Editado por: Sonia Roman
Última actualización: Enero 2023
Más datosNonalcoholic fatty liver disease (NAFLD) is currently the most common cause of chronic liver disease worldwide affecting a third of adults and 12% of children in Western countries. In around 50–60%% of cases, NAFLD and type 2 diabetes mellitus (T2DM) coexist and act synergistically to increase the risk of adverse hepatic and extra-hepatic outcomes. T2DM is a strong risk factor for rapid progression of NAFLD to nonalcoholic steatohepatitis (NASH), cirrhosis or hepatocellular carcinoma (HCC), which have become frequent indications of liver transplantation.
The pathophysiology of NAFLD is complex and its relationship with T2DM is bidirectional, where lipotoxicity and insulin resistance (IR), act as the strongest pillars.
To date, no pharmacological treatment has been approved for NAFLD. However, there is an intense research with numerous drugs focused on reversing inflammation and liver fibrosis through modulation of molecular targets without good results.
It has been known for some time that weight reduction >10% is associated to histological improvement of NAFLD. Recently, glycemic control has been shown to induce similar results. Diet and physical exercise for weight reduction have limitations, so alternative methods (pharmacologic, endoscopic or surgical) may be required. Currently, new antidiabetic drugs inducing weight loss, have been recently approved for the treatment of obesity. Nevertheless, their therapeutic effects on NAFLD have not been extensively studied.
We will review here, recently published data on the effects of weight loss and glycemic control on the histological and metabolic parameters of NAFLD and recent published data on therapeutic studies of NAFLD with new antidiabetic drugs.
Nonalcoholic fatty liver disease (NAFLD) is currently the most common cause of chronic liver disease worldwide affecting both adults and children. It affects around a third of adults in Western countries [1]. NAFLD is tightly associated with obesity and type 2 diabetes mellitus (T2DM). The global prevalence of NAFLD in people with T2DM is high of around 56%. [2] NAFLD and T2DM frequently coexist and act synergistically to increase the risk of hepatic and extra-hepatic complications [3,4]. Growing evidence indicates that patients with NAFLD are at substantial risk of increased cardiovascular morbidity and mortality [5]. In addition, T2DM is also a strong risk factor for the rapid progression of NAFLD to nonalcoholic steatohepatitis (NASH), cirrhosis or hepatocellular carcinoma (HCC) becoming one of the most frequent indication of liver transplantation worldwide. The economic burden of NAFLD is high worldwide. In the United States, over 64 million people are projected to have NAFLD, with annual direct medical costs of about $103 billion and in Europe there are over 52 million people with NAFLD with an annual cost of about €35 billion [6].
2Pathophysiology of NAFLDThe pathophysiological relationship between NAFLD and T2DM seems to be bidirectional. It is quite complex and not completely understood [7]. So, it is not going to be described here in detail. It has also been suggested that NAFLD might precede the development of T2DM, particularly in lean or non-obese patients and that the risk of developing T2DM parallels the severity of NAFLD. Whether NAFLD or T2DM precedes remains largely unknown; they may move in tandem, interplaying with each other.
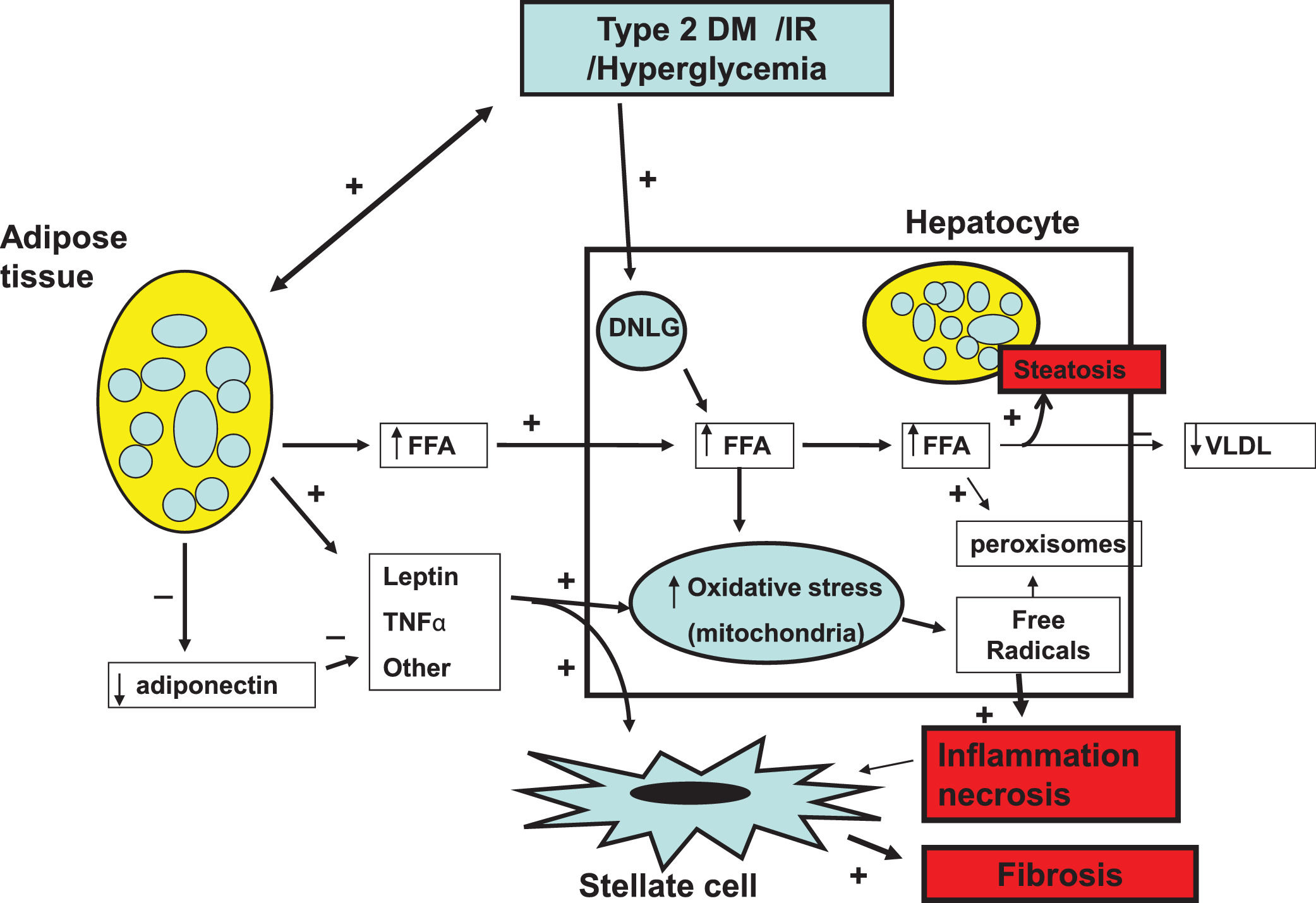
