Tin oxide has been extensively studied due to its wide variety of applications. However, its poor sinter ability requires the use of sintering aids for its processing. The sintering behaviour of three different SnO2-based powder mixtures, containing Bi2O3 in amounts between 1 and 2mol%, has been analyzed. The effects of thermal treatment parameters (heating rate, maximum temperature and soaking time) on the densification were obtained by a factorial experimental design 23. Bi2O3 adequate proportion (around 1.5%) combined with a fast heating (15°Cmin−1) and a high maximum temperature (1300°C), allows reaching densifications around 45%. However, soaking time has no significant effect over densification. An interpretation of the significant effects has been proposed based on thermodynamic behaviour of Bi-containing compounds and the mass transport mechanisms.
El óxido de estaño es un material ampliamente estudiado dada su gran variedad de aplicaciones. Sin embargo, debido a que sinteriza sin densificar, su procesado requiere la incorporación de promotores de la sinterización. Se ha estudiado el comportamiento de 3 mezclas a base de óxido de estaño que contenían óxido de bismuto como promotor de la sinterización, en proporciones 1-2%mol. A través de un diseño factorial de experimentos 23, se han evaluado los efectos de los parámetros del tratamiento térmico (velocidad de calentamiento, temperatura máxima y tiempo de permanencia) sobre la densificación. La combinación de una adecuada proporción de Bi2O3 (alrededor del 1,5%), una velocidad de calentamiento rápida (15°C·min−1) y una temperatura de sinterización elevada (1.300°C), permite alcanzar una densificación del 45%. Sin embargo, el tiempo de permanencia no ejerce un efecto significativo. Se propone una interpretación de los efectos significativos sobre la densificación, basada en el comportamiento termodinámico de los compuestos que contienen Bi y en los mecanismos de transporte de materia.
Tin oxide exhibits many attractive physical and chemical properties, such as high conductivity (n-type semiconductor) and corrosion resistance. Traditionally, SnO2 has been used as raw material for some pigments [1] and as opacifier in ceramic glazes [2]. Nowadays, it is broadly used in the production of gas sensors [3,4], as well as components requiring high chemical corrosion resistance in chemical industry applications [5]. In the last field, an important application is obtaining electrodes for the processing of aluminium by electrolysis [6,7] and electric glass melting furnaces [8].
One of the main drawbacks of SnO2 is its poor sinter ability since hinders its use [9,10]. According to Kimura et al. [11], two different phenomena can occur during the sintering process in ceramic bodies: densification and particle coarsening. High densification is obtained when bulk transport mechanisms, as grain-boundary diffusion, are predominant. By contrast, surface transport mechanisms, as surface diffusion or evaporation–condensation, generates a non-densified body because of the particle coarsening. In the case of pure tin oxide, the studies describe a decomposition of SnO2 in SnO and O2 at temperatures above 1100°C. In consequence, the evaporation–condensation mechanism predominates during sintering, whereby the electrodes obtained from this material showed a very low densification [12,13].
Different approaches have been used to improve densification, namely, hot isostatic pressing [14], Field Activated Sintering Technique (FAST) [15] or the addition of other metallic oxides as “sintering aids” [16,17], those promote the formation of a eutectic liquid between SnO2 and the “sintering aid” at low temperature favouring a liquid-phase sintering [18,19]. Between the oxides proposed as “sintering aids” for tin oxide, bismuth oxide has been proposed as a non-toxic alternative. The Bi2O3–SnO2 phase diagram contains three stable solid phases: bismuth oxide (m.p. 840°C), tin oxide (m.p. 1800°C) and Bi2Sn2O7 (melts incongruently near 1400°C and decomposes to solid SnO2 and a Bi2O3-rich liquid). In addition, a low-temperature eutectic was present for a 2mol% SnO2 and 98mol% Bi2O3 (825°C). In addition, the presence of Bi2O3 suppresses SnO2 sublimation owing to the high pressure of oxygen resulting from Bi2O3 or Bi2Sn2O7 sublimation [20]. In consequence, the sintering mechanism of SnO2 through the gas phase is partially blocked.
In this work, a factorial experimental design 23 has been used to analyze the effect of thermal cycle parameters (heating rate, maximum temperature and soaking time) over the performance of bismuth oxide as sintering aid for tin oxide. Thermodynamic data have been used to interpret the obtained results.
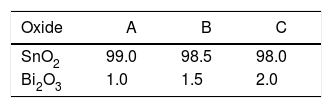
Experimental procedureRaw materials were SnO2 (purity 99.85%, Quimialmel S.A., Spain), and Bi2O3 as sintering aid (purity 98%, Fluka AG, Germany). Three different compositions were formulated to evaluate the effect of bismuth oxide proportion over the sintering behaviour of tin oxide (Table 1). 0.8% in weight of polivinylalcohol (Mowiol 8-88, Clariant Iberica S.A. Spain), was added to each composition as a ligand.
Firstly, raw materials were mixed in a planetary mill (Pulverisette 5, Fritsch GmbH, Germany), at 230rpm during an hour using water as a fluid and the suspension was dried at 110°C for 24h. Secondly, the dried powder was sieved trough a 600μm mesh and was moistened to 5% (kg water/kg dry solid). Thirdly, disc specimens of 2cm diameter and 0.5cm thickness were dry-pressed at 450kgcm−2 in a laboratory uniaxial press (Nanneti Spa, Italy). Finally, eight different thermal treatments were carried out in a laboratory furnace in air atmosphere (RHF1600, Carbolite Furnaces, UK) with the experimental design showed in Table 2.
