Metabolic (dysfunction) associated fatty liver disease (MAFLD) and cholelithiasis are highly prevalent and are associated with common risk factors such as obesity, hypertriglyceridemia, and fasting glucose levels; however, it is not clear whether cholelithiasis is associated with MAFLD or fibrosis.
ObjectiveTo determine MAFLD severity and associated risk factors in patients diagnosed with cholelithiasis.
Materials and methodsObservational, cross-sectional and prolective study (from October 2018 to March 2020) of patients undergoing elective laparoscopic cholecystectomy with liver biopsy, excluding other causes of hepatic disease or significant alcohol consumption. MAFLD detection was based on histology using the Kleiner score and one of the following criteria: overweight/obesity, T2DM, or evidence of metabolic dysregulation. The AST to Platelet Ratio Index, the NAFLD Fibrosis Score, the fibrosis-4 index and the hepatic steatosis index were performed to assess the relationship of non-invasive hepatic scores with histopathology.
Results80 patients median age (interquartile range) was 42 (18) years, with a BMI of 27.9 (6.11) Kg/m2. Of all patients, 58.8% had MAFLD, 78.7% were women, and 13.8% had the severe form (formerly named NASH). No substantial correlation between biochemical parameters and histopathological analysis of MAFLD and fibrosis was observed.
ConclusionBecause cholelithiasis and MAFLD are highly prevalent diseases, it is essential to conduct studies on the relationship between both pathologies. Currently, liver biopsy is the best diagnostic method since the predictive biochemical models did not show a substantial correlation to classify MAFLD. Its early detection is relevant since a considerable percentage of advanced fibrosis (8.7%) was found.
Metabolic (dysfunction) associated fatty liver disease (MAFLD) is an updated and novel term proposed for hepatic steatosis, which supplies a comprehensive definition for its independent diagnosis of other liver diseases. The proposal of MAFLD is endorsed by the key liver societies and patients associations [1–6]. MAFLD represents the hepatic manifestation of a heterogeneous multisystemic disorder, which handles a significant clinical and economic burden worldwide, and does not significantly change its prevalence compared to non-alcoholic fatty liver disease [7].
MAFLD's current diagnosis includes evidence of hepatic steatosis, plus one of the following three criteria: overweight/obesity, metabolic dysregulation, or type 2 diabetes mellitus (T2DM) [6]. Furthermore, MAFLD does not require excluding patients with other chronic liver diseases or alcohol use [8]. As well, MAFLD criteria can identify a greater number of people with significant hepatic fibrosis [9], cardiovascular conditions [10], and chronic kidney disease [11].
Cholelithiasis corresponds to one of the most common gastrointestinal tract disorders, characterized by cholesterol gallstones formation, which implies an alteration in the bile composition and high concentrations of supersaturated cholesterol along with proteins that promote the nucleation of cholesterol crystals, as well as the gallbladder malfunction through the modification of its contractility and epithelial secretion [12]. In western societies, cholesterol gallstones account for 80 to 90% of the cholecystectomy stones. Cholelithiasis risk increase with age, obesity, T2DM, dyslipidemia, hypertriglyceridemia, low levels of high-density lipoprotein, elevated serum cholesterol, hyperinsulinemia, to be a female, and a sedentary life; also, all of these conditions are risk factors for metabolic syndrome, where cholesterol gallstones represent a complication [13, 14].
Despite MAFLD's high incidence and close association with metabolic disorders, there are currently no non-invasive markers with high predictive value for screening aggressive forms or early detection. Consequently, liver biopsy remains the standard test for diagnosing and staging the patient with MAFLD. By evaluating the degree of steatosis, inflammatory activity, parenchymal damage, and the presence of tissue remodeling/fibrosis, the histopathology study helps to distinguish healthy subjects from those with mild or severe MAFLD and their potential to evolve to more advanced forms of liver damage such as cirrhosis.
The high prevalence of cholelithiasis among patients with MAFLD compared with patients without MAFLD (47% vs. 26%, respectively; p <0.0001) [15] and their metabolic relationship make a probable coincidence in many cases since they share risk factors such as overweight/obesity, hypertriglyceridemia, insulin resistance, and T2DM [16]. However, it is not clear if gallstones are a risk factor for MAFLD because most studies do not have a liver biopsy. This work aimed to determine MAFLD severity and associated risk factors in a series of histologically diagnosed patients in a cross-sectional cohort of Mexican inhabitants.
