A high prevalence of steatotic liver disease has been described in psoriasis. However, the influence of genetic polymorphisms has yet to be investigated in this scenario. This study aims to determine the frequency of steatosis, advanced liver fibrosis and PNPLA3/TM6SF2 genotypes in individuals with psoriasis and to evaluate the impact of genetic polymorphisms, metabolic parameters and cumulative methotrexate dose on steatosis and fibrosis.
Materials and MethodsCross-sectional study that prospectively included psoriasis outpatients, submitted to clinical and laboratory analysis, transient elastography (FibroScan®, Fr) and PNPLA3/TM6SF2 genotyping. Steatosis was defined by CAP ≥275 dB/m and advanced liver fibrosis as transient elastography ≥10 kPa. Logistic regression analysis evaluated the independent variables related to steatosis and fibrosis; p-value< 0.05 was considered significant.
ResultsOne hundred and ninety-nine patients were enrolled (age 54.6 ± 12.6 years, 57.3% female). Metabolic syndrome (MetS), steatosis and advanced liver fibrosis prevalence were 55.8%, 54.8% and 9%, respectively. PNPLA3 and TM6SF2 genotypes frequencies were CC 42.3%/CG 49.5%/GG 8.2% and CC 88.7%/ CT 11.3%/ TT 0%. MetS (OR3.01 95%CI 1.51-5.98; p = 0.002) and body mass index (OR1.17 95%CI 1.08-1.26; p < 0.01) were independently associated with steatosis. Diabetes Mellitus (T2DM) (OR10.76 95%CI 2.42-47.87; p = 0.002) and harboring at least one PNPLA3 G allele (OR5.66 95%CI 1.08-29.52; p = 0.039) were associated with advanced fibrosis, but not TM6SF2 polymorphism or cumulative MTX dose.
ConclusionsMetS and T2DM confer higher odds for steatosis and advanced fibrosis in individuals with psoriasis. PNPLA3 G allele, but not TM6SF2 polymorphism, impacts a 5-fold odds of advanced liver fibrosis.
Psoriasis (Pso) is a chronic, immune-mediated inflammatory skin disease whose severity depends on environmental and inherited factors [1]. Prevalence in Western countries is around 2–4% of the population, depending on age and ethnicity [2], and it is associated with metabolic syndrome (MetS) [3,4]. Psoriatic patients have a two-fold risk of Steatotic Liver Disease (SLD) compared to non-psoriatic controls [5]. SLD is bi-directionally associated with MetS and its components, which increases the risk of advanced fibrosis and cirrhosis [6], especially in specific populations like patients with type 2 Diabetes Mellitus (T2DM) and medically complicated obesity [7]. A new perspective of the liver as “a non-innocent bystander” in the steatosis scenario has emerged, as hepatic lipid accumulation also impacts glucose and lipid metabolisms [8].
Observational studies have demonstrated associations between Pso and SLD in recent decades, even independently of MetS [9,10]. In retrospective studies, liver fibrosis in Pso has been linked to MetS and SLD more than the cumulative methotrexate (MTX) dose despite difficulties in histological differentiation since both MTX and SLD share common histological aspects [11–15]. A recent large longitudinal cohort study found no association between cumulative MTX dose and elevated liver stiffness or serologic markers of fibrosis, showing that T2DM was the most significant risk factor associated with liver stiffness [16].
Since 2016, a Hepato-Dermal Axis hypothesis has emerged, demonstrating multiple inflammatory and cytokine-mediated mechanisms shared by the steatotic liver and Pso, coming from a unique trigger, such as the expanded and inflamed adipose tissue [17]. Although it appears reasonable, there may be additional factors to justify the independent association between SLD and Pso and, especially, fibrosis.
