Many interventions have been investigated for the treatment of nonalcoholic steatohepatitis (NASH). This study aims to summarize all investigated options to date and review the use of specific endpoints at different stages of ongoing trials of noncirrhotic NASH treatments. Using a horizon scanning approach, evidence were identified including meta-analyses of randomized controlled trials (RCTs) in PubMed, EMBASE, Cochrane, and AMED (up to February 2020), recently published RCTs in PubMed (2015-April 2020), RCTs presented at conferences (AASL and EASL, 2015–2020), and ongoing RCTs in ClincalTrials.gov (2015-November 2020). We included 6 meta-analyses of RCTs, 30 published RCTs, 11 conference abstracts, and 62 ongoing RCTs. An evidence map was created to demonstrate the treatment effects of 49 therapeutic modalities for NASH. Only six interventions (6/49, 12.24%) met the histological surrogate endpoints for potential conditional FDA approval. Obeticholic acid is the only therapy demonstrating positive benefits in ≥1-point improvement in fibrosis with no worsening of NASH in a phase 3 trial. The other therapies were all phase 2 studies. ≥1-point improvement in fibrosis with no worsening of NASH was shown in patients treated with cenicriviroc. NASH resolution with no worsening of fibrosis was shown in patients treated with liraglutide, semaglutide and resmetirom. Lanifibranor achieved both surrogate histological endpoints. Five ongoing RCTs (5/62, 8.06%) will investigate histological progression to cirrhosis, death, or liver-related clinical outcomes. In conclusion, some therapeutic modalities showed promising benefits, but further studies are warranted to find a definite treatment of NASH which prevents progression to cirrhosis and adverse liver outcomes.
Nonalcoholic steatohepatitis (NASH) is the progressive form of nonalcoholic fatty liver disease (NAFLD) which leads to cirrhosis and hepatocellular carcinoma, and independently is associated with both increased cardiovascular and liver related mortality [1]. The prevalence of NASH is estimated at 1.5–6.45% in the general population and is expected to increase along with the rise in obesity and other components of the metabolic syndrome [2,3].
Optimal pharmacological treatment has not yet been established for NASH. Currently, lifestyle changes with weight reduction remain the foundation of NASH treatment [4], however, significant weight loss is difficult to achieve and maintain
Over the past five years, elucidation of the basic mechanisms underlying NASH has been rapidly translated to treatment strategies. The effectiveness of several such approaches has been tested or are under investigation in phase 2b and phase 3 trials. Moreover, a wide range of endpoints is currently being employed in these trials in an attempt to find the best predictors of a treatment’s biological activity and efficacy [5]. Herein, by using a horizon scanning approach, we summarize the overall landscape of current treatment modalities that have been investigated and review the use of specific endpoints at different stages of ongoing trials of noncirrhotic NASH treatments.
2Materials and methods2.1Overall study designWe undertake this study using a horizon scanning approach [6,7]. All relevant evidence related to any therapeutic modalities ranging from meta-analyses of randomized controlled trials (RCTs), recently published RCTs, unpublished RCTs, and ongoing RCTs were identified from various sources and summarized to provide the overall landscape of current treatment modalities for noncirrhotic NASH.
2.2Data source and search strategyWe performed a systematic review to identify meta-analyses of RCTs investigating therapeutic modalities for NASH. Electronic databases including PubMed, EMBASE, Cochrane, and AMED were searched for meta-analyses of RCTs published from inception up to February 2020. The search term used was (nonalcoholic fatty liver disease OR non-alcoholic fatty liver disease OR NAFLD OR nonalcoholic steatohepatitis OR non-alcoholic steatohepatitis OR NASH) AND (“systematic review” OR systematic review OR meta-analysis). To be included, studies must be a systematic review with meta-analyses of RCTs investigating NASH treatments. The meta-analysis with the largest number of the RCTs included was chosen when more than one meta-analysis was found for the same therapeutic modalities.
To identify recently published RCTs investigating NASH treatments, which were not part of the included meta-analyses, we performed an updated search in PubMed (2015-April 2020). We also performed a manual search of conference abstracts presented at the American Association for Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) (2015–2020) to identify unpublished RCTs, while a search in ClincalTrials.gov (2015-November 2020) was conducted to determine any relevant ongoing RCTs.
2.3Study selection and data extractionThe identified studies were screened and selected based on the eligibility criteria by two independent reviewers (CP and SKV). Data extraction were independently performed by two reviewers (CP and SKV) using a standardized data extraction form. Discrepancies were discussed among reviewers and resolved by the third reviewer (NC). We extracted the following data from meta-analyses of RCTs: number of included RCTs, population, intervention (s), comparator (s), and treatment effects of interest including histological outcomes, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and hepatic fat content assessed by magnetic resonance imaging proton density fat fraction (MRI-PDFF), which were reported as relative risk (RR), odd ratio (OR), or mean difference (MD). For recently published RCTs and conference abstracts, we extracted the following data: study phase, population, intervention, comparator, sample size, duration, title acronym, and treatment effects of interest. For ongoing RCTs registered at ClinicalTrials.gov, we extracted phase, intervention, comparator, target sample size, duration, and study endpoints.
2.4Data analysesWe categorized treatment effects of interest into (1) currently accepted histological endpoints for conditional US Food and Drug Administration (FDA) approval i.e., ≥1 improvement in fibrosis with no worsening of NASH or NASH resolution with no worsening of fibrosis [8], (2) other histological endpoints, (3) laboratory outcomes, and 4) imaging outcomes. An evidence map was created to demonstrate the treatment effects of each therapeutic modality for NASH compared to placebo or active comparator which were investigated in the included meta-analyses of RCTs, recently published RCTs, and unpublished RCTs. The results of treatment effects are only available from meta-analyses, published RCTs, and conference abstracts. Thus, only the study endpoints of the ongoing RCTs of NASH treatments were summarized to provide the summary of what treatment effects will be investigated.