2MethodsTo determine MAFLD frequency and severity in patients with cholelithiasis, a cross-sectional observational study was carried out in patients from the Department of Hepatopancreatic and Biliary Surgery of the Hospital General de México “Dr. Eduardo Liceaga”. Observational, cross-sectional and prolective study with patients of either gender, aged between 18 and 60 years, who underwent laparoscopic cholecystectomy from October 2018 to March 2020, signed informed consent to obtain a liver biopsy and store their information in the database. (Supplementary figure 1)
2.1PatientsConsecutive patients undergoing elective laparoscopic cholecystectomy were included, from which a biopsy (0.5-1.0 cm of liver tissue from the right lobe) was obtained to corroborate the MAFLD diagnosis. The final cohort included 80 patients, excluding patients due to other causes of liver disease (viral, autoimmune, drug-induced, hereditary hemochromatosis, Wilson's disease) and whose confirmed daily alcohol intake was ≥20 g, conducting the Alcohol Use Disorders Identification Test (AUDIT), which assesses patients for hazardous and harmful alcohol consumption.
2.2Cholelithiasis diagnosisAn ultrasound of the liver and bile ducts was performed to diagnose gallstones or bile sludge at the time of clinical evaluation. Cholelithiasis was analyzed by the presence of one of the following criteria: (I) sonographic evidence of gallstones (one or more echogenic structures, distal shadow, possibly mobile in the gallbladder), (II) echogenic material within the gallbladder with constant shading and little or no visualization of the gallbladder.
2.3Clinical evaluationAnthropometric variables such as age (years), weight (Kg), height (m), and personal pathological history (T2DM, hypertension, dyslipidemia) were obtained from the patient's clinical record in the electronic file. Laboratory test data were obtained: complete blood count, alanine, and aspartate aminotransferase (ALT-AST), gamma-glutamyl transpeptidase (GGT), lactic dehydrogenase (LDH), alkaline phosphatase (ALP), total cholesterol, triglycerides (TG), fasting glucose, albumin, medium corpuscular volume (MCV), hematocrit, hemoglobin, and platelets available in all subjects.
2.4Predictive model assessmentsDifferent hepatic predictive models were calculated based on routine laboratory parameters, which reflect alterations in hepatic function. The AST to Platelet Ratio Index (APRI)= [(AST (U/L)/ULN)/PLT count (109/L)] × 100, a score less than 0.5 predicts the absence of fibrosis, while a score greater than 1.5 predicts the presence of fibrosis. The NAFLD Fibrosis Score (NFS)= -1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio -0.013 × platelet count (x109/L) -0.66 × albumin (g/dL), a score less than -1.455 predicts the absence of advanced fibrosis, whereas a score greater than 0.675 predicts the presence of advanced fibrosis. The fibrosis-4 index (FIB-4)= (Age x AST) / (Platelets x √ALT), a score less than 1.45, predicts the absence of advanced fibrosis, while a score greater than 3.25 predicts the presence of advanced fibrosis. The hepatic steatosis index (HSI)= 8*ALT/AST + BMI (+2; if DM2; +2; if a woman), a score less than 30 predicts the absence of steatosis, while a score greater than 36 predicts the presence of steatosis [25].
2.5Liver histologyLiver biopsies were formalin-fixed, paraffin-embedded, and examined for study using hematoxylin-eosin staining. Two expert pathologists independently diagnosed the biopsy specimens blindly, without knowing the patients' clinical data. Any difference in observations was rectified by consensus. The Kleiner score includes three semi-quantitative parameters, which are added to obtain a score of 0-8 points: steatosis according to the hepatocytes percentage with lipid droplets (grade 0: <5%, 1: 5–33%, 2: 33–66%, and 3: >66%), lobular inflammation measured in foci per field (grade 0: none, 1: <2, 2: 2-4, 3: >4), hepatocytes ballooning (grade 0: none, 1: few cells, 2: many cells). Liver fibrosis was also assessed (grade 1: perisinusoidal or periportal, 1A: mild perisinusoidal fibrosis in zone 3, 1B: moderate perisinusoidal fibrosis in zone 3, 1C: only portal/periportal fibrosis, 2: perisinusoidal fibrosis in zone 3, with portal/periportal fibrosis, 3: fibrosis bridges, 4: cirrhosis).