Patatin-like phospholipase domain-containing 3 gene (PNPLA3) polymorphism I148M (rs738409) has emerged as one of the key genetic determinants of SLD, metabolic steatohepatitis and progressive liver fibrosis, irrespective of body mass index (BMI), insulin resistance and dyslipidemia [18,19]. In addition, a polymorphism of the transmembrane 6 superfamily member 2 gene (TM6SF2), variant E167K (rs58542926), has also been associated with SLD independently of PNPLA3 variant I148M effect, but equally not associated with BMI and insulin resistance [20]. So far, the impact of both polymorphisms has not been evaluated in Pso patients.
Considering the potential association between Pso and SLD, this study aimed to determine the frequency of steatosis, advanced liver fibrosis, PNPLA3 and TM6SF2 genotypes in this population. Additionally, we sought to evaluate the impact of genetic polymorphisms, metabolic parameters and cumulative MTX dose on steatosis and advanced liver fibrosis in individuals with psoriasis.
2Material and methods2.1Study design and patientsThis bicentric cross-sectional study included outpatients with established Pso diagnosis (clinically and/or histologically), at least 18 years old, regardless of the type of Pso-specific treatment, from dermatology divisions at tertiary hospitals, Federal Hospital of Bonsucesso and Clementino Fraga Filho University Hospital of the Federal University of Rio de Janeiro, Brazil.
Exclusion criteria were: HIV, hepatitis B and hepatitis C, as well as those with other etiologies for chronic liver diseases; use of hepatotoxic drugs in the last six months, steatogenic drugs (except MTX) like systemic corticosteroids, amiodarone, valproic acid and tamoxifen in the last two years or systemic chemotherapy in the last five years; daily alcohol intake greater than 20g for women and 30g for man in the last five years; conditions that could interfere with liver stiffness analysis (liver congestion, ascites, serum aminotransferase values greater than five times the upper standard limit, cholestasis and pregnancy).
2.2Study proceduresIndividuals included in the study were submitted to clinical and laboratory evaluation and liver stiffness /controlled attenuation parameter (CAP) measurements using transient elastography (TE) (FibroScan® TOUCH 502, Echosens, Fr). Blood sample collection for PNPLA3/TM6SF2 genotyping, metabolic evaluation, and TE were performed on the same day.
2.3Demographic, clinical and laboratory variablesDemographic (sex, age), anthropometric (BMI, abdominal circumference) and clinical data (diagnosis of T2DM, systemic arterial hypertension, dyslipidemia and MetS according to ATPIII criteria [21] were collected. Data regarding clinical psoriasis characteristics were: time since psoriasis onset (time since the first cutaneous lesion onset, reminded by the patient), time of dermatological follow-up (defined as the time since any dermatologic treatment/follow-up until the study enrollment date), use of immunobiological (anytime) and cumulative MTX dose. Cumulative MTX doses ≥1500 mg were considered at risk for steatosis and fibrosis [13].
Laboratory data included alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, glycated hemoglobin and platelet count. Liver enzymes (AST, ALT and GGT) were analyzed as absolute values and indexes (absolute value/ upper standard limit).
2.4Liver stiffness measurement (LSM) and controlled attenuation parameter (CAP)Liver stiffness measurement was performed with at least 3 hours of fasting by a single experienced operator using Fibroscan® TOUCH 502 (Echosens, France) with M and XL probes designed for this device. The technique used was previously described [22]. Only results with ten valid shots, success rate > 60% and an interquartile interval (IQR) /median liver stiffness ratio ≤30% were included in the analysis. The results were expressed in kilopascals (kPa). The CAP was simultaneously evaluated within valid LSM and was expressed in decibels per meter (dB/m). The XL probe, designed to evaluate measurements between 35 mm and 75 mm in depth (against 25–65 mm in the M probe), was used in those patients who failed to obtain valid measurements with the M probe. The best cut-off for advanced liver fibrosis was 10 kPa [23]. CAP results equal to or greater than 275 dB/m are defined as the presence of steatosis [24].
