbA1C - hemoglobin A1C, HDL – high density lipoprotein, LDL – low density lipoprotein, TG - triglycerides.
We also evaluated the representation of different grades of fibrosis in patients at each follow-up. Because individual fibrosis grades differed slightly among different methods, we developed a categorical assessment based on METAVIR scores, as follows: no fibrosis (F0 with Samsung RS85 vs. F0–1 with Fibroscan), minimal fibrosis (F1 with Samsung RS85 vs. F2 with Fibroscan), significant fibrosis (F2–3 with Samsung RS85 vs. F3 with Fibroscan), and cirrhosis (F4 with both Samsung and Fibroscan). The results are shown in Fig. 3. The representation of liver steatosis grades according to fat quantification using the CAP method is shown in Fig. 4.
3.5Metabolic and biochemical characteristicsWe observed no significant changes at 6 or 12 months in liver enzymes (ALT, AST, and ALP), a significant decrease in GGT at 6 months but no significant change at 12 months, and significant decreases at 6 months in fasting glucose and glycated haemoglobin (HbA1C). Total cholesterol levels had improved significantly at the 6-month and 12-month assessments; however, we observed no significant changes in HDL, LDL, or TGs levels (Table 5). The percentage of laboratory-measured abnormal metabolic abnormalities at each follow-up is shown in Fig. 5. Except for HDL, the percentage of abnormal metabolic values was lowest at the 6-month evaluation.
Change in biochemical parameters before and after intervention.
| Biochemical parameters | |||||
|---|---|---|---|---|---|
| Baseline (n = 33) | Follow-up at 6 months (n = 31) | P value | Follow-up at 12 months (n = 29) | P value | |
| ALT (µkat/L) | 0.53 ± 0.30 | 0.49 ± 0.29 | 0.621 | 0.51 ± 0.30 | 0.780 |
| AST (µkat/L) | 0.40 ± 0.13 | 0.39 ± 0.13 | 0.703 | 0.42 ± 0.14 | 0.616 |
| ALP (µkat/L) | 1.31 ± 0.33 | 1.26 ± 0.31 | 0.497 | 1.16 ± 0.28 | 0.059 |
| GGT (µkat/L) | 0.50 ± 0.33 | 0.34 ± 0.18 | 0.017 | 0.39 ± 0.29 | 0.168 |
| Glycaemia (mmol/L) | 5.72 ± 0.97 | 5.29 ± 0.46 | 0,027 | 5.39 ± 0.79 | 0.155 |
| HbA1C (mmol/mol) | 40.1 ± 6.3 | 36.7 ± 3.3 | 0.009 | 37.1 ± 5.4 | 0.052 |
| Total cholesterol (mmol/L) | 5.05 ± 0.96 | 4.51 ± 0.89 | 0.021 | 4.58 ± 0.68 | 0.029 |
| HDL (mmol/L) | 1.33 ± 0.32 | 1.28 ± 0.32 | 0.532 | 1.38 ± 0.34 | 0.517 |
| LDL (mmol/L) | 3.43 ± 0.97 | 3.09 ± 0.97 | 0.170 | 3.19 ± 0.79 | 0.277 |
| TG (mmol/L) | 1.58 ± 0.58 | 1.34 ± 0.57 | 0.099 | 1.40 ± 0.64 | 0.246 |
In the treatment of NAFLD, only two drugs have histologically demonstrable effectiveness: the antidiabetic drug pioglitazone, useful in selected patients with T2DM, and vitamin E (800 IU/day) for patients with NASH without cirrhosis and without T2DM [14]. However, these drugs are not yet indicated directly for NAFLD, and thus, the recommendations are to adequately treat individual comorbidities [15]. In the presence of T2DM, we use antidiabetic drugs. Glucagon-like peptide-1 (GLP1) agonists, newer dual agonists (GLP1 and GIP), or even triple agonists (GLP1 and GIP and glucagon receptor agonists) have shown promising drugs to improve not only liver fat but also fibrosis in some [16–19]. Another drug [20] sodium/glucose cotransporter 2 (SGLT-2) inhibitors are promising too, with an effect on the reduction of liver fat content [21–24]. Dyslipidaemia should be treated mainly with statins, which are considered safe in NAFLD, and they have indications to slow the progression of liver fibrosis and prevent liver decompensation in liver cirrhosis [25]. Obesity therapy is equally important, with promising GLP1 agonists mentioned above [26,27], or orlistat leading to improvement in steatosis and signs of NASH [28]. A new surprising mechanism of liver specific thyromimetic resmetirom showed resolution of NASH without worsening liver fibrosis [29], and this drug was recently approved by the FDA [30].
Patients with obesity are more likely to have NAFLD and thus are more at risk for disease progression compared to patients without obesity, and other appropriate treatments are being sought to effectively lead to weight reduction in this population, especially when drug therapy fails. In one meta-analysis, bariatric surgery proves [31] histological improvement of steatosis, NASH, and liver fibrosis [32]. However, a small subset of patients in that analysis had newly developed liver fibrosis, so caution is needed regarding some particularly malabsorptive surgical procedures because they may be rarely associated with liver failure after bariatric surgery [33,34].