2.6MAFLD diagnosisMAFLD diagnosis was based on histology (biopsy) in addition to one of the following three criteria: overweight/obesity, T2DM, or evidence of metabolic dysregulation. For this study, metabolic dysregulation was defined by the presence of the following two abnormalities: plasma triglycerides ≥150 mg/dL (≥1.7 mmol/L) and prediabetes (fasting glucose levels of 100 to 125 mg/dL (5.6 to 6.9 mmol/L) [6]. Cases with a Kleiner score of 0-2 were classified as patients without MAFLD, while those with scores of 3-4 were arranged as mild MAFLD, and those with a score greater than five were classified as severe MAFLD.
2.7Statistic analysisFrequencies and percentages described the qualitative categorical variables. The quantitative variables were defined using the median and interquartile range. After analyzing and determining that the variables' distribution and behavior were non-parametric through the Kolmogorov-Smirnov test; the Mann-Whitney U test was used to define which group had the difference. The odds ratio (OR) for MAFLD was calculated using multivariate logistic regression analysis; a p-value lower than 0.05 was considered statistically significant.
A dichotomous division was performed to carry out a correlation analysis that defined MAFLD and fibrosis stages, which assess the diagnostic accuracy of the liver predictive scales with histology. The receiver-operating characteristic (ROC) curve was calculated to describe the relationship of the true positive rate (sensitivity) versus the false-positive rate (specificity) as the cut-point on the scales was moved. The AUROC with the associated approximate 95% confidence interval (CI) was estimated using the Mann–Whitney statistic. The results were summarized by presenting the AUROC with 95% CI and, where applicable, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at the optimal cut-point.
Spearman's Rho correlation and Cohen's Kappa coefficient were also determined to find the relationship between hepatic predictive biochemical models and histological data from liver biopsies. Statistical analysis was performed with IBM SPSS Statistics software for Macintosh, Version 24.
2.8EthicsThe ethics and research committee of the Hospital General de México approved this study (DI/18/304/03/078) and the ethics committee of Medica Sur Hospital (2017-EXT-238). The patients signed the informed consent to obtain the liver biopsy and store the information in the database that was used in the research, which follow the basic principles of human research in the Helsinki Declaration (Helsinki Finland 1975, last amendment at the 52nd General Assembly in Fortaleza, Brazil, October 2013). The collected data were treated confidentially, with an attached privacy notice to the informed consent.
3Results3.1Patient's characteristicsEighty patients who underwent laparoscopic cholecystectomy by elective surgery were recruited; 77.5% (n= 62) were women (77.5%) and 22.5% (n= 18) were men, median age of 42 (IR=18) years. Regarding body weight, the median was 70 Kg (IR=18.8), and the BMI was 27.9 Kg/m2 (IR=6.11).