2.5Genetic analysis of PNPLA3 and TM6SF2 polymorphismsDNA extraction was performed according to the procedures recommended by the manufacturer of the “Qiamp DNA Mini Kit” (Qiagen), followed by quantification and determination of the degree of purity via spectrophotometric readings at 260 and 280 nm in an Epoch equipment (BioTek Instruments). The polymorphisms rs738409 (C / G) of the PNPLA3 gene and rs58542926 (C / T) of the TM6SF2 gene were genotyped using specific allele TaqMan® (Applied Biosystems) probes via real-time polymerase chain reaction (qPCR), using the CFX96 thermocycler (Bio-Rad), under universal amplification conditions. The identified alleles characterized three genotypes of PNPLA3 (CC, CG and GG) and TM6SF2 (CC, CT and TT). After genotyping, the SNP analysis tool (https://www.snpstats.net/start.htm) was used to determine whether the studied sample was in Hardy-Weinberg equilibrium. The presence of at least one allele G for the PNPLA3 gene and at least one allele T for the TM6SF2 gene were considered for analysis.
2.6Statistical analysisData was recorded in case report forms and entered in SPSS 21.0 software (IBM Corp, Armonk, New York). Categorical and continuous variables were analyzed and expressed as frequencies for categorical variables. Continuous variables were expressed as means with standard deviation or medians with interquartile intervals in the case of parametric or non-parametric distribution, respectively. Univariate analysis was performed using the Chi-square or Fisher test for categorical variables and Student's t-test or Mann-Whitney test for continuous variables as appropriate. Binary logistic regression analysis was performed to identify variables independently