In contrast to the widely assessed bariatric surgery, fewer studies have evaluated the effect of various endoscopic bariatric techniques on NAFLD, which are called endoscopic bariatric and metabolic therapies (EBMT). The significant decrease in weight, BMI, hip circumference and waist in our study is consistent with studies with IGB[35,36]; if we compare our results of weight loss at 6 months which was 11.3 kg, our BMI decrease was 3.7 kg/m2, EWL 36.5 %, we can say that these data are consistent with the metaanalysis of Moura et al. with decrease in weight 12.9 kg, BMI decrease was 5.2 kg/m2 and EWL 33.8 %[35]. Another metaanalysis Kotinda et al. show results from analysed studies with EWL 23.9, 34.0, 21.5, 31.8, 25.1 %. If we focus on comparing our data with respect to liver parameters, we can look at a meta-analysis of Freitas et al. [37] with GGT decrease 0.16ukat/l, which was similar to our results, reduction of HbA1c of 0.17 % (our results after conversion 0.3 %). Other metaanalysis Chandan et al. [38] focused on liver steatosis, analysed studies were variable by a diagnosis of steatosis and showed improvement in steatosis in 79.2 % assessed by imaging after 6 months in our results; we recorded an improvement in steatosis assessed by CAP in 69.2 % of patients, in this metaanalysis was EWL 32.1 %. Another study focused on IGB and hepatic steatosis by Forlano et al. [39] evaluated the percentage of patients with steatosis evaluated only by ultrasound B-mode, where after 6 months, there was an improvement from 43 % to 8 % of patients; comparison with our data is difficult when trying to compare them, we recorded an improvement from stage S3 steatosis (CAP ≥ 280 dB/m) from 51.5 % of patients to 26.9 % after 6 months.
The improvement in non-invasively measured liver fibrosis values in our study was in line with Bazerbachi et al. [40], who found that liver fibrosis measured non-invasively by magnetic resonance elastography improved at month 6 from 3.52 kPa to 3.2 kPa, which was similar to our pSWE results from 3.88 kPa to 3.37 kPa. Another study by Salomone et al. [41]., demonstrated an improvement in non-invasively measured liver fibrosis and steatosis using the TE method on the Fibroscan device after Orbera IGB implantation in patients with significant fibrosis (≥9.7 kPa). Liver stiffness improved from 13.3 kPa to 11.3 kPa at month 6, and CAP decreased from 355 to 296 dB/m. Dijk et al. [42] showed an improvement in liver elasticity measured by Fibroscan at month 6 from 6.0 kPa to 4.9 kPa and a significant improvement in CAP from 328 dB/m to 272 dB/m after IGB administration in 19 patients compared to 10 controls with lifestyle change. They had 5 % of patients in the S0 category of steatosis and 53 % in this category after the intervention.
If we compare the results with another endoscopic method, such as endoscopic sleeve gastroplasty (ESG), according to the metaanalysis Nunes et al. the effect on NAFLD was evaluated after 12 months, when according to the NAFLD fibrosis score there was an average improvement of 0.5, according to the hepatic steatosis index there was a significant decrease of 4.85, TWL was significantly better by 17.3 % and EWL by 48.0 %. Unfortunately, they did not evaluate steatosis or fibrosis either according to histology or imaging examinations. [35,36,39–41,43-46].
Three important limitations of our study were the absence of a control group and a small cohort of patients, and it included only non-invasive measurements of liver fibrosis and steatosis. However, liver biopsy is a problematic invasive procedure with certain complications and the possibility of sampling error; in addition, fear of this procedure discourages many patients from participating in studies. Fortunately, non-invasive ultrasound methods for the evaluation of liver fibrosis and steatosis are well-established and correlate well with histological findings [47]. Contrary to expectations, we had a small number of patients with advanced fibrosis [48], where we see the reason in the representation of younger patients, and since we were not able to validly measure liver elastography in patients mostly exceeding BMI 40–45 kg/m2, in whom we would expect a higher incidence of significant fibrosis. One reason for the latter may be the increasing SLD, which is larger in more obese patients due to the fat layer. Larger SLD is associated with lower elastography accuracy [49–51]; in our work, SLD decreased at the months 6 and 12 after intervention, which corresponded to an improvement in the IQR/Med elastography quality parameter.
5ConclusionsWe can conclude that performing bariatric endoscopy, specifically with IGB, leads to weight reduction and improved liver stiffness, as measured by ultrasound hepatic elastography, and liver fat reduction is measured by CAP. Results for reduced liver stiffness were statistically significant at the 6-month follow-up with the point SWE method and at the 6- and 12-month follow-up with the TE method. A statistically significant reduction of steatosis according to the CAP parameter was achieved at the 6- and 12-month follow-up. The less favorable results at month 12 were probably influenced by a subset of participants who regained at least some weight at 6 months after Orbera IGB extraction (and 12 months of follow-up), affecting 12-month findings for the entire study population. Increasing the number of participants and strengthening the design with biopsy assessment of fibrosis and steatosis would address some of these limitations.
FundingThis work was supported by the Ministry of Health, Czech Republic – conceptual development of research organisation (FNOs/2021). Publication of this work was financial supported by Endowment Fund of the Czech Society of Hepatology.
Data availabilityThe data sets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Authors contributionsStudy concept and design: AV, MB, and EM; acquisition of data: AV, JM, and ZB; analysis and interpretation of data: AV, LP, and MB; drafting of the manuscript: AV, MB; critical revision of the manuscript for important intellectual content: OU, MB, ZŠ and OU; statistical analysis LP and JM; obtained funding: AV; study supervision: OU. All the authors have read and approved the final manuscript.