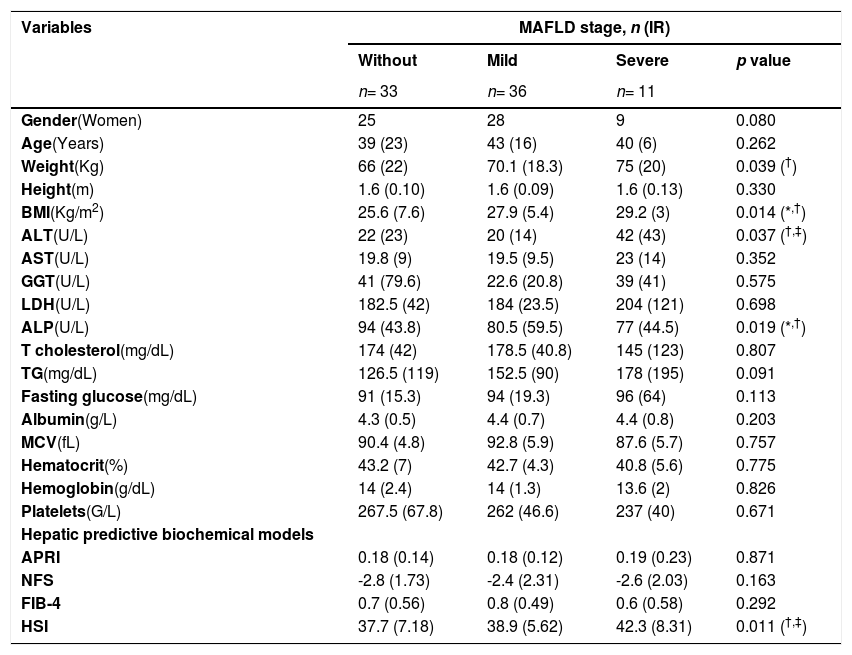
Within the analyzed population, 41.3% (n= 33) did not present MAFLD, with a median age of 39 years, a bodyweight of 66 Kg, a BMI of 25.6 Kg/m2, and 75.76% (n= 25) were women. Patients with mild MAFLD accounted for 45% (n= 36), with an average age of 43 years, a bodyweight of 70.1 Kg, a BMI of 27.9 Kg/m2, and 77.78% (n= 28) were women. Finally, patients with severe MAFLD are 13.8% (n= 11) of the population, with a median age of 40 years, a bodyweight of 75 Kg, a BMI of 29.2 Kg/m2, and 81.82% (n=9) were women. (Table 1)
Anthropometric, biochemical, and clinical variables by MAFLD stage.
| Variables | MAFLD stage, n (IR) | |||
|---|---|---|---|---|
| Without | Mild | Severe | p value | |
| n= 33 | n= 36 | n= 11 | ||
| Gender(Women) | 25 | 28 | 9 | 0.080 |
| Age(Years) | 39 (23) | 43 (16) | 40 (6) | 0.262 |
| Weight(Kg) | 66 (22) | 70.1 (18.3) | 75 (20) | 0.039 (†) |
| Height(m) | 1.6 (0.10) | 1.6 (0.09) | 1.6 (0.13) | 0.330 |
| BMI(Kg/m2) | 25.6 (7.6) | 27.9 (5.4) | 29.2 (3) | 0.014 (*,†) |
| ALT(U/L) | 22 (23) | 20 (14) | 42 (43) | 0.037 (†,‡) |
| AST(U/L) | 19.8 (9) | 19.5 (9.5) | 23 (14) | 0.352 |
| GGT(U/L) | 41 (79.6) | 22.6 (20.8) | 39 (41) | 0.575 |
| LDH(U/L) | 182.5 (42) | 184 (23.5) | 204 (121) | 0.698 |
| ALP(U/L) | 94 (43.8) | 80.5 (59.5) | 77 (44.5) | 0.019 (*,†) |
| T cholesterol(mg/dL) | 174 (42) | 178.5 (40.8) | 145 (123) | 0.807 |
| TG(mg/dL) | 126.5 (119) | 152.5 (90) | 178 (195) | 0.091 |
| Fasting glucose(mg/dL) | 91 (15.3) | 94 (19.3) | 96 (64) | 0.113 |
| Albumin(g/L) | 4.3 (0.5) | 4.4 (0.7) | 4.4 (0.8) | 0.203 |
| MCV(fL) | 90.4 (4.8) | 92.8 (5.9) | 87.6 (5.7) | 0.757 |
| Hematocrit(%) | 43.2 (7) | 42.7 (4.3) | 40.8 (5.6) | 0.775 |
| Hemoglobin(g/dL) | 14 (2.4) | 14 (1.3) | 13.6 (2) | 0.826 |
| Platelets(G/L) | 267.5 (67.8) | 262 (46.6) | 237 (40) | 0.671 |
| Hepatic predictive biochemical models | ||||
| APRI | 0.18 (0.14) | 0.18 (0.12) | 0.19 (0.23) | 0.871 |
| NFS | -2.8 (1.73) | -2.4 (2.31) | -2.6 (2.03) | 0.163 |
| FIB-4 | 0.7 (0.56) | 0.8 (0.49) | 0.6 (0.58) | 0.292 |
| HSI | 37.7 (7.18) | 38.9 (5.62) | 42.3 (8.31) | 0.011 (†,‡) |
Differences in the median were considered significant at p ≤ 0.05 between groups.