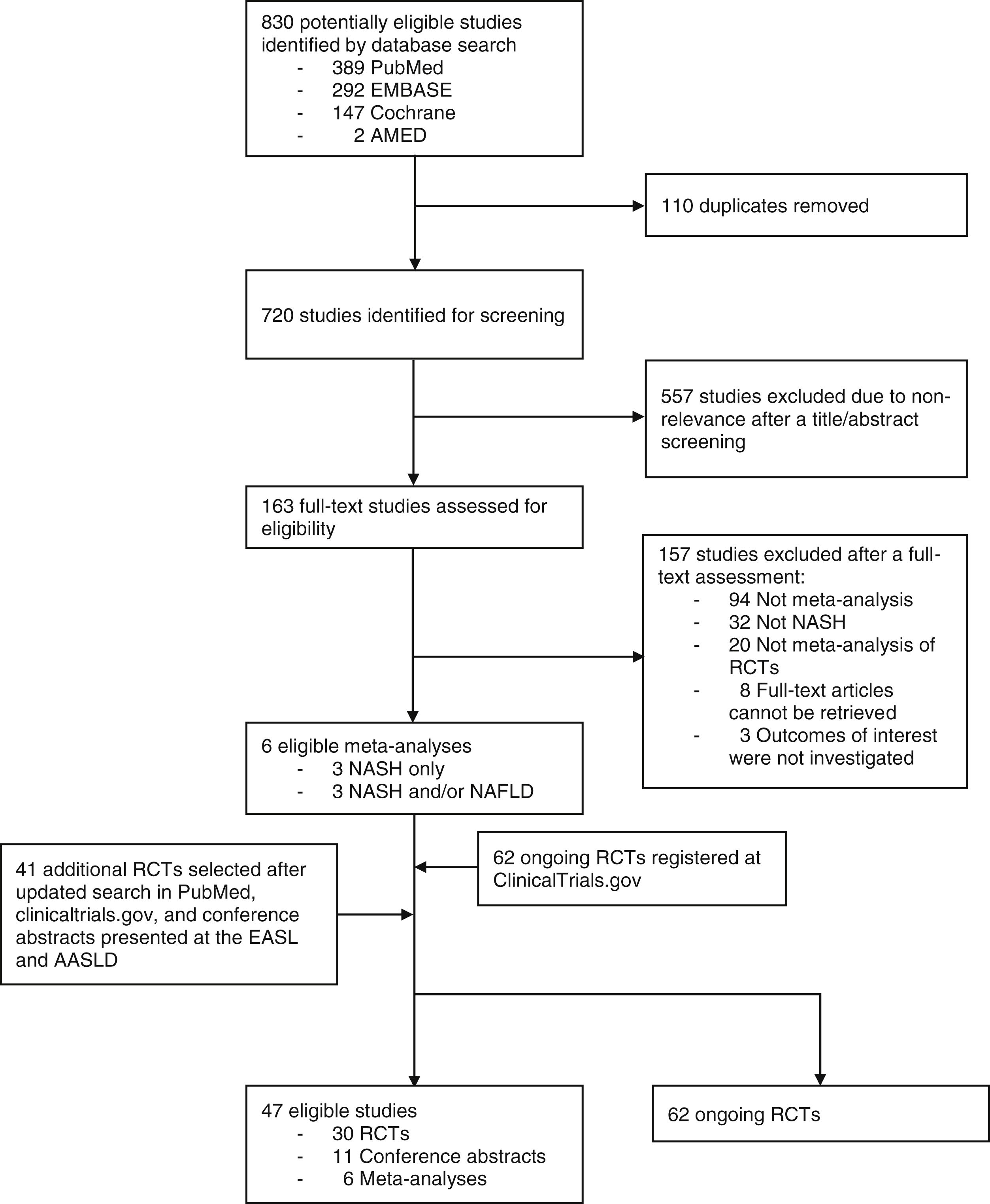
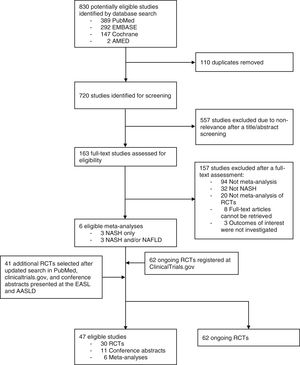
3ResultsOur horizon scanning included 6 meta-analyses of RCTs, 30 published RCTs, 11 conference abstracts, and additional 62 ongoing RCTs, as shown in Fig. 1 with details of study selection provided in the Supporting information Figs. S1–S3. Characteristics of the included studies are summarized in the Supporting information Table S1–S3.
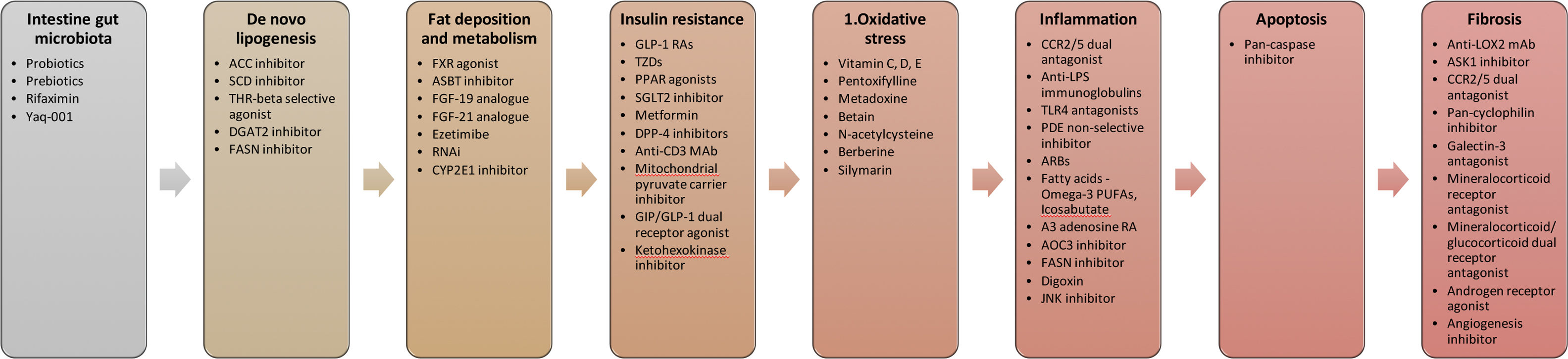
Based on 6 meta-analyses of RCTs, 30 RCTs, and 11 conference abstracts, a total of 49 therapeutic modalities for NASH were evaluated of which comprised 3 non-pharmacological interventions (moderate aerobic exercise, dietary intervention plus nutritional orientation, and fast weight loss method) and 46 pharmacological interventions (Fig. 2). They included firsocostat, muromonab-CD3, simtuzumab, IMM-124E, pentoxifylline, rifaximin, angiotensin receptor blockers (ARBs), volixibat, selonsertib, betain, cenicriviroc, ezetimibe, sitagliptin, TVB-2640, aldafermin, pegbelfermin, efruxifermin, cilofexor, EDP-305, MET409, nidufexor, obeticholic acid, tropifexor, ursodeoxycholic acid, liraglutide, semaglutide, metadoxine, metformin, metformin plus thiazolidinediones (TZDs), metformin plus N-acetylcysteine, metformin plus N-acetylcysteine and ursodeoxycholic acid, omega-3 polyunsaturated fatty acids (PUFAs), emricasan, elafibranor, MSDC-0602K, lanifibranor, prebiotics, probiotics, silymarin, TZDs, TZDs plus ARBs, resmetirom, JKB-121, vitamin D, vitamin E, and vitamin E plus vitamin C.