Fundamentally, obesity and insulin resistance (IR) are important cofactors in the generation of liver damage [8–10]. The noxious process begins with hepatic steatosis (HS) as a result of the increase in the uptake of circulating triglycerides (TGL), ceramides and diacylglycerols. Enlarged fat cells in obese adipose tissue diminish the capacity to store fat and are resistant to the anti-lipolytic effect of insulin [11]. Lipid saturation in the liver is worsened by increased intracellular de novo lipogenesis (DNLG) stimulated by IR and the reduced secretion of very low-density lipoproteins (VLDL) by hepatocytes. The deleterious effect caused by the accumulation of lipids in the liver is called as “lipotoxicity”. Cellular liver damage occurs as a result of mitochondrial oxidative stress due to the high content of liver fat, the concurrence of adipokines, microbiota and glucocorticoids [12–14] generating free radicals and peroxisomes [15,16]. The progression from HS to NASH occurs when the protective mechanisms of free fatty acids (FFA)-mediated lipotoxicity become exhausted, and the rate of hepatocyte death exceeds the rate of hepatocyte regeneration. This triggers the activation of stellate cells and induce them to increase the production of collagen, connective tissue growth and extracellular matrix favoring in turn progressive fibrosis and cirrhosis [17,18] (Fig 1).
Pathophysiology of NAFLD. Insulin resistance (IR) and obesity are the most important elements involved in the pathophysiology of NAFLD. They synergistically operate to induce hepatic steatosis (HS) initiating the lipotoxicity process. This results in mitochondrial oxidative stress giving rise to free radicals and peroxisomes generation inducing process of ballooning cell degeneration and necrosis and inflammation enhanced by inflammation mediators and adipokines. The inflammation process stimulates stellate cells to produce collagen and extracellular matrix favoring progression to liver cirrhosis.
In addition, there are some genetic variants involved in the genesis of HS in the absence of IR. Some polymorphisms of patatin-like phospholipase domain-containing protein 3 (PNPLA3) and the transmembrane 6 superfamily member 2 protein (TMS6F2) are associated with impairment of lipid metabolism favoring development of NAFLD [19,20].
3T2DM increases the risk of NAFLD progressionT2DM plays an important role in the progression of NAFLD, as T2DM increases the risk of NASH by approximately two to three times [21]. Based on liver histology, about 37.3% of patients with T2DM develop NASH, and 17% advanced fibrosis [22]. According to long term studies with NASH patients, liver fibrosis (not inflammation) is the main predictor of mortality. The presence of significant fibrosis (≥ F2) is associated to increased mortality [23]. In addition, the risk of HCC increases two to three times with the presence of T2DM [24].
4The effects of body weight loss on liver histologyWeight loss is associated to significant improvements in HS, NASH and fibrosis in individuals with NAFLD [25,26] and is also highly effective in preventing or delaying the onset of T2DM in people at high risk [27,28]. Weight loss can be achieved by different methods ranging from lifestyle changes (diet and physical exercise), anti-obesity drug use and bariatric surgery. Recently, bariatric endoscopic methods have boomed. All these therapies have different degrees of effectiveness for weight loss and invasiveness as well as advantages and disadvantages.
4.1Diet and physical exerciseIn a classical study with 261 biopsy-proven NASH patients (66% with prediabetes or DM and 100% with overweight or obesity) the effect of weight loss through diet and physical exercise during 52 weeks on histologic features was evaluated. Globally, 30% of patients lost >5% of weight, among them 25% achieved resolution of NASH, 47% had reduction of NAFLD activity score (NAS), and 19% had regression of liver fibrosis. A higher proportion of the subjects with >5% weight loss, had NASH resolution and NAS reduction than those who lost <5% of weight. Most patients who lost from 5 to 6.9% of weight had reduction of NAS (65%); most patients who lost from 7 to 9.9% of weight had resolution of NASH (64%) and all patients who lost >10% of weight had reduction of NAS, 90% had resolution of NASH, and 45% had regression of fibrosis. In patients with body mass index (BMI) >35 kg/m2 and fasting glucose >5.5 mmol/L, NAS decreased more significantly in those who achieved weight reduction >10%. In the total group, the highest rates of NAS reduction, NASH resolution, and fibrosis regression occurred in patients with weight loss >10% [26].