patients with mild MAFLD versus patients with severe MAFLD. Abbreviations: Metabolic associated fatty liver disease (MAFLD), interquartile range (IR), body mass index (BMI), alanine aminotransferase (ALT) aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), triglycerides (TG), medium corpuscular volume (MCV), AST to Platelet Ratio Index (APRI), NAFLD Fibrosis Score (NFS), fibrosis-4 index (FIB-4), hepatic steatosis index (HSI).
Medians of the serum parameters were determined to see the variation between MAFLD severity in patients with cholelithiasis. The median ALP values showed statistical significance (77 U/L and 80.5 U/L vs. 94 U/L p= 0.019) between the group with severe and mild MAFLD versus the group without MAFLD. On the other hand, patients with severe and mild MAFLD showed higher medians for BMI (29.2 Kg/m2 and 27.9 Kg/m2 vs. 25.6 Kg/m2 p= 0.014) versus patients without MAFLD. (Table 1)
Logistic regression according to MAFLD presence was performed with the statistically significant data from the univariate analysis. Gender was dichotomized into female and male with an OR of 1.1 (95% CI 0.60-2.01, p= 0.390), the BMI as >25 and <25kg/m2 with an OR of 6.8 (95% CI 2.07-22.40, p= 0.002), the ALP as >100 mg/dL and <100 mg/dL with an OR of 0.5 (95% CI 0.18-1.33, p= 0.162), and the fasting glucose as >126 mg/dL and <126 mg/dL with an OR of 1.8 (95% CI 0.374-8.79, p= 0.460). (Table 2)
Logistic regression of the statistically significant variables according to MAFLD presence.
Abbreviations: Metabolic associated fatty liver disease (MAFLD), standard deviation (SD), odds ratio (OR), confidence interval (CI), body mass index (BMI), alkaline phosphatase (ALP).
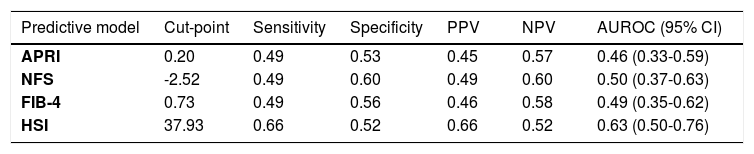
The accuracy of classifying fibrosis and hepatic steatosis based on clinical characteristics and biomarkers was assessed (Table 3). The cut-points were determined by maximizing the Youden index, and the sensitivity and specificity were evaluated at the specified cut-point.
Hepatic predictive biochemical models to classify fibrosis and MAFLD.
Abbreviations: Positive predictive value (PPV), negative predictive value (NPV), area under the receiver operating characteristic curve (AUROC), confidence interval (CI), AST to Platelet Ratio Index (APRI), NAFLD Fibrosis Score (NFS), fibrosis-4 index (FIB-4), hepatic steatosis index (HSI).
The highest AUROC of 0.63 was observed for HSI for accuracy in classifying hepatic steatosis, with a sensitivity of 66% and specificity of 52% at a cut-point of 37.93. The next highest AUROC of 0.50 was reported for fibrosis by NFS, with a sensitivity of 49% and specificity of 60% at a cut-point of -2.52. For FIB-4, the AUROC was 0.49 with a sensitivity of 49%; specificity 56% at a cut-point of 0.73. An AUROC of 0.46 was also observed for APRI for accuracy in classifying fibrosis with a sensitivity of 49% and specificity of 53% at a cut-point of 0.2. (Table 3)
The correlation and concordance between MAFLD and histopathological data of liver fibrosis were also performed, using contingency tables. The Rho values for the fibrosis indices (APRI, NFS, and FIB-4) were 0.09, and the Kappa coefficients were 0.04, which means very poor correlation and negligible agreement. For hepatic steatosis, both the Rho value (0.23) and the Kappa index (0.22) had poor correlation and agreement. (Supp. table 1)
3.4Histopathological evaluationLiver biopsies histopathological evaluation classified the MAFLD phenotype with the Kleiner score, which assesses the degree of steatosis, inflammatory activity, parenchymal damage, and the presence of fibrosis. (Fig. 1)
MAFLD histological representation. A) Liver without steatosis, B) Liver with mild MAFLD, C) Liver with severe MAFLD, D) Liver with fibrosis. Liver tissue obtained from human biopsies, stained with H&E. Hepatic steatosis and steatohepatitis was diagnosed by standard criteria using the NAFLD activity score. Original magnification 100X. E) Liver without steatosis, F) Liver with mild MAFLD, G) Liver severe MAFLD, H) Liver with fibrosis. Liver tissue obtained from human biopsies, stained with Masson trichome. Original magnification 100X.