Target of pharmacological therapies for NASH.
Abbreviations: ACC – acyl-CoA carboxylase, SCD – stearoyl Coenzyme A desaturase, THR – thyroid hormone receptor, FASN – fatty acid synthase, FXR – farnesoid X receptor, ASBT – apical sodium-dependent bile acid transporter, FGF – fibroblast growth factor, RNAi – RNA interference, CYP2E1 – cytochrome P450 family 2 subtype E member 1, GLP-1 RAs – glucagon-like peptide-1 receptor agonists, TZDs – thiazolidinediones, PPAR – peroxisome proliferator-activated receptor, SGLT2 – sodium glucose co-transporter type 2, DPP-4 – dipeptidyl peptidase-4, MAb – monoclonal antibody, GIP – glucagon-dependent insulinotropic polypeptide, CCR2/5 – chemokine receptor 2/5, LPS – lipopolysaccharide, TLR4 – toll-like receptor 4, PDE – phosphodiesterase, PUFAs – polyunsaturated fatty acids, RA – receptor agonist, AOC3 – amine oxidase copper containing 3, FASN – fatty acid synthase, JNK – Jun N-terminal kinase, LOX2 – lysyl oxidase-like 2, ASK1 – apoptosis signal-regulating kinase 1, CCR2/5 – chemokine receptor 2/5, ARBs – angiotensin receptor blockers.
There are 62 ongoing RCTs of treatment modalities for NASH registered in ClinicalTrials.gov of which comprised 54 RCTs investigating pharmacological interventions and 8 RCTs investigating non-pharmacological interventions (nutritional intervention and surgical procedures). Most trials are in phase 2 (32 RCTs) with only 6 phase 3 RCTs.
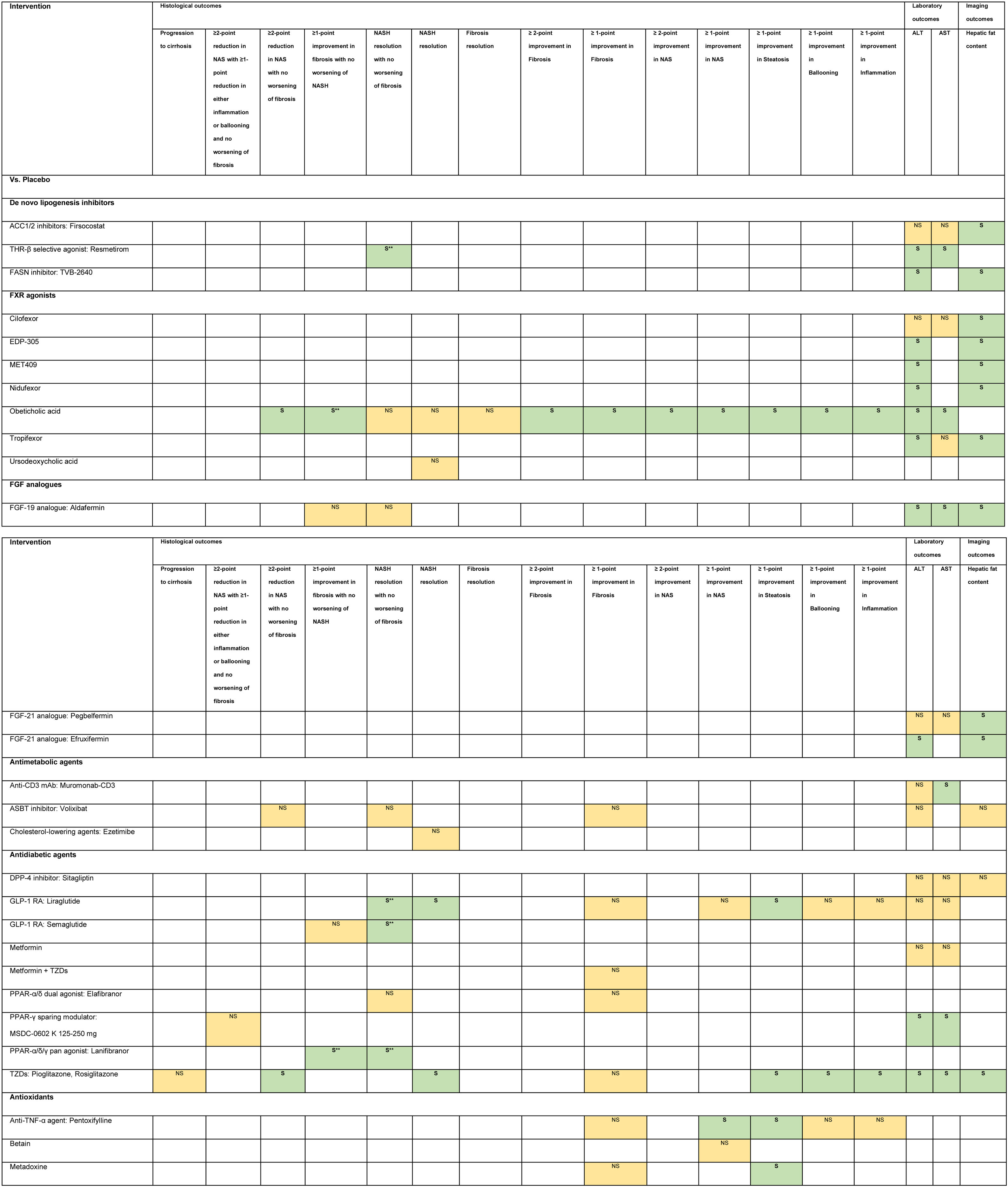
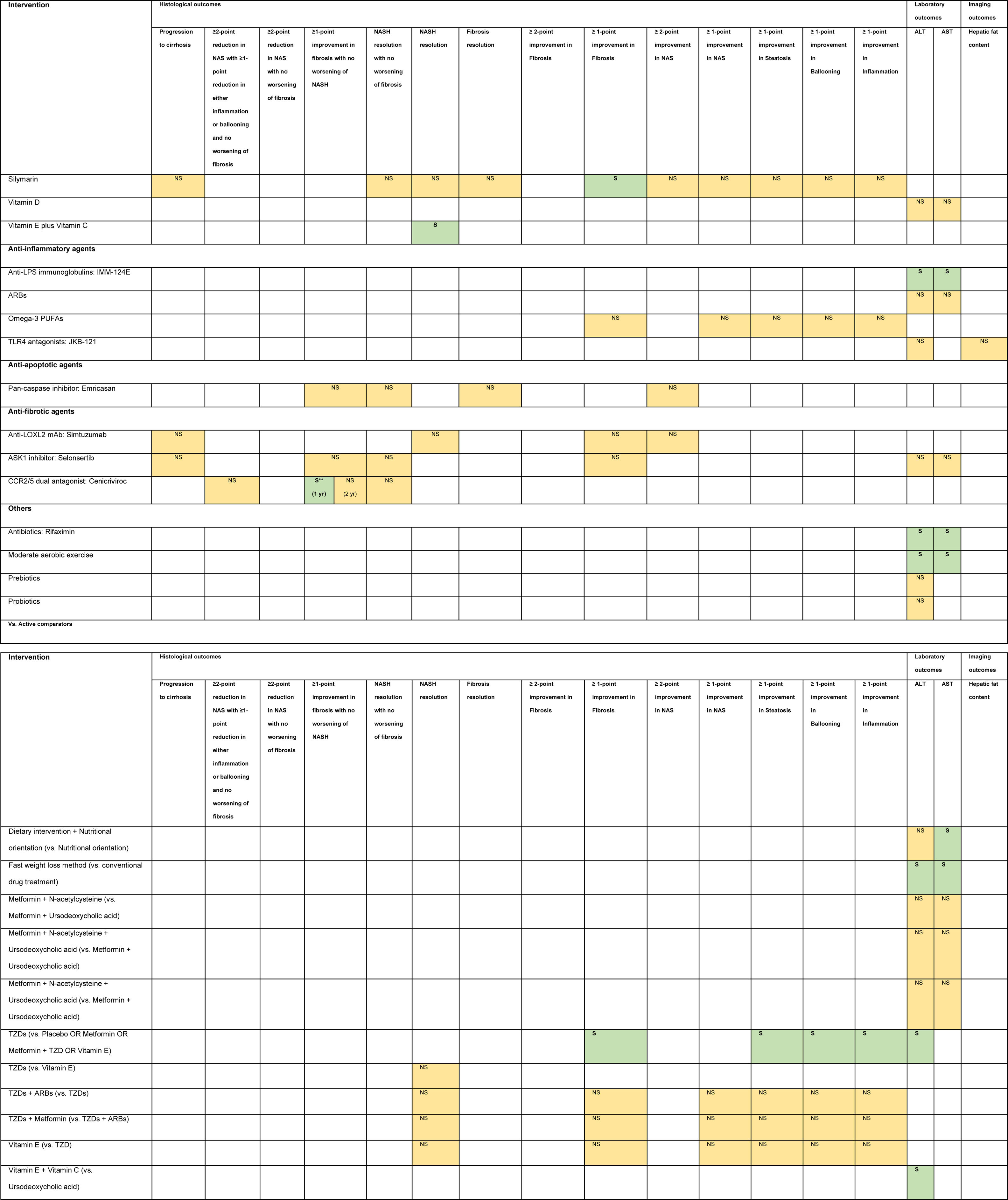
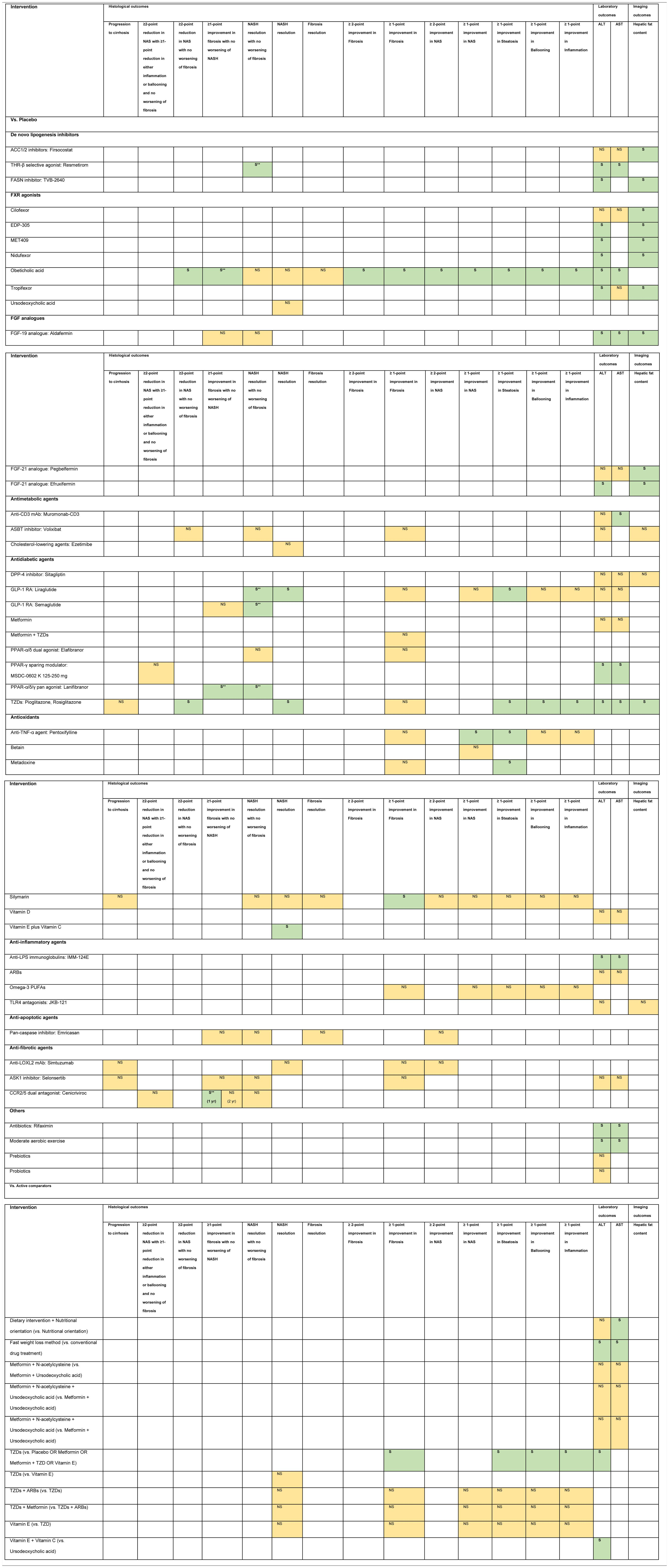
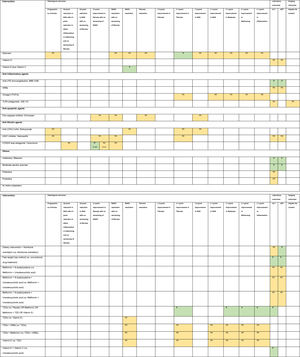
3.1Evidence map of therapeutic modalities for NASHAn evidence map summarizing treatment effects of therapeutic modalities for NASH is shown in Table 1 based on 6 meta-analyses of RCTs, 30 RCTs, and 11 conference abstracts. The details of estimates provided in the Supporting information Table S4. The significant treatment effects are summarized below.