In other RCTs, the results of this study have been reproduced. In two studies, diet and physical exercise administered for 12 and 26 weeks, compared with only physical exercise, produced significant weight loss and improvement in NAS and HS on liver biopsy [29,30]. Other RCTs that compared hypocaloric diets combined with physical exercise to only physical exercise or hypocaloric diet reported that the combined regimen was superior in terms of weight loss and improvement of metabolic parameters such as glycated hemoglobin (HbA1c), homeostatic model assessment of insulin resistance (HOMA-IR), low density lipoprotein cholesterol (LDL – cholesterol) and serum TGL [31–34] which are tightly linked to the development and progression of NASH.
Notwithstanding, diet and physical exercise regimen as a method of weight reduction has several disadvantages. Its implementation can be difficult because of time-consuming, lack of motivation, high costs and poor adherence. The usual protocol includes repeated hypocaloric diets and at least 210 min of exercise a week, motivational interviewing and multifactorial care composed of physicians, nutritionists and psychologists. According to some studies only 20 to 30% of patients can achieve a significant body weight reduction (> 5–10%) [35]. Therefore, alternative therapies may be required.
4.2Drug therapy (DT)Before 2014 FDA- approved anti-obesity drug therapy included: phentermine, phentermine/topiramate, bupropion/ naltrexone and orlistat. These medications showed an average efficacy of 5–6% weight loss based on clinical trials. Nevertheless, their use was limited by their toxicity. In 2014, liraglutide and in 2021, semaglutide (both Glucagon -Like Peptide 1- receptor agonists (GLP-1Ra), were approved in USA by the FDA for weight management in patients with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 plus at least one weight-associated comorbidity (diagnosis of T2DM not required) [36,37]. In a RCT, the daily SC administration of 3 mg of liraglutide for one year in obese patients produced a mean decrease of 8% body weight compared to 2.6% in controls receiving placebo [36] with approximately two thirds of patients treated with liraglutide achieving >5% body weight reduction and one third experiencing >10%. In a phase III clinical trial in overweight patients without T2DM, once-weekly treatment with 2.4 mg SC semaglutide significantly decreased body weight after 68 weeks of treatment by −14.9% compared to −2.4% in placebo-treated controls [37]. In patients with dia betes and obesity, semaglutide decreased body weight by −9.6% compared to −3.4% in placebo controls [38]. Reduction of body weight was associated with reduction of IR, serum TGL and blood pressure. Liraglutide and semaglutide were well tolerated in clinical trials and most side effects were minor, particularly on the digestive tract.
It is relevant to note that the effects of high doses of liraglutide and semaglutide (as those used for weight reduction) in histological parameters of NASH have not been assessed up to date. Notwithstanding, some studies specifically designed for assessing the effects of lower doses of both drugs on NASH have demonstrated histological significant improvement as it is going to be punctually discussed in following sections.
4.3Bariatric endoscopic therapy (BET)There are many endoscopic procedures recently developed for the treatment of obesity. However, only five have been approved by the FDA based on randomized controlled trials. They include gastric and duodenal devices and techniques such as: intragastric balloons (IGB), endoscopic sleeve gastroplasty (ESG), primary obesity surgery endoluminal (POSE), aspiration therapy and transpyloric shuttle [39]. In a recent systematic review and metanalysis of 18 studies with 863 patients treated with these BET techniques, it was reported weight reduction of 14.5% at a 6 month follow- up. Liver fibrosis significantly reduced (p = 0.02). There were also significant improvement in other NAFLD surrogates such as alanine aminotransferase (p <0.001), hepatic steatosis (p < 0.0001) and NAS (p < 0.0001) [39]. In two recent studies, one non-comparative observational with IGB and one small RCT comparing IGB to sham in patients with obesity and NAFLD or NASH it was reported changes in metabolic parameters and significant reduction of NAS and liver fibrosis after 6 months of follow-up [40,41]. In a study with ESG, it was reported significant decrease in liver enzymes, hepatic steatosis index and FIB-4 in obese NAFLD patients at a 12- and 24-month follow-up [42]. ESG has proved to be a safe and efficient method for significant weight reduction within 12 to 18 months of follow up [43]. So, it is currently considered a valid less invasive alternative method to the surgical sleeve gastrectomy.
4.4Bariatric surgery (BS)BS (mainly Roux-en-Y Gastric Bypass (RYGB) and sleeve gastrectomy) is a highly effective therapy for long term weight reduction in patients with morbid obesity. Patients may show >30% rapid weight loss. In studies focused on weight loss, operated patients with NASH improved all histological parameters, including HS, NASH and fibrosis [44]. Lassailly G et al. demonstrated that 1 year after surgery, resolution of NASH occurred in 85% of cases [45]. A metaanalysis carried out on 15 studies including 766 patients with paired liver biopsies, demonstrated that steatosis was improved in 91.6%, NASH in 81.3%, and fibrosis in 65.5% [46]. Notwithstanding, BS is a major procedure that can pose serious complications, side effects and mortality. Additionally, in some patients, rapid weight loss after BS may result in worsening of liver histology with liver dysfunction [47]. Therefore, it has been reserved to patients with morbid obesity (BMI >40) or to less obese patients (BMI >35) with high risks of metabolic, cardiovascular, respiratory or hepatic complications.
In conclusion, the effects of DT, BET and BS have not been specifically studied in the treatment of NAFLD and NASH. Nevertheless, all these therapies are superior to diet and physical exercise, each with increasing effectiveness for weight loss: DT (10–15%), BET (15–25%) and BS (>30%) and increasing frequency and severity of adverse side effects so they may be used consecutively based on the degree of obesity, the severity of NASH and the number and type of comorbidities. Further research with well-designed prospective and controlled studies using these therapies specifically for the treatment of NAFLD and NASH, prioritizing changes in liver histological parameters and modulation of metabolic, cardiovascular and hepatic complication rate outcomes are urgently needed in order to determine the best use of these therapies for the treatment of NAFLD and NASH.