Fig. 2 shows that in the group without MAFLD, only 3% of the patients had more than 5% liver fat content; in those with mild MAFLD, 41.7% presented lipid compromise of 5%, and 8.3% presented lipid compromise greater than 33%; while the group with severe MAFLD, 9.1% of the patients had a lipid compromise lower than 5%, 36.4% of the subjects had a lipid infiltrate higher than 33%, and 54.5% had more than 66 % lipids in hepatocytes.
Regarding inflammation, 48.5% of patients without MAFLD had less than two foci per field. In the group with mild MAFLD, 50% had fewer than two foci per area, 30.6% had 2-4 foci per optic field, and 8.3% of the subjects had more than four foci per area. Furthermore, in the group with severe MAFLD, 36.4% of the patients had less than two foci per area, 45.4% had 2-4 foci per optic field, and 18.2% had more than four foci. (Fig. 2)
Hepatocyte ballooning evaluation showed that in the group without MAFLD, none of the patients had ballooned cells. In the mild MAFLD group, 72.2% had few ballooned cells, and 5.6% of subjects had many ballooned hepatocytes. In the severe MAFLD group, 54.5% of the patients presented few ballooned cells, and 45.5% had many ballooned hepatocytes. (Fig. 2)
Liver fibrosis showed that in the group without MAFLD, 27.3% of the individuals presented mild fibrosis, 6.1% portal and perisinusoidal fibrosis, while 9.1% presented fibrosis bridges; the mild MAFLD group had 22.2% of patients with mild fibrosis, 16.7% with a portal and perisinusoidal fibrosis, and 11.1% fibrosis bridges. Finally, in the group with severe MAFLD, 18.2% of the patients had mild fibrosis, and 9.1% had portal and perisinusoidal fibrosis. (Fig. 2)
To determine the type of gallstones in the patients, the medical records were examined, which showed that 27.45% had pigmentary lithiasis and 72.55% had cholesterol stones; these results are similar to what is already reported in western societies [17].
4DiscussionCholelithiasis and MAFLD may coexist; their association is determined by the presence of shared risk factors such as age, ethnicity, obesity, insulin resistance, metabolic syndrome, atherosclerosis, and cardiovascular disease risk [18,19]. Of the 80 patients in the cohort, the overall MAFLD prevalence was 58.8%; it should be noted that the prevalence is due to the histopathological diagnosis, which gives great strength to the study, of which 78.7% were women, and only 11 of the 47 patients with MAFLD had a severe phenotype; however, no associated risks were found for developing the severe phenotype. Through histopathology, it was observed that 8.75% of the studied population had advanced fibrosis, and 35% suffer from some degree of fibrosis diagnosed by biopsy; nevertheless, fibrosis does not appear to be related to MAFLD's severity.
As in our research, a study conducted by Dr. Yilmaz's group did not show an association between cholelithiasis, steatohepatitis, and fibrosis, through liver biopsy [20]. So far, with our study, it can be said that there is no causality between MAFLD and cholelithiasis, which corresponds to a bidirectional association with an independent risk factor. However, metabolic disorder could be considered the common risk factor between both entities [21,22].
There are some clinical predictor models to identify the risk of NASH and advanced fibrosis, including obesity, age, hypertension, and hypertriglyceridemia, but their accuracy in diagnosing NASH or fibrosis remains poor [23]. Also, APRI, NFS, and FIB-4 are based on biochemical variables: age, body mass index, hyperglycemia, platelet count, albumin, AST, and ALT, with an accuracy to predict advanced fibrosis of 0.85, 0.84, and 0.80 respectively [24,25]; however, in our study these values were not reached, probably because the scales are used mainly for viral liver disease.