Evidence map of therapeutic modalities for nonalcoholic steatohepatitis.
Note: NS Non-significant effect (yellow) S Significant positive effect (green), S** Interventions showed significant positive effect in the currently accepted histological endpoints for conditional approval (i.e. ≥1 improvement in fibrosis with no worsening of NASH, or NASH resolution with no worsening of fibrosis.
Abbreviations: ACC1/2 – acyl-CoA carboxylase isoform 1 and 2, ALT – alanine aminotransferase, ARBs – angiotensin receptor blockers, ASBT – apical sodium-dependent bile acid transporter, ASK1 – apoptosis signal-regulating kinase 1, AST – aspartate aminotransferase, CCR2/5 – chemokine receptor 2/5, DPP-4 – dipeptidyl peptidase-4, FASN – fatty acid synthase, FGF – fibroblast growth factor, FXR – farnesoid X receptor, GLP-1 RA – glucagon-like peptide-1 receptor agonist, LOXL2 – lysyl oxidase-like 2, LPS – lipopolysaccharide, mAb – monoclonal antibody, NAS – nonalcoholic fatty liver disease activity score, NASH – nonalcoholic steatohepatitis, PPAR – peroxisome proliferator-activated receptor, PUFAs – poly unsaturated fatty acids, THR – thyroid hormone receptor, TLR4 – toll-like receptor 4, TNF – tumor necrosis factor, TZDs – thiazolidinediones.
Compared to placebo, six interventions were found to have positive treatment effects on the currently accepted histological endpoints for conditional approval (6/49, 12.2%) demonstrated in one phase 3 RCT and five phase 2 RCTs.
≥1-point improvement in fibrosis with no worsening of NASH was shown in patients treated with obeticholic acid (RR 1.9, 95%CI 1.4–2.8) in the phase 3 clinical trial (REGENERATE) [9] and cenicriviroc (OR 2.2, 95%CI 1.1–4.3) in the phase 2b clinical trial (CENTAUR) [10]. NASH resolution with no worsening of fibrosis