associated with steatosis and fibrosis. The variables included in the model were those with clinical plausibility or p-values < 0.20 in the univariate analysis. The significance level adopted was 5%, with descriptive levels (p) below this value being considered statistically significant.
2.7Ethical statementsWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of Hospital Universitário Clementino Fraga Filho, Federal University of Rio de Janeiro (CAAE 13459019.2.0000.5257) and Hospital Federal de Bonsucesso (CAAE 13459019.2.3001.5253).
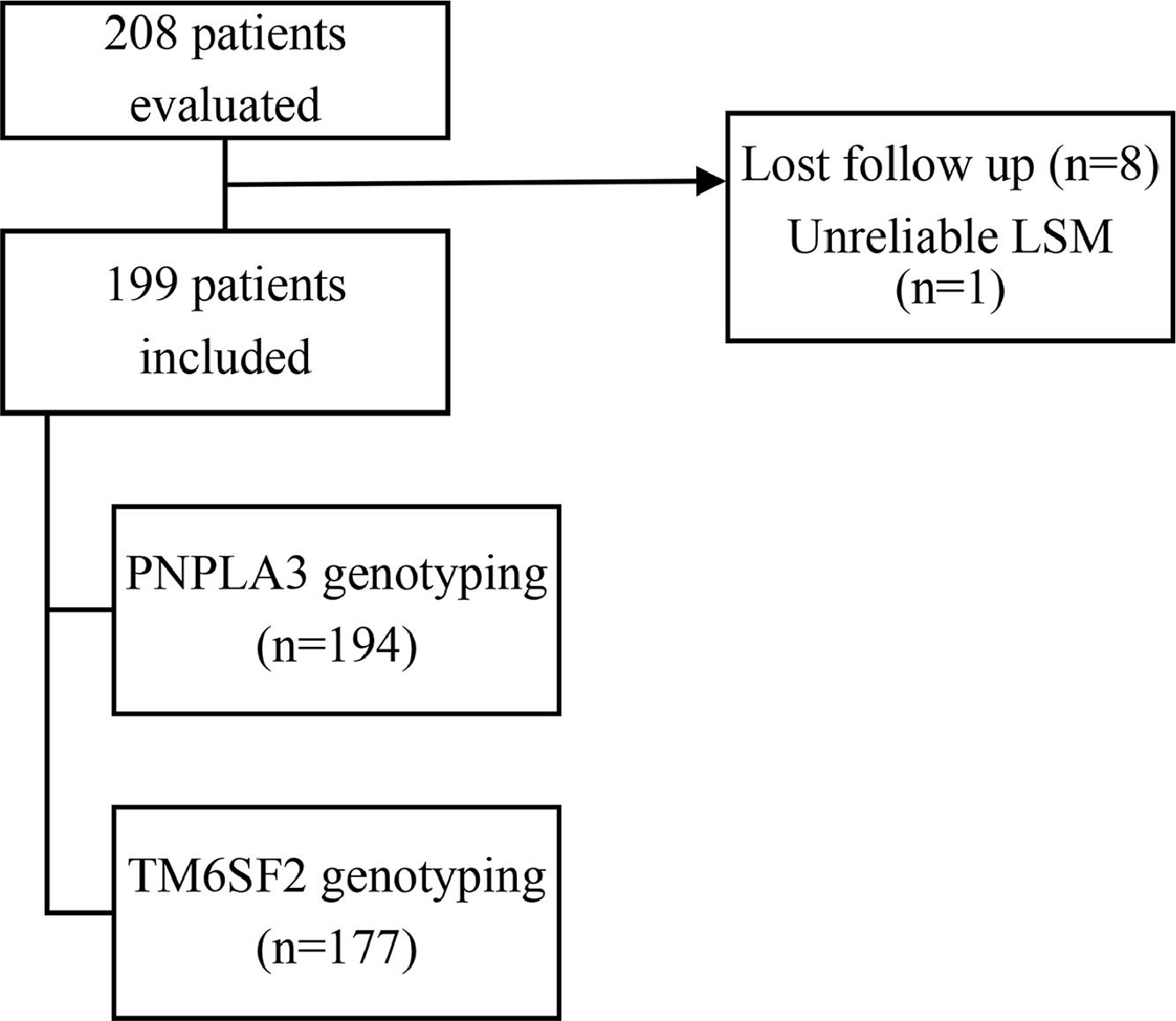
3ResultsTwo hundred and eight patients were selected; eight lost follow up, and one was excluded due to unreliable LSM. Thus, one hundred and ninety-nine patients were enrolled for the analysis. All patients had blood collected adequately. Unequivocal PNPLA3 and TM6SF2 genotyping data were achieved in 194 and 177 patients, respectively (Fig. 1). Demographic, clinical and laboratory data, PNPLA3/TM6FS2 genotypes, TE and CAP data were shown in Table 1. The distribution of genotypes for PNPLA3/TM6FS2 on this studied sample was in Hardy-Weinberg equilibrium (data not shown).
Study population characteristics.
| Variables | n = 199 |
|---|---|
| Demographic, anthropometric and clinical data | |
| Female sex (%) | 57.3 |
| Age (years) | 54.6 ± 12.6 |
| BMI (Kg/m2) | 29.3 ± 5.7 |
| BMI>25 Kg/m2 (%) | 75.0 |
| BMI>30 Kg/m2 (%) | 37.7 |
| Abdominal circumference (cm) | 103.0 ± 13.2 |
| Type 2 Diabetes Mellitus (%) | 30.7 |
| Arterial hypertension (%) | 60.8 |
| Dyslipidemia (%) | 69.8 |
| Metabolic Syndrome (%) | 55.8 |
| Clinical psoriasis characteristics | |
| Time since psoriasis onset (months) | 146.0 (78.6-264.0) |