Hepatic steatosis can be diagnosed using predictive biochemical models, such as the HSI, which comprises three variables, aspartate aminotransferase AST/ALT ratio, BMI, and the presence of diabetes with an AUROC of 0.81 [24,25]; however, in our study, these values are probably not reached because the cut-off values were different. Since clinical predictor models are a quick and easy method for clinicians to recognize patients at risk for cirrhosis, their improvement is crucial since they are not very good at detecting MAFLD in early stages, nor in patients with gallstones.
Anticipated detection importance lies in identifying associated risk factors and recognizing patients at higher risk of complications [26]. It is important to note that most non-invasive tests are not very sensitive or specific to differentiate between fibrosis early stages; therefore, liver biopsy cannot be substituted until the appropriate biomarker is available. Also, it is considered essential to give medical follow-up to patients who underwent a cholecystectomy, in which non-invasive studies should be performed to evaluate their liver health and see if they develop any malignancy.
The study's main limitations are the small group of samples with severe MAFLD, which did not evaluate a different liver result. Similarly, the dwindling number of patients did not allow a separate analysis of risk factors in this cohort. The study's strengths are the number of patients diagnosed with cholelithiasis who underwent laparoscopic cholecystectomy with MAFLD proven by biopsy, applying the NAFLD Activity Score, and the presence of metabolic disorders that allow inclusion in the new MAFLD definition.
Because cholelithiasis and MAFLD have a high prevalence in the Mexican population, it is imperative to carry out studies that provide information about both pathologies and their relationship. This study reveals the importance of considering the severity of cholelithiasis and MAFLD to avoid complications during surgery and postoperatively.
Today, the best diagnosis is given by liver biopsy since the pathology score represents the quantity and the location and alteration of the parenchyma and vascular alterations. However, it would be beneficial to seek better non-invasive tests that capture both disease activity and fibrosis stage, which would allow reserving liver biopsy for complicated cases or ruling out other forms of liver disease.
5SourcesThis research received no specific grant from funding agencies in public, commercial, or non-profit sectors. The Hepato-Pancreato and Biliary Surgery unit of the Hospital General de México Dr. Eduardo Liceaga and the Medica Sur Clinic and Foundation have partially subsidized this work.
Authors contributionsAll authors have contributed to the article's realization and improvement and agreed on the manuscript's content. Dr. Rodríguez-Antonio, Dr. López-Sánchez, and Dr. Nuño-Lámbarri design carried out the study and drafted the article. Dr. Montalvo-Javé was the surgeon who provided the patient's biopsies, contributed with diverse ideas, and corrected the final version of the manuscript; Dr. Reyes-Gómez, Dr. Contreras-Flores, Dr. Farías-García, and Dr. Espejel-Deloiza collaborated with the biopsies obtainment, data acquisition management and treatment. Dr. Durán-Padilla and Dr. Chablé-Montero are the pathologists who performed the histological analysis; Dr. Chávez-Tapia and Dr. Uribe revised, contributed with diverse ideas, and corrected the final version of the manuscript. The definitive version has been read and approved by all authors.
Supporting informationSupplementary figure 1. Flow chart
Supplementary table 1. Correlation and concordance between hepatic predictive models and histological data.
List of abbreviationsALT Alanine aminotransferase Alcohol Use Disorders Identification Test alkaline phosphatase aspartate aminotransferase AST to Platelet Ratio Index area under the receiver operating characteristic curve body mass index confidence interval fibrosis-4 index gamma-glutamyl transpeptidase hepatic steatosis index interquartile range lactate dehydrogenase medium corpuscular volume metabolic (dysfunction) associated fatty liver disease NAFLD Fibrosis Score negative predictive value odds ratio positive predictive value receiver operating characteristic standard error triglycerides type 2 diabetes mellitus
We appreciate the support of the Department of Surgery, Division of Hepato-Pancreato and Biliary Surgery of the Hospital General de México “Dr. Eduardo Liceaga” and Medica Sur Hospital so that this article can be carried out.