was shown in the phase 2 RCTs involving patients treated with liraglutide (LEAN, RR 4.3, 95%CI 1.0–17.7) [11], semaglutide (NN9931-4296, OR 6.87, 95%CI 2.60–17.63) and resmetirom (OR 4.5, 95%CI 1.0–21.9) [12]. Moreover, patients treated with lanifibranor achieved both ≥1-point improvement in fibrosis with no worsening of NASH (RR 1.80, 95%CI 1.13–2.87) and NASH resolution with no worsening of fibrosis (RR 2.40, 95%CI 1.43–4.03) in a phase 2 RCT (NATIVE) [13].
3.3Other histological endpointsEight interventions were found to have positive treatment effects in the histological outcomes (8/49, 16.3%). Compared to placebo, pentoxifylline showed benefits in ≥1-point improvement in NASH activity score (NAS) (RR 2.7, 95%CI 1.2–6.0), and steatosis (RR 2.4, 95%CI 1.1–5.0) [14]. Obeticholic acid 25 mg showed benefits in ≥2-point improvement in NAS with no worsening of fibrosis (RR 1.5, 95%CI 1.2–1.9), ≥2-point improvement in NAS (RR 2.0, 95%CI 1.1–3.7), and ≥1-point improvement in fibrosis (RR 1.9, 95%CI 1.1–3.1), NAS (RR 2.1, 95%CI 1.4–3.2), steatosis (RR 1.6, 95%CI 1.2–2.3), ballooning (RR 1.5, 95%CI 1.0–2.3), and inflammation (RR 1.6, 95%CI 1.1–2.2) [9,14]. Liraglutide showed benefits in NASH resolution (OR 6.2, 95%CI 1.2–34.4) and ≥1-point improvement in steatosis (RR 1.8, 95%CI 1.1–3.0) [11]. Metadoxine improved steatosis by at least 1 point (RR 2.9, 95%CI 1.5–2.6) [14]. Silymarin showed benefit in ≥1-point improvement in fibrosis (RR 3.7, 95%CI 1.1–12.6) [15]. TZDs showed benefits in ≥2-point reduction in NAS with no worsening of fibrosis (RR 3.2, 95%CI 1.7–6.2), NASH resolution (RR 2.6, 95%CI 1.4–4.9), and ≥1-point improvement in steatosis (RR 2.7, 95%CI 1.6–4.5), ballooning (RR 2.1, 95%CI 1.2–3.7), and inflammation (RR 2.3, 95%CI 1.2–4.1) [16,17]. Vitamin E plus Vitamin C showed benefit in NASH resolution (OR 2.1, 95%CI 1.1–4.1) [18].