| Time of dermatological follow-up (months) | 45.0 (16.0-97.5) |
| MTX treatment (%) | 79.3 |
| Cumulative MTX dose (mg) | 1365 (663.7-3570) |
| Cumulative MTX dose ≥ 1500mg (%) | 38.6 |
| Cumulative MTX dose ≥ 1500mg (mg) | 3600 (2320-5785) |
| Immunobiological treatment (%) | 35.2 |
| Laboratory results | |
| ALT (U/L) | 21.0 (17.0-32.5) |
| ALT index | 0.45 (0.29-0.66) |
| AST (U/L) | 21.0 (18.0-27.0) |
| AST index | 0.52 (0.45-0.69) |
| GGT (U/L) | 29.0 (21.2-40.0) |
| GGT index | 0.46 (0.35-0.73) |
| LDL (mg/dL) | 119.0 ± 36.7 |
| HDL (mg/dL) | 45.0 (39.0-55.0) |
| Triglycerides (mg/dL) | 127.0 (91.0-169.0) |
| Glycated hemoglobin (%) | 5.8 (5.5-6.4) |
| Platelet count (x103) | 251.2 ± 68.8 |
| Genetic characteristics | |
| PNPLA3 CC/CG/GG (%)¶ | 42.3/49.5/8.2 |
| G allele frequency (%) | 32.9 |
| TM6SF2 CC/CT/TT (%)¶¶ | 88.7/11.3/0 |
| T allele frequency (%) | 5.6 |
| Elastographic characteristics | |
| LSM (kPa) | 5.3 (4.2-6.7) |
| LSM (kPa) ≥ 10 (%) | 9 |
| IQR (%) | 15.0 (10.0-20.0) |
| CAP (dB/m) ≥ 275 (%) | 54.8 |
| CAP (dBm) | 275.8 ± 55.6 |
| Success Rate (%) | 100.0 (87.0-100.0) |
| M Probe (%) | 63.3 |
Values are proportion for categorical data, mean (SD) for normally distributed data and medians (interquartile interval) for non-parametric data. BMI, Body Mass Index; ALT, AST, and GGT indexes absolute value/ upper normal limit; LDL, low-density lipoprotein; HDL, high-density lipoprotein; LSM, liver stiffness measurement; CAP, controlled attenuation parameter.
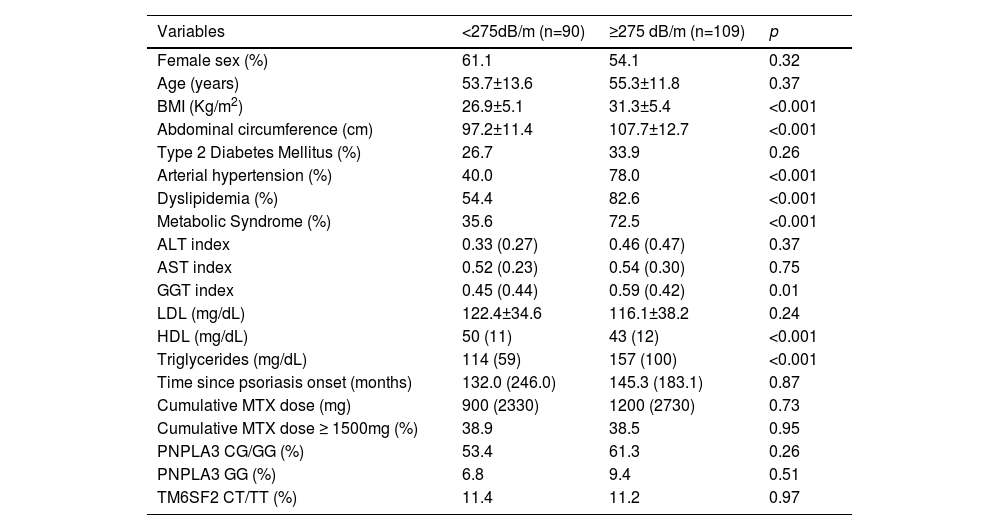
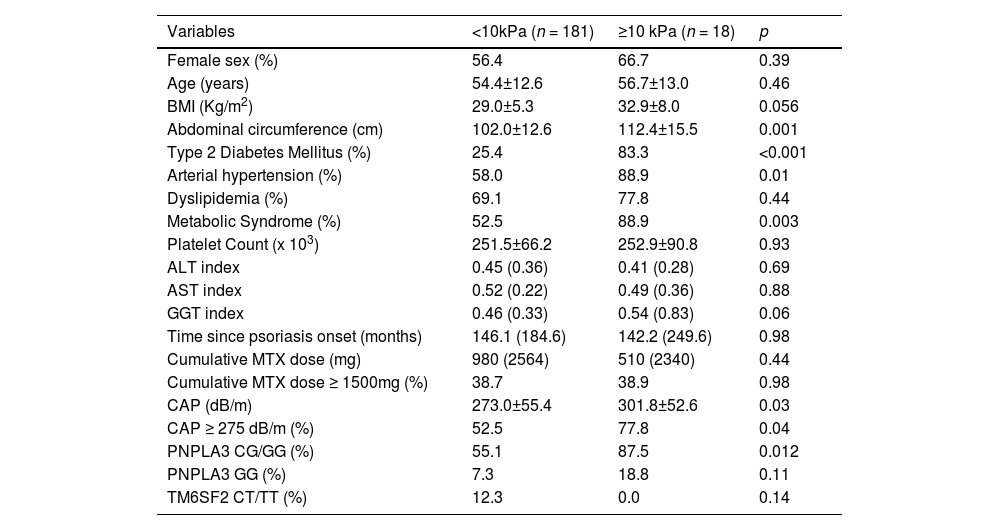
The comparative analysis regarding patients with and without steatosis and advanced liver fibrosis evaluated by TE are shown in Table 2 and Table 3, respectively.