5The effects of glycemic control on liver histologyHyperglycemia promotes the progression of NAFLD by increasing oxidative stress, triggering an aberrant inflammatory response, and inducing mitochondrial dysfunction [48,49]. Based on this, it was assumed that, in diabetic patients with NAFLD, optimal glycemic control could have a beneficial effect on histological parameters (particularly fibrosis) as compared to those with poor glycemic control. This issue was demonstrated until recently in several studies [50–55] (Table 1). In a study with 713 NAFLD patients, 49% with DM, 51.3% with advanced fibrosis/cirrhosis, and 69.6% with NASH, a higher mean HbA1c was associated with higher grade of steatosis and ballooned hepatocytes, but not with lobular inflammation. Every 1% increase in mean HbA1c was associated with 15% higher odds of increased fibrosis stage (OR: 1.15, 95% CI: 1.01- 1.31). As compared with good glycemic control, moderate control was significantly associated with increased severity of ballooned hepatocytes (OR: 1.74, 95% CI: 1.01- 3.01) and hepatic fibrosis (OR: 4.59, 95% CI: 2.33- 9.06). The authors concluded that optimizing glycemic control, as opposed to diabetic status, may be a means of modifying the risk of NASH-related inflammation, and fibrosis progression [50]. Other studies have obtained similar findings. In a very recent study with 568 with biopsy-proven NAFLD, the association between mean HbA1c and hepatic histological features was investigated with logistic regression. A non-linear association between HbA1c and increased fibrosis stage (F ≥ 3) was observed, inflection point was 9.2%. Similarly as described in the previous study, every 1% increase in mean HbA1c was associated with 16% higher odds of increased fibrosis stage (OR: 1.16, 95% CI: 1.04–1.30), even after adjustment for confounding factors [51]. In another study with 39 NAFLD patients who had undergone consecutively liver biopsies in a median follow up of 2.4 years, decrease of HbA1c and the use of insulin were significantly associated with improvement of liver fibrosis independently of age, sex, and BMI [52]. In another study with 221 biopsy-proven NAFLD patients (70% with T2DM). SAF score (that includes steatosis, grade of inflammation and grade of fibrosis) and NAFLD activity score (NAS) were higher in patients with T2DM than in those without T2DM. HbA1c was positively associated with SAF and fibrosis stage. Additionally, FPG, 2 h oral glucose tolerance test (OGTT), and HOMA-IR were positively associated with SAF, NAS and fibrosis stage [53]. In another study with 1935 NAFLD subjects, HbA1c level ≥6.5% was significantly associated to potential liver fibrosis assessed by Fib-4. The prevalence of NAFLD and liver fibrosis increased according to glycemia up to 8.0% HbA1c [54]. Finally, in a cross-sectional study with 1630 NAFLD patients, a strong association between HbA1c levels and the risk of fibrosis assessed by NAFLD fibrosis score was observed using a multivariate analysis (OR: 2.69, 95% CI: 1.60–4.53). This association remained significant, even in subjects without T2DM [55].
Description of studies that assess association between glycemic control and outcomes of NAFLD in patients with and without T2DM.
| Author | Design | N | DM/Fibrosis/NASH% | Method evaluation | Results |
|---|---|---|---|---|---|
| Alexopoulos AS [48] | P/R longitudinal cohort study | 713 | 49/ 51/70DM Treated with diverse treatment | Liver biopsy | Lower HbA1c was significantly associated to lower severity of ballooned hepatocytes and liver fibrosis. |
| Gong F [49] | P longitudinal cohort study | 568 | 26.4/19.5/76 | Liver Biopsy | A non-linear association between HbA1c and increased fibrosis stage (F ≥ 3) and HbA1c was observed |
| Hamaguchi E [50] | R/P longitudinal cohort study | 39 | 77/100/NRDM treated with diverse treatment | Liver biopsy | In a follow up of 2.4 years, decrease of HbA1c was associated with improvement of liver fibrosis independently of age, sex, and BMI |
| Bian H [51] | Cross-sectional study | 221 | 70/50/95DM treated with diverse treatment | Liver biopsy | HbA1c, FPG, OGTT, and HOMA-IR were positively associated with SAF, NAS and fibrosis stage. |
| Tanaka K [52] | General population Cross-sectional study | 1935 | NR/25/NR | Fib-4 | HbA1c ≥6.5% was significantly associated to potential liver fibrosis. The prevalence of NAFLD and liver fibrosis increased according to glycemia up to 8.0% HbA1c. |
| Yu C [53] | General population Cross-sectional study | 1630 | NR/NR/NR | NAFLD fibrosis score | A strong association between HbA1c levels and NAFLD fibrosis score (OR: 2.69, 95% CI: 1.60–4.53, p <0.001). The association remained significant, even in subjects without T2DM |
P: Prospective, R: Retrospective; NR: non-reported; FPG: fasting plasma glucose; OGTT: oral glucose tolerance test; SAF: Steatosis, inflammatory activity and fibrosis score; NAS: NAFLD activity score.
These studies show that tight glycemic control may modify the risk of NASH-related inflammation, and fibrosis progression. Values of HbA1c recommended by the American Diabetes Association (ADA) and the American College of Physicians (ACP) may be adopted (<7%) [56,57]. However, further prospective studies are necessary in order to confirm these findings.