Compared to active comparators of placebo or metformin or metformin plus TZD or vitamin E, TZDs showed histological benefits in ≥1-point improvement in fibrosis, steatosis, ballooning, and inflammation [19].
3.4Laboratory outcomesNineteen interventions were found to have positive treatment effects in the improvement of ALT and/or AST (19/49, 38.8%): Compared to placebo, IMM-124E [20], rifaximin [21], aldafermin [22,23], obeticholic acid [24], moderate aerobic exercise [25], MSDC-0602K [26], resmetirom [12], and TZDs [16,17] significantly improved both ALT and AST. Fast weight loss method compared to conventional drug treatment significantly improved both ALT and AST [27].
TVB-2640 [28], efruxifermin [29], EDP-305 [30], MET409 [31], and nidufexor [32] significantly improved ALT compared to placebo. TZDs (vs. placebo or metformin or metformin plus TZD or vitamin E) [19], vitamin E plus vitamin C (vs. ursodeoxycholic acid) [33], and tropifexor (vs. placebo) [34] significantly improved only ALT but not AST. Muromonab-CD3 (vs. placebo) [35] and dietary intervention plus nutritional orientation (vs. nutritional orientation) [36] significantly improved only AST but not ALT.
3.5Imaging outcomesEleven interventions were found to have positive treatment effects in the improvement of hepatic fat content assessed by MRI-PDFF (11/49, 22.4%): firsocostat [37], TVB-2640 [28], aldafermin [22,23], pegbelfermin [38], efruxifermin [29], cilofexor [39], EDP-305 [30], MET409 [31], nidufexor [32], tropifexor [34], and TZDs [16,17].
3.6Non-statistically significant treatment effectsTwenty interventions were found to have non-statistically significant treatment effects for NASH in all of the investigated endpoints (21/49, 42.9%).
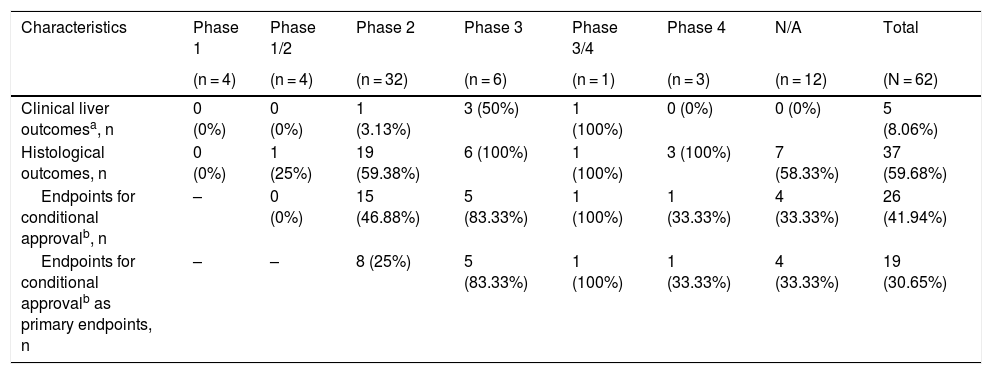
3.7Outcome characteristics of ongoing RCTs of treatment modalities for NASHOutcome characteristics of ongoing RCTs of treatment modalities for NASH are summarized in Table 2. It was found that 5 out of 62 RCTs (8.06%) will investigate clinical liver outcomes i.e., histological progression to cirrhosis, death, or liver-related clinical outcomes. Moreover, liver biopsy-based outcomes will be investigated in 37 out of 62 RCTs (59.68%), of which 26 RCTs (41.94%) use the currently accepted histological endpoints for conditional approval.
Summary of outcome characteristics of ongoing randomized controlled trials of interventions for nonalcoholic steatohepatitis.
| Characteristics | Phase 1 | Phase 1/2 | Phase 2 | Phase 3 | Phase 3/4 | Phase 4 | N/A | Total |
|---|---|---|---|---|---|---|---|---|
| (n = 4) | (n = 4) | (n = 32) | (n = 6) | (n = 1) | (n = 3) | (n = 12) | (N = 62) | |
| Clinical liver outcomesa, n | 0 (0%) | 0 (0%) | 1 (3.13%) | 3 (50%) | 1 (100%) | 0 (0%) | 0 (0%) | 5 (8.06%) |
| Histological outcomes, n | 0 (0%) | 1 (25%) | 19 (59.38%) | 6 (100%) | 1 (100%) | 3 (100%) | 7 (58.33%) | 37 (59.68%) |
| Endpoints for conditional approvalb, n | – | 0 (0%) | 15 (46.88%) | 5 (83.33%) | 1 (100%) | 1 (33.33%) | 4 (33.33%) | 26 (41.94%) |
| Endpoints for conditional approvalb as primary endpoints, n | – | – | 8 (25%) | 5 (83.33%) | 1 (100%) | 1 (33.33%) | 4 (33.33%) | 19 (30.65%) |
Abbreviations: N/A – not applicable, NASH – nonalcoholic steatohepatitis.
We provide the overall landscape on the horizon for treatment of NASH ranging from 6 meta-analyses of RCTs, 30 recently published RCTs, 11 unpublished RCTs and 62 ongoing RCTs. Using an evidence map based on 49 therapeutic modalities for NASH, we found that 6 promising interventions showed significant treatment effects on the histological surrogate endpoints for conditional approval. Obeticholic acid and cenicriviroc treated patients achieved ≥1-point improvement in fibrosis with no worsening of NASH. Liraglutide, semaglutide, and resmetirom treated patients achieved NASH resolution with no worsening of fibrosis. While lanifibranor treated patients achieved both histological surrogate endpoints. However, none of the investigated treatment modalities so far has been approved by FDA for the indication of NASH. They are either still in clinical development trials or have failed to show benefits in phase 3 trials.