Bulk density of green and sintered specimens was measured by mercury immersion (Archimedes’ method), and densification (change in bulk density due to sintering divided by the change needed to attain a pore-free solid), was calculated according to German [21].
Characterization of crystalline structures present on some specimens was performed using an X-ray diffractometer (Theta-Theta D8 Advance, Bruker, Germany), with CuK radiation (λ=1.54183Å). The generator applied an intensity light source of 45kV and 40mA. XRD data were collected by means of a VÅNTEC-1 detector in a 2θ from 5 to 90° with a step width of 0.015° and a counting time of 1.2s/step. SEM images were taken with a FEG-SEM (QUANTA 200F, FEI Co, USA) from polished sections of some samples.
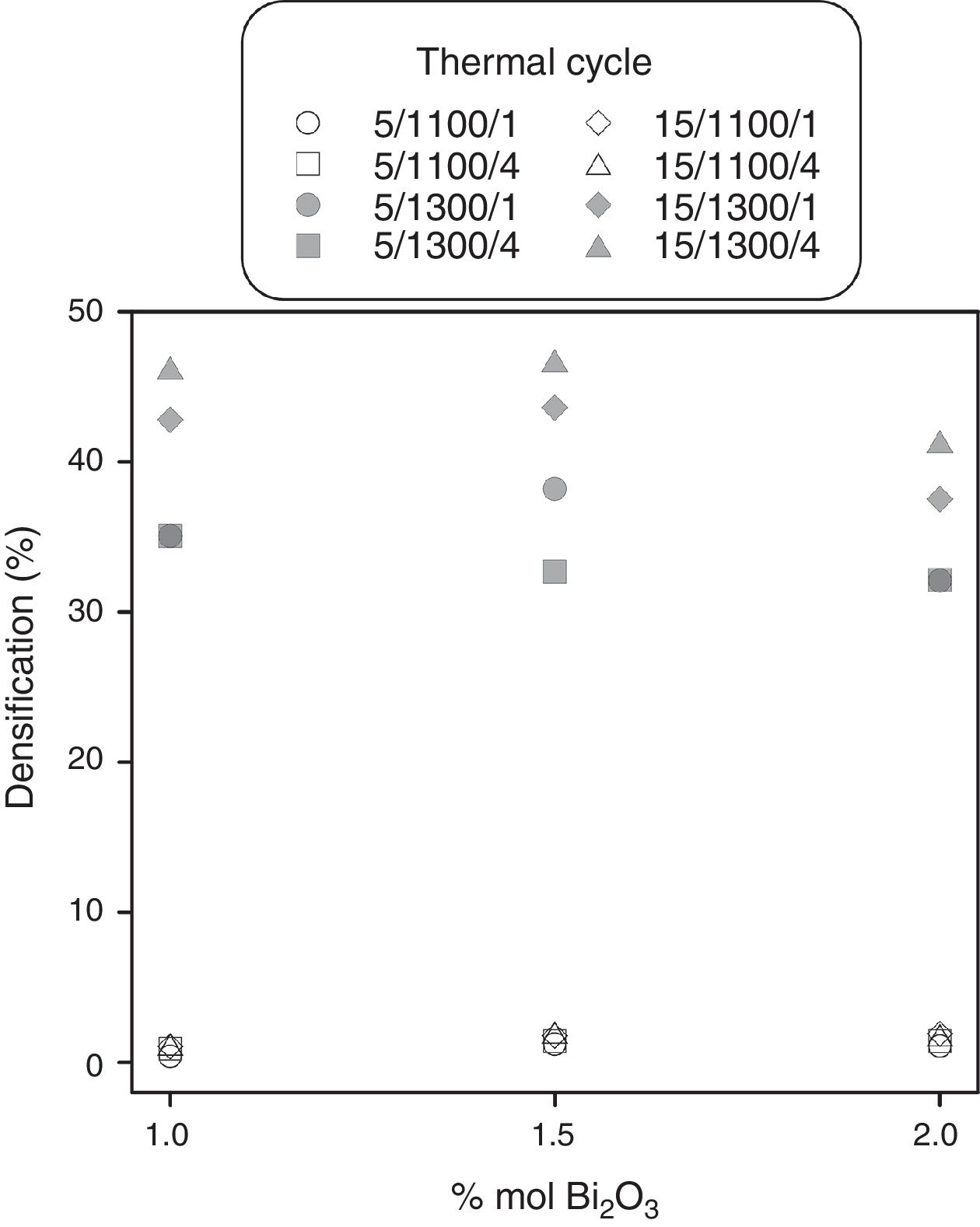
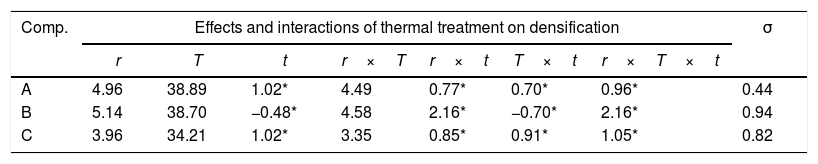
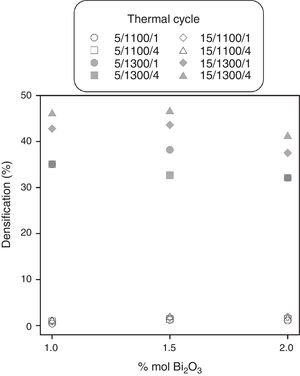
Experimental results and discussionResults showed that the addition of bismuth oxide allows to reach densifications of 45% (corresponding to a relative density of 73.8% with respect to pore-free SnO2). However, sintering aid was only effective at temperatures around 1300°C