6Pharmacologic treatment of NAFLDTo date, no pharmacological agent has been approved by the FDA for the treatment of NAFLD/NASH. Vitamin E in non-diabetic patients and pioglitazone in diabetic and non-diabetic patients have been recommended because they have been associated with improvement of NASH in some studies. However, there have been some safety concerns regarding the long-term use of vitamin E. Although, robust data are insufficient for a conclusive answer, the use of vitamin E has been suggested to increase the risk of stroke and all-cause mortality [58], and pioglitazone may give rise to weight gain, edema, osteoporosis and heart failure as side effects [59,60].
Due to the above, an intensive research is currently ongoing, focused mainly on agents targeting different pathogenic pathways in NASH, specifically liver inflammation and/or fibrosis, such as: a) modulators of bile acid agents, such as farnesoid x receptor agonists, liver x receptor α inhibitors, fibroblast growth factor 19 and 21 analogues; b) modulators of lipid metabolism, such as acetyl-coA carboxylase inhibitors, stearoyl-coA desaturase inhibitors, diacylglycerol acyltransferase 2 inhibitors, thyroid hormone receptor β selective agonists; c) direct antifibrotic agents such as CC chemokine receptor 2 and 5 inhibitor [61]. However, up to date, many studies testing these agents have yielded limited or non-good results and many of them are still in early phases or research.
7Antidiabetic agentsConcomitantly, newer antidiabetic agents, mainly glucagon-like peptide-1 receptor agonist (GLP-1 Ra), sodium-glucose cotransporter 2 inhibitors (SGLT2 in), and dipeptidyl-peptidase 4 inhibitors (DPP-4 in) have been recently assessed specifically for the treatment of NAFLD in diabetic and non-diabetic patients and have yielded interesting and promising results. Most of them have been evaluated by means of non-invasive methods and only few have been assessed by means of liver biopsy (Table 2). In this section some of these drugs will be discussed.
Pharmacological effects of the most used antidiabetic drugs in NAFLD.
| Antidiabetic drugs | Molecular mechanism | Weight change | Effect on NAFLD |
|---|---|---|---|
| Thiazolidinediones | -PPAR-γ and PPAR-α activation-IR in adipose tissue, liver and skeletalmuscle reversion- Glucose and lipid metabolism improvement-HbA1c reduction-Plasma adiponectin increase | Weight gain 3–5% | Pioglitazone:- Resolution of NASH in47% without T2DM and 60% with T2DM- ALT and AST reduction-Fibrosis progression prevention.Rosiglitazone:-No effect on lipotoxicity-ALT and AST reduction-No effects of liver histology |
| GLP-1Ra | -Glucose- dependent insulinrelease from pancreatic islets stimulation-Gastric emptying reduction-Post-meal glucagon release inhibition- Food intake reduction- HbA1c lowering | Liraglutide: weight loss: ∼8% (3 mg/d)Semaglutide: weight loss: ∼14.9% (2.4 mg/w) | Liraglutide and Semaglutide : the only GLP-1Ra associated tohistological efficacy-Resolution of NASH-Prevention of fibrosis progression-ALT and AST reduction |
| DPP-4 in | -Inhibition of DPP-4increasing effect of incretins GLP-1 and GIP-Postprandial glucose levels reduction- HbA1c moderate lowering | Neutral | Sitagliptin:-The most tested DPP-4 in-Moderate reduction of hepatic lipids-Inconstant reduction of ALT-No clear effects of liver histology |
| SGLT2 in | -Reduction of renal tubular glucose reabsorption, without stimulating insulin release-HbA1c reduction | Weight loss ∼3–5% | Canagliflozin and dapagliflozin-ALT and AST reduction-IHTG reduction assessed with non-invasive procedures- Effects on liver histology non-demonstrated Ipragliflozin:-Significant improvement of fibrosis and NASH by liver biopsy |
PPAR: receptor peroxisome proliferator-activated receptor gamma; IHTG: intrehepatic triglycerides; TGL: triglycerides.
This drug belongs to the group of thiazolidinediones and selectively stimulates the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) and to a lesser extent PPAR-α. It modulates the transcription of the genes involved in the control of glucose and lipid metabolism in the muscle, adipose tissue, and the liver [62]. For more than a decade, it has been the only antidiabetic agent recommended for the treatment of diabetic NASH patients following the results of a RCT in which significant improvement of NAS and fibrosis prevention progression were observed in liver biopsies of NASH patients treated with pioglitazone compared to those receiving placebo [63]. In another study, in patients with T2DM and prediabetes, liver histological improvements were accompanied by significant reduction in serum glucose levels, normal OGTT and reduction of HbA1c (the latter only in DM patients). However, patients receiving pioglitazone significantly increased body weight (+2.5 kg, p<0.020) [64]. A recent metanalysis of 5 RCTs with 392 NASH patients treated with pioglitazone for 6 to 24 months, showed significant improvement of liver fibrosis at any stage, advanced fibrosis (F3, F4), > 1 stage of fibrosis and NASH resolution in both patients with and without T2DM [65,66].
The major drawback of pioglitazone is weight gain which may worsen lipotoxicity and glucose metabolism. In addition, it is associated with other adverse side effects: peripheral edema, osteoporosis and heart failure [59,60]. Conversely, some of the newer antidiabetic drugs induce weight loss and are safer, therefore they may be better for the treatment of NAFLD patients particularly those with T2DM and obesity/overweight.