Based on the successes demonstrated in early and late phase 2 trials, the six promising drug interventions have advanced to phase 3 trials, which are either in development or currently on-going. Therefore, future FDA approval for any potential drug will be incumbent on the sum of the completed phase 1–3 results with more attention on the long-term benefits on final outcomes of all-cause mortality and liver-related clinical outcomes.
The array of pharmacologic therapeutic targets that we reviewed span all stages implicated within the pathophysiology of NASH development. This includes the initial dysmetabolic stage accompanied by insulin resistance and hepatic steatosis, and the later stages featuring oxidative stress, apoptosis, inflammation, and hepatic fibrosis. However, since increasing hepatic fibrosis appears to be the single most important predictor of mortality in patients with NASH [40], those candidate pharmacologic therapies that directly affect fibrogenesis or upstream inflammation have emerged as frontrunners in the single drug pipeline.
Obeticholic acid is a semisynthetic derivative of chenodeoxycholic acid, a primary bile acid found in human and rodents and natural ligand of FXR. The FXR pathway is involved in regulating hepatic gluconeogenesis and lipogenesis and hepatic inflammation to maintain metabolic homeostasis in the liver [41]. Administration of obeticholic acid reduces hepatic steatosis and insulin resistance in obese rats [42], while in a phase 2 trial of patients with type 2 diabetes mellitus and NAFLD obeticholic acid administration also demonstrated improvement in insulin sensitivity and biochemical markers of fibrosis [43]. Targeting FXR in rodent models have also revealed other possibilities potentially relevant to NAFLD through implications of the gut-liver axis [44], including reduced intestinal inflammation and bacterial translocation [45,46], and protection against PAMP-induced inflammation through anti-NF-kB properties [47]. Despite meeting its primary histologic endpoint at the planned 18-month interim analysis, obeticholic acid was rejected by the FDA in June 2020 for an accelerated approval pathway. In the response letter issued, the FDA cited its uncertainty surrounding the use of a surrogate histopathologic endpoint as sufficiently outweighing the potential risks, along with further recommendations to Intercept Pharmaceuticals to submit additional post-interim analysis of safety and efficacy. The end-of-study analysis for REGENERATE, encompassing an anticipated 7-year follow-up, will evaluate the effect of obeticholic acid on an all-cause mortality and liver-related clinical outcomes as well as long-term safety.
Cenicriviroc is a small molecule, dual antagonist of the C–C chemokine receptor type 2 (CCR2) and 5 (CCR5), through which action is meant to disrupt recruitment of inflammatory monocytes into the liver and hepatic stellate cell activation, respectively. In human liver biopsies, Krenkel et al. demonstrated significantly increased CCR2+ macrophage infiltration of the hepatic parenchyma in the setting of cirrhosis and fibrosing steatohepatitis, compared to non-fibrotic controls [48]. In turn, use of cenicriviroc in a western-diet induced murine NASH model has been shown to ameliorate fibrosis and steatohepatitis through inhibiting monocyte infiltration of the liver [48]. It is important to note that the anti-fibrotic benefit of cenicriviroc demonstrated in the phase 2b clinical trial (CENTAUR) was a secondary endpoint. The primary endpoint of CENTAUR – a ≥2‐point improvement in NAS and no worsening of fibrosis at year 1 – was not met. However, based on the significant anti-fibrotic benefits observed with subjects receiving cenicriviroc, especially among those with more advanced fibrosis, this drug was advanced to phase 3 clinical trial. The results of the phase 3 AURORA study are not yet available, as the study is still ongoing.
Liraglutide and semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), have a high potential role in NASH management. More than 10 years ago, GLP-1 RA has been used for glycaemic control in overweight with type 2 diabetes patients. Because it does not only provide the effect on systemic insulin resistance, but it does also provide the effects on obesity. The rationale which seems to best explain those favourable effects is that it can centrally suppress appetite, delay gastric emptying, and induce weight loss. Together with the well-established knowledge that NASH has strong association with metabolic syndrome, especially obesity and type 2 diabetes. It has been proven that liraglutide met the histological resolution of NASH with no worsening in fibrosis [11]. Liraglutide might has an effect on cardiovascular diseases that are found to be the common cause of death among NASH patients. Regarding to its effects, liraglutide has high potential to be included in a strategy for improving the outcomes in NASH patients. Liraglutide provides the direct hepatic effect along with weight loss which are synergistically improve NASH outcomes, based on the mechanism of action of GLP-1. The researchers revealed that GLP-1 analogues improve the ability of hepatocytes to handle excess non-esterified fatty acids and lipid production by modulating lipid transport, beta-oxidation, and de-novo lipogenesis in previous in-vitro studies [49–51], all of which have been implicated in the pathogenesis of NASH. Liraglutide are considered safe. Its adverse effects were mild and tolerable such as transient and predictable gastrointestinal symptoms. Moreover, theoretically, a higher dose of liraglutide may provide more magnitude of effect in NASH patients. However, to our knowledge, the optimal dose has not yet been discovered. Nevertheless, further studies of liraglutide should be conducted in the longer period of time to confirm its efficacy and to determine its cardiovascular implications in NASH patients. Subcutaneous semaglutide once daily has also demonstrated its promising benefits in NASH patients in a phase 2 RCT with a safety profile consistent with that observed in its previous trials in diabetic patients [52]. Within our understanding, Novo Nordisk has not proceeded with studying liraglutide in a phase 3 trial for the primary treatment of NASH but has chosen instead to focus its efforts on semaglutide, a similar GLP-1 agonist agent with a longer half-life and oral dosing availability.
Resmetirom is a highly selective thyroid hormone receptor-β agonist, the primary thyroxine receptor in the liver. Activation of thyroid hormone receptor-β is believed to favourably affect cholesterol and lipoprotein levels via multiple mechanisms, including increasing the expression genes associated with lipid metabolism and clearance, while avoiding the unwanted systemic actions of thyroid hormone in heart and bone that are largely mediated through THR-α [53]. In a phase 2 trial, resmetirom-treated patients showed a 22% relative reduction of hepatic fat content compared to placebo after 12 weeks of treatment, with an even greater improvement seen after 36 weeks of treatment (29%) [12]. In addition to resmetirom, a phase 2 trial of VK2809 – a THR-β agonist being developed by Viking Therapeutics – has shown ≥ 30% reduction in liver fat content in 88% of patients receiving the compound compared to patients receiving placebo, as well as improvements in plasma low-density lipoprotein cholesterol and triglyceride levels [54]. Safety and efficacy of VK2809 as treatment for biopsy-proven NASH is currently being investigated in a phase 2b trial (VOYAGE), while resmetirom is in phase 3 development.
Lanifibranor is a pan-peroxisome proliferator-activated receptor (PPAR) agonist in which the activation of all three PPAR isoforms (alpha/delta/gamma) involves key regulatory functions in metabolism, inflammation, and fibrogenesis [55]. Lanifibranor is the first drug candidate to demonstrate statistically significant benefits on both ≥1 improvement in fibrosis with no worsening of NASH and NASH resolution with no worsening of fibrosis which will be further investigated in a phase 3 clinical trial. While elafibranor, a dual-PPAR agonist, had previously showed benefit of NASH resolution with no worsening of fibrosis in the phase 2 RCT (GOLDEN-505) [56]. However, the interim analysis of its subsequent phase 3 RCT (RESOLVE-it) revealed that elafibranor failed to meet the primary efficacy endpoint at week 72, and so has officially been terminated.
The benefits of NASH treatments on clinical endpoint of histological progression to cirrhosis was not extensively studied. Our horizon scanning found that four interventions were studied, but none of them showed significant benefits on the prevention of histological progression to cirrhosis. These include simtuzumab [57], selonsertib [58], silymarin [15,18], and TZDs [18]. A number of different endpoints have been selected in clinical trials in NASH, ranging from histologic improvement (e.g., NAS score), resolution of NASH, >1 stage regression of fibrosis and prevention of progression to cirrhosis. Given the long natural history of liver disease, it remains unclear which of these criteria is most likely to predict in prolonging survival or reduction in risk of liver-related complications. However, it is clear that the single most important predictor of liver-related complications is fibrosis stage, particularly stage 2 or 3 [40]. The Phase 3 trials completed thus far (REGENERATE, STELLAR) have used fibrosis stage improvement (without worsening of NASH) as the primary endpoint [9,58] while RESOLVE-IT (NCT02704403) used resolution of NASH without worsening of fibrosis as the primary endpoint. A more clinically relevant endpoint may be improvement in fibrosis without worsening of NASH, as progressive fibrosis has been shown to be the most important predictor of liver-related and overall survival in NASH [40]. It is particularly important that improvement in fibrosis and resolution are independent variables, since the natural history of histologic progression with increasing fibrosis stage in NASH is characterized by a reduction in degree and severity of NASH [59]. Over time, active steatohepatitis may “burn out” with more fibrosis and less active ballooning or even steatosis [60]. Others have argued that endpoints in clinical trials of NASH should focus on clinical endpoints such as survival, liver-related complications or symptoms or signs of liver decompensation such as increased MELD score, increased Child-Pugh class, or listing for liver transplantation [8]. The REGENERATE trial is the only Phase 3 study to demonstrate a statistically significant >1 stage improvement in fibrosis among patient with NASH with fibrosis stage 2 or 3 [9]. A significant challenge in NASH clinical trials is the highly variable placebo response rate [61]. Nevertheless, there remains a critical need to identify surrogates reasonably likely to predict clinical benefit due to the long natural history of liver disease. It remains unclear from a regulatory standpoint which surrogate endpoint might be considered the most likely to predict or associated with clinical outcomes. However, improvement in fibrosis stage, especially from stage 3 or 4 to stage 2 or lower, appears intuitively to be likely to predict improvement in clinical outcomes, as is reduction in risk of progression to cirrhosis. Therefore, it would appear important for future clinical trials to be powered to show in improvement in fibrosis stage and/or progression to cirrhosis.
Our horizon scanning has several limitations. Benefits of the therapeutic modalities for NASH were determined solely based on the statistical significance of surrogate endpoints because most studies could not investigate the benefits on the prevention of progression to cirrhosis and other liver outcomes. This raised a question whether these treatment effects were clinically meaningful for NASH patients. Moreover, most of the treatment effects in the evidence map were derived from a single RCT due to the limited number of studies conducted so far. The treatment effects shown in this horizon scanning could change as many more RCTs for NASH are being conducted.
In conclusion, our horizon scanning found that some drugs could significantly improve surrogate endpoints for NASH patients. However, after attempts spanning for many years until now, the definite treatment for NASH has yet to be found. With emerging pharmacological interventions and advances in technology, it is expected that we could eventually see the light at the end of the tunnel for NASH patients. AbbreviationsAASLD American Association for Study of Liver Diseases Alanine aminotransferase Angiotensin receptor blockers Aspartate aminotransferase European Association for the Study of the Liver Food and Drug Administration Glucagon-like peptide-1 receptor agonist Mean difference Magnetic resonance imaging proton density fat fraction Non-alcoholic fatty liver disease Nonalcoholic fatty liver disease activity score Nonalcoholic steatohepatitis Odd ratio Peroxisome proliferator activated receptor Polyunsaturated fatty acids Randomized controlled trials Relative risk Thiazolidinediones
Conceived and designed the study: CP, SKV, PP, TP, KVK, and NC. Performed horizon scanning: CP, SKV, and NC. Wrote the paper: CP, SKV, PP, TP, KVK, and NC. All authors have approved the final draft of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflict of interestKV Kowdley received research support from Intercept, Gilead Sciences, Novartis, Glaxo Smith Kline, Genfit and Enata; serves as a consultant and speaker for Gilead Sciences and Intercept. NC was an advisor on the advisory board of Intercept. The other authors have no conflict to declare.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to thank Chayanis Kositamongkol for her editorial assistance throughout the project.