Comparative analysis between patients with Pso with and without steatosis (CAP≥ 275dB/m) (n = 199).
Values are proportion for categorical data, mean (SD) for normally distributed data and medians (interquartile range) for non-parametric data. BMI, Body Mass Index; ALT, AST and GGT indexes (absolute value/ upper standard limit); LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Comparative analysis between patients with Pso with and without advanced liver fibrosis (LSM≥10kPa) (n=199).
Values are proportion for categorical data, mean (SD) for normally distributed data and medians (interquartile range) for non-parametric data. BMI, Body Mass Index; ALT, AST and GGT indexes (absolute value/ upper standard limit), and CAP, controlled attenuation parameter.
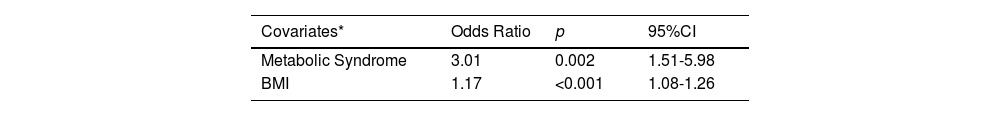
Variables included on logistic regression for steatosis were BMI, MetS, GGT index and cumulative MTX dose (the latter was included based on clinical plausibility). Abdominal circumference, arterial hypertension, dyslipidemia, HDL and triglycerides were also statistically significant but not included as they were competitive with BMI and MetS. MetS and BMI were independently associated with steatosis (Table 4).
Model for independently associated variables with the presence of steatosis (CAP≥275dB/m).
| Covariates* | Odds Ratio | p | 95%CI |
|---|---|---|---|
| Metabolic Syndrome | 3.01 | 0.002 | 1.51-5.98 |
| BMI | 1.17 | <0.001 | 1.08-1.26 |
Regarding liver stiffness, variables included in logistic regression were PNPLA3 CG/GG, TM6SF2 CT/TT, T2DM, Arterial Hypertension, BMI and cumulative MTX dose (the latter also for clinical plausibility). Abdominal circumference, GGT index and CAP were also not included as they were competitive with variables included in the initial model. Both models were adjusted for age and gender. T2DM and at least one G allele of the PNPLA3 genotype were independently associated with advanced liver fibrosis in this population (Table 5).