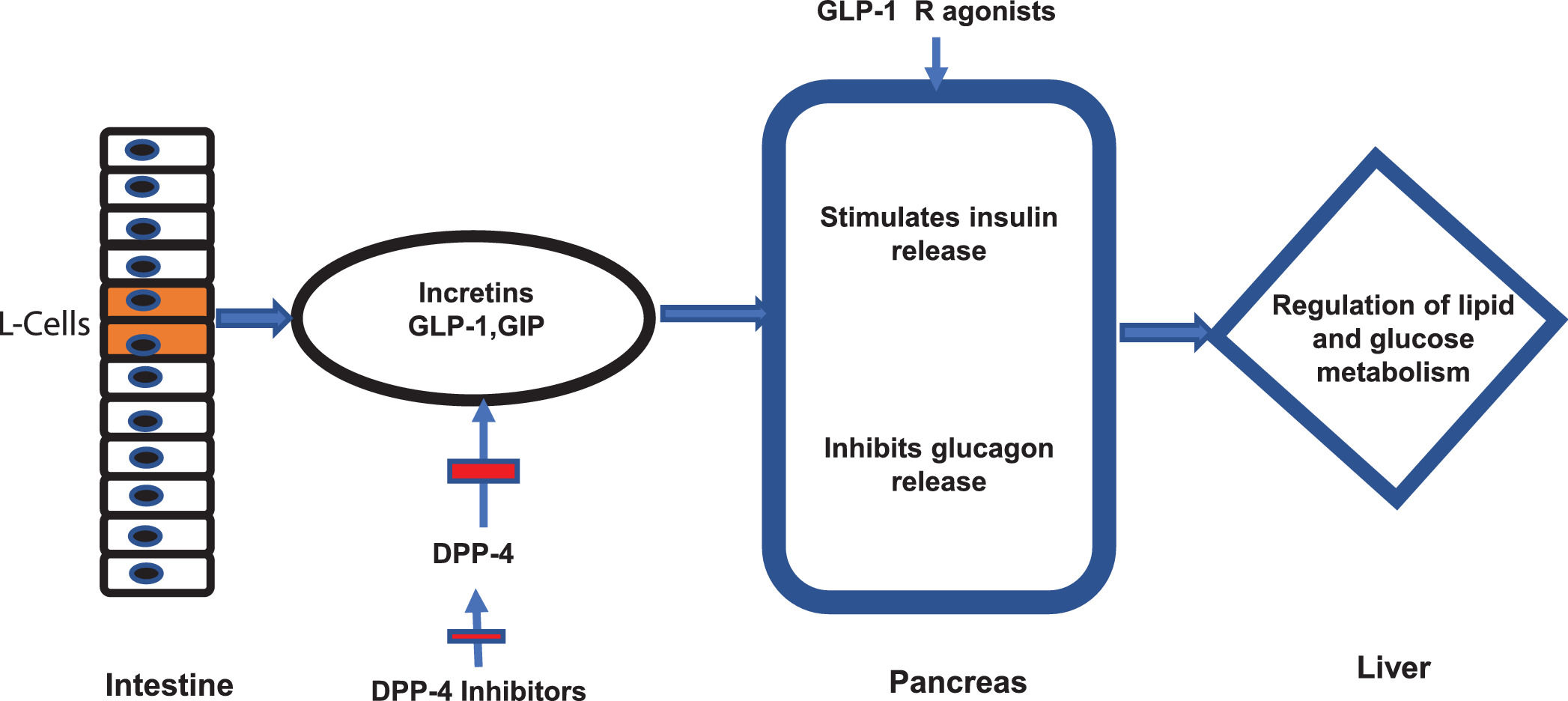
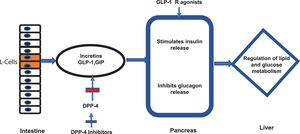
7.2Newer antidiabetic agentsNewer antidiabetic agents, such as GLP-1 Ra, SGLT2 in and DPP-4 in have been recently evaluated for the treatment of NAFLD and NASH in patients with or without DM. GLP-1 is a hormone produced by the intestinal L cells due to the presence of nutrients and exerts its main effect by stimulating glucose-dependent insulin release from the pancreatic islets [67], also slowing gastric emptying [68], inhibiting post-meal glucagon release and reducing food intake [69]. The GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) (both incretins) are degraded by the enzyme DPP-4 [70] (Fig 2). Therefore, DPP-4 in have their effect through incretins. On the other side, SGLT2 in function through a novel mechanism of reducing renal tubular glucose reabsorption, producing a reduction in blood glucose without stimulating insulin release [71]. These agents have an effective glycemic control without risks of hypoglycemia.
Incretins and mechanism of action of new antidiabetics. The figure illustrates the mechanism of action of intestinal incretins (GLP-1 and GIP) on the regulation of glucose and lipid metabolism. It also illustrates the mechanism of action of DPP-4 inhibitors and GPL-1 receptor agonists on incretin effects.
Not all these newer antidiabetic agents have comparable improvement on liver histology of NAFLD patients. In a recent retrospective study with 637 diabetic NAFLD patients, 472 were treated with DPP-4 in, GLP-1 Ra or SGLT-1 in for 12 months and 165 treated with other active antidiabetic treatment served as controls. BMI, HbA1c and aminotransferases serum levels significantly decreased in the groups treated with GLP-1Ra and SGLT2 in, compared with both controls and DPP-4 in. Fat Liver Index and FIB-4 were reduced only on GLP-1Ra and SGLT2 in. The shift of FIB-4 values towards the category ruling out advanced fibrosis was maintained after additional adjustment for confounders [72].
In a systematic review, 25 studies with diabetic and non-diabetic patients with NAFLD were analyzed. HS and NASH were assessed with imaging and liver biopsy. From eight GLP-1 Ra trials, 7 showed improvement in HS and 2 in NASH by histology. From 7 SGLT2 in studies, 6 demonstrated improvement in NAFLD, five improvement in NASH, only one by liver biopsy and finally from 6 DPP-4 in studies only 2 demonstrated improvement in NASH with imaging procedures [73].