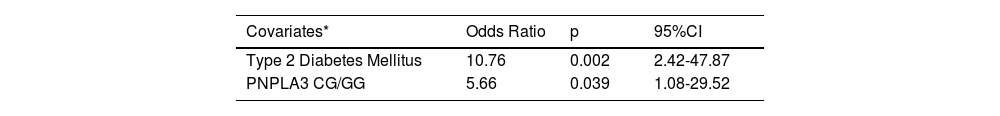
Model for independently associated variables with the presence of advanced liver fibrosis as outcome (LSM≥10kPa).
| Covariates* | Odds Ratio | p | 95%CI |
|---|---|---|---|
| Type 2 Diabetes Mellitus | 10.76 | 0.002 | 2.42-47.87 |
| PNPLA3 CG/GG | 5.66 | 0.039 | 1.08-29.52 |
This is the first study to evaluate the frequency and impact of PNPLA3 and TM6SF2 polymorphisms on SLD in a population diagnosed with psoriasis. We demonstrated the association between the PNPLA3 rs738409 C > G polymorphism and advanced liver fibrosis assessed by LSM higher than 10 kPa. On the other hand, no impact was observed either in steatosis or fibrosis due to TM6SF2 polymorphism.
The prevalence of SLD was elevated in this Brazilian multi-ethnic population of Pso patients. At least one PNPLA3 G allele was associated with a 5.7-fold increased odds of advanced liver fibrosis. The present study has also shown that the distribution of PNPLA3 and TM6SF2 genotypes is similar to those regarding non-psoriatic SLD South American populations [25–27]. The expanded adipose tissue/dysmetabolism seems to be the main trigger for the hepato-dermal axis [17], and we may hypothesize if the PNPLA3 polymorphism in Pso may have a role in the direct hepato-dermal interplay, resulting in a phenotype of high risk of liver disease [28]. In parallel, there is evidence of the association between spleen longitudinal diameter and duration of disease in psoriasis; this reinforces the hepato-dermal axis hypothesis, also pointing to the enlarged spleen as an indicator of systemic inflammation in psoriasis [29].
Data from a well-powered meta-analysis showed that PNPLA3 rs738409 C > G polymorphism impacts fibrosis in Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD) patients, where GG homozygous had a 3.2-fold higher odds of developing liver fibrosis [30]. A similar study investigating the relationship between advanced liver fibrosis and genetic polymorphisms in T2DM patients has also described the impact of harboring the GG PNPLA3 genotype [27]. This finding may suggest that SLD in Pso patients may have similar multifactorial aspects as in other populations with MASLD. In our study, T2DM was also independently associated with advanced liver fibrosis, as reported in previous studies involving Pso patients [12,13,16,31]. This result reinforces the evidence that T2DM is the major risk factor for developing MASLD and fibrosis progression in the general population [7].
Interestingly, our study showed no association with steatosis despite the observed relationship between PNPLA3 polymorphism and advanced liver fibrosis. Although PNPLA3 rs738409 C > G polymorphism is related to loss of lipase activity towards triglycerides, increasing liver fat levels [19,32], there is evidence of direct activation of specific fibrogenic signaling pathways [33,34]. On the other hand, TM6SF2 rs58542926 C>T polymorphism had no impact on steatosis or fibrosis in our population, despite the fact of being a variant known for decreasing hepatocyte lipid secretion [20] resulting in more steatosis and liver fibrosis [35,36]. This lack of association might be due to one or both of these factors: first, genotyping was not possible in twenty-two patients due to obtaining dubious results, impairing the power of a possible association; and second, this is a low-frequency polymorphism in our population, with only 11% harboring the T allele, any with the homozygotic genotype. A larger population may be necessary to find a potential impact. This way, further studies may better investigate the potential association of TM6SF2 polymorphism with steatosis and fibrosis in Pso individuals, confirming or not our finding.
Although some studies addressed the factors associated with steatosis in Pso patients, with most showing steatosis prevalence ranging between 44 and 47%, the diagnosis of steatosis was always based on ultrasonography [37]. Ultrasonography generally detects only moderate steatosis (20 and 30%), has inter-operator variability and has reduced accuracy in patients with obesity [24]. In the present study, we used the CAP measurement to diagnose steatosis, adopting the recent cut-off recommendation of 275 dB/m with over 90% sensitivity [24], and we detected a higher prevalence of steatosis of 54.8%. Of note, our study also had a slightly higher prevalence of MetS of 55.8%, compared to previously published studies that described a prevalence of 20 to 50% in Pso patients [4].
Importantly, we once again highlighted the absence of an association between cumulative MTX dose and steatosis or advanced liver fibrosis, even when considering cumulative doses of MTX > 1500 mg [13]. Less than half (38.6%) of the patients had a cumulative MTX dose ≥1500 mg despite a total frequency of MTX use of 79.3%. In recent years, there has been a greater availability of immunobiological therapies frequently used with MTX in lower doses in more severe Pso disease. Our study did not evaluate the impact of immunobiological as an indirect protective factor in these patients since they help maintain lower doses of MTX in more severe cases. This hypothesis may also be better investigated in further studies with more patients using immunobiological therapies. However, our study's expressive prevalence of liver fibrosis and steatosis occurred in a population with a median cumulative MTX dose lower than 1500 mg, reinforcing the possible low impact of long-term MTX on liver fibrosis [16,38]. Previous studies based on TE in patients using MTX due to Pso and other chronic inflammatory diseases [39,40,31,41,42,16] adopted lower cut-offs to detect liver fibrosis. They showed a similar prevalence of liver fibrosis from 4.8 to 15.8% compared to our findings (9%) using a cut-off of 10 kPa (none of the patients with LSM≥10 kPa (n = 18) had liver failure. Nonetheless, three of them had clinically significant portal hypertension). Cumulative MTX dose in these studies ranged from 1.38 g [39] to 4.8 g [16], including studies with no MTX use or reported cumulative MTX dose. Those who reported cumulative MTX doses had no association with TE values. Noteworthy, MTX-induced liver fibrosis has been described based on serial liver biopsies when SLD and even viral hepatitis were not recognized [43]. Finally, the most relevant concern would be whether MTX can worsen the natural history of SLD in Pso [43,44]. In vitro research has recently shown that PNPLA3 rs738409 polymorphism loss of function can also increase sensitivity to hepatotoxins [45], an exciting finding, especially if added to the association between the presence of the PNPLA3 rs738409 C > G polymorphism and advanced liver fibrosis found in our study.
There are some limitations in our study. First, it was a cross-sectional study, where causality cannot be proven. Second, steatosis and liver fibrosis were not histologically proven. However, there was no rationale to perform liver biopsy in these patients since TE and CAP are non-invasive tools used mainly to screen steatosis and liver fibrosis [24,46,47] and have progressively replaced liver biopsy in clinical practice. Third, disease severity on Pso was not measured, and associations were not performed regarding this variable. However, all patients were followed at outpatient clinics from tertiary hospitals, with long-term dermatological follow-up (median: 45.0 months [16.0-97.5]), and mostly on systemic therapy (79.3% on methotrexate and 35.2% on immunobiological therapy), suggesting more severe disease. Lastly, TM6SF2 genotyping was not feasible in 22 patients, which might have underpowered our results regarding its association with liver steatosis or advanced liver fibrosis. On the contrary, this was the first study that has shown the impact of PNPLA3 polymorphism in psoriasis patients, and longitudinal studies evaluating long-term liver outcomes may be valuable to confirm it as a prognostic factor regarding fibrosis in individuals with psoriasis. As the factors related to steatosis in the present study were all metabolic, these individuals may also be prone to lifestyle modifications to control metabolic syndrome and possibly steatosis.
5ConclusionsThere is a clear-cut relationship between the G allele of PNPLA3 polymorphism and the presence of advanced liver fibrosis evaluated by TE, in addition to T2DM, independently of cumulative MTX dose, in patients with psoriasis. TM6SF2 was not associated with SLD in Pso patients. Metabolic risk factors are prevalent in Pso and contribute to steatosis and fibrosis development, but a new perspective emerges, placing PNPLA3 polymorphism as an additional hypothesis for the hepato-dermal axis.
FundingThis study was supported by grants 429965/2018-4 from the National Council for Research and Technology, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and E-26/202627/2019 from Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil. The sponsors have no role in study design, data collection and analysis, interpretation or preparation of results, or manuscript review and approval.
Data availability statementThe data supporting this study's findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.