The above suggests that GLP-1Ra and SGLT2 in are more effective than DPP-4 in for improving histological (HS and NASH not fibrosis) and metabolic parameters (BMI, serum glucose and aminotransferase levels) in diabetic and non-diabetic NAFLD patients.
7.2.1GLP-1R agonistsNot all GLP-1Ra have similar effects on NAFLD. In a recent metanalysis of 11 studies involving 936 patients liraglutide was used in 6, exenatide in 3, dulaglutide in 1 and semaglutide in 1. NAFLD or NASH was assessed by liver biopsy in 2 studies and by imaging techniques in 9. Compared to placebo or reference therapy, treatment with GLP-1 Ra for a median of 26 weeks was associated with significant reduction in liver fat content on magnetic resonance-based techniques (pooled weighted mean difference: - 3.92%, 95% CI −6.27% −1.56%) and serum aminotransferases levels [74–81]. Greater histological resolution of NASH without worsening of liver fibrosis was observed only for liraglutide and semaglutide (OR :4.06, 95% CI: 2.52–6.55) [82,83].
Another metanalysis of 8 RCTs with 615 NAFLD patients with T2DM (297 treated with GLP-1Ra and 318 in the control arm) showed in patients treated with GLP1-Ra significant improvement in serum ALT, (− 0.56, 95% CI − 0.88 to − 0.25), reduction in liver fat content (LFC) (− 0.43, 95% CI − 0.74 to − 0.12), reduction of HbA1c (− 0.40, 95% CI, − 0.61 to − 0.19) and body weight (− 0.66, 95% CI, − 0.88 to − 0.44) compared to those treated with standard of care or placebo. Significant improvement in histological NASH parameters was also seen in the GLP1-Ra arm (RR: 6.60, 95% CI 2.67 to 16.29). This was the first meta-analysis conducted exclusively in patients with T2DM and NAFLD which presented a strong signal that GLP1-Ra improve liver function and histology through glycemic control, body weight loss and improvement of metabolic parameters [84].
Studies with liraglutide and semaglutide have been reported recently. In a multicenter phase II RCT with 26 patients treated with 1.8 mg daily SC and 26 treated with placebo for 48 weeks, 39% of those treated with liraglutide had resolution of definite NASH compared to 9% in the placebo group (RR :4.3, 95% CI: 1.0–17.7). The 9% in the liraglutide group versus 36% in the placebo group had progression of fibrosis (RR: 0.2, 95%CI: 0.1–1.0). Patients in the liraglutide group also showed significant weight loss, HbA1c reduction and serum glucose levels compared to placebo group patients. Liraglutide was well tolerated [82].
In a recent study, 320 biopsy-proven NASH patients (62% with T2DM and 72% with advanced fibrosis) were treated with semaglutide at doses of 0.1, 0.2 and 0.4 mg/d SC or placebo for 72 weeks. NASH resolution was achieved with no worsening of fibrosis in 40% in the 0.1-mg group, 36% in the 0.2-mg group, 59% in the 0.4-mg group, and 17% in the placebo group (p<0.001 for semaglutide 0.4 mg vs. placebo). However, fibrosis improvement was not different between patients in the 0.4mg group and placebo group. At the end of treatment patients in the 0.4 mg group showed higher weight loss and decreased HbA1c levels than those with 0.1, 0.2 mgs and placebo. (weight loss: −12.5% vs −4.8%, −8.9% and −0.61% respectively and HbA1c: −1.15% vs −0.63%, −1.07% and −0.01% respectively). HbA1c reduction was reported only in T2DM patients [83].
7.2.2SGLT2 inhibitorsFinally, from SGLT2 in, empagliflozine, dapagliflozin, ipragliflozine and canagliflozine have been assessed in NAFLD with T2DM. Most of them reported beneficial effects on HS, assessed with non-invasive procedures (CAP or MRI techniques) [85–88]. In another study with canagliflozin, histological improvement was reported, however, the number of patients was poor (N = 9) and no controls were used [88]. In a randomized, controlled trial conducted in patients with T2DM and NAFLD, 25 were treated with ipragliflozin (IPR) 72 weeks and 26 were managed without IPR(CTR). Liver fibrosis assessed by liver biopsy was ameliorated in 70.6% in the IPR group and in 22.2% in CTR group (p < 0.01) while NASH resolved in 66.7% vs 27.3% of the IPR and CTR groups respectively. Authors concluded that long-term ipragliflozin treatment may ameliorate hepatic fibrosis in patients with NAFLD [89].
In most studies, significant reduction of weight, visceral mass fat, HbA1c and serum glucose levels was observed [85-87,90]. Based on the above, more studies with SGLT2 in, especially on liver histology with larger number of patients are required in order to get a clear conclusion.
In conclusion, based on the above discussed on the new antidiabetic drugs, GLP-1Ra (mainly liraglutide and semaglutide) and SGLT2 in seem to be more effective than DPP-4 in for the improvement of histologic parameters and metabolic abnormalities of NAFLD. Their beneficial effect may be related to better glycemic control and weight reduction. GLP-Ra can produce weight loss from 8 to 15%, SGLT2 in from 3 to 5% and DPP-4 are weight loss neutrals. More studies are required to confirm these findings.
8Conclusions and recommendationsIn accordance with the above discussed, the fundamental cornerstone in the treatment of NAFLD patients with T2DM is weight loss. Tight glycemic control is emerging as an independent relevant factor associated with improvement of histologic parameters of NASH. Both factors can act synergistically, although it remains to be confirmed in prospective studies.
Weight loss through diet and physical exercise is initially recommended. Alternative bariatric therapies may be useful in refractory patients. The sequential use of DT, BET and BS can be carefully evaluated for selected patients based on the degree of obesity, the severity of NASH and the number and type of comorbidities. Further clinical studies assessing these therapies specifically indicated for NASH patients are urgently needed in order to define their best use.
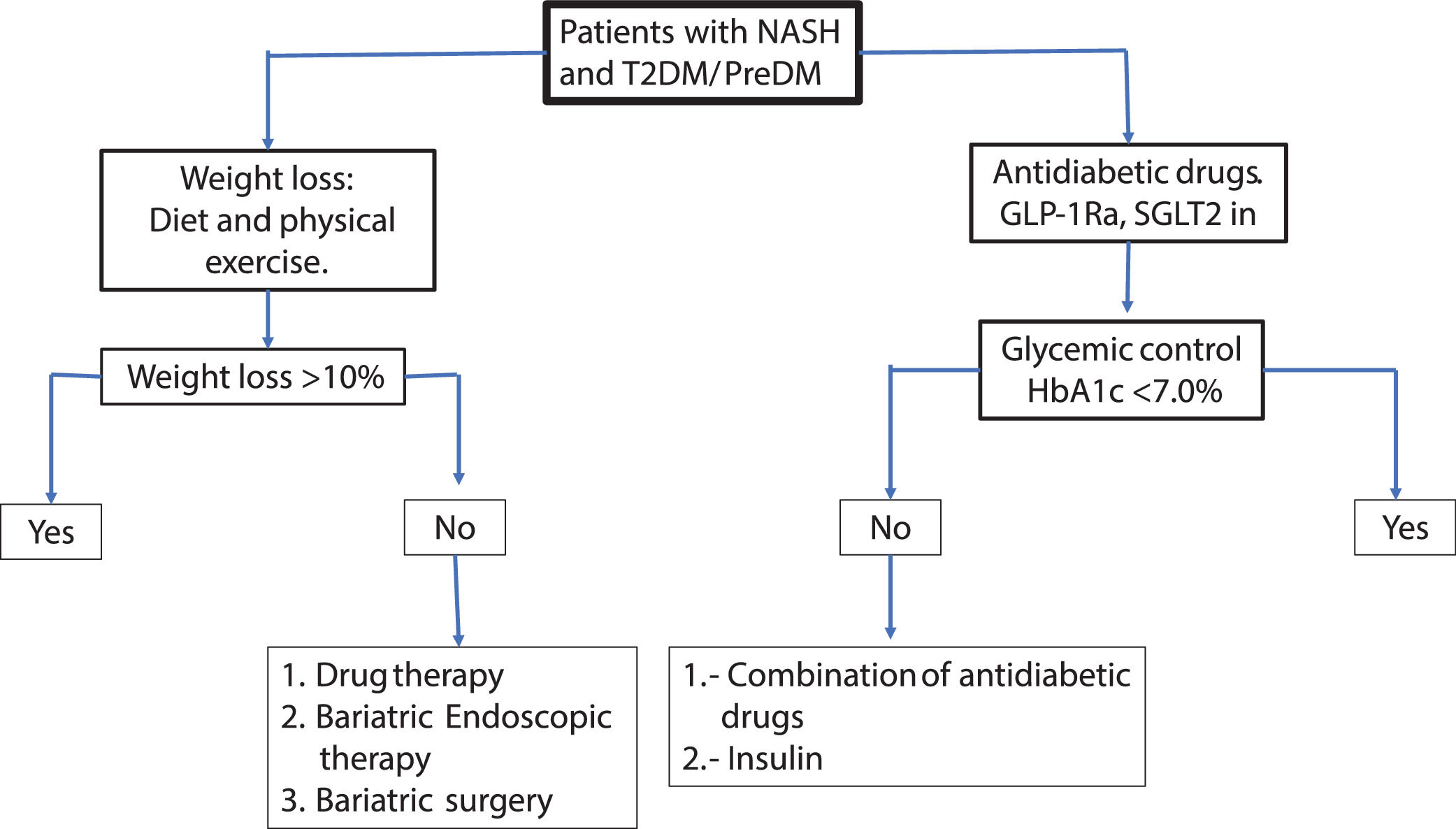
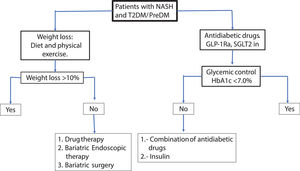
For glycemic control, pioglitazone, GLP-1 Ra or SGLT2 in can be used preferentially. In patients with obesity the pioglitazone should be avoided or carefully used. GLP-1Ra and to a lesser extent SGLT2 in can be useful for glycemic control and weight loss simultaneously. It is recommended to set as therapeutic goals >10% weight loss maintaining HbA1c <7% to ensure close control of glycemia. The therapeutic effect on NAFLD can be assessed with liver enzymes serum levels, HS by controlled attenuation parameter score (CAP) or magnetic resonance spectroscopy and fibrosis by elastography (transient, US or magnetic resonance technique). Less-expensive non-invasive methods for the measurement of HS and liver fibrosis such as fatty liver index (FLI), NAFLD fibrosis score, Fib-4, etc., maybe be useful. In the Fig 3a practical algorithm is proposed for a real-world setting.
Considering the current intense research in the field of pharmacological therapy of this disease, in the near future, probably highly effective and safer drugs for the treatment of NAFLD / NASH will be available. In the meantime, effective weight loss and tight glycemic control may be the paradigms on the current treatment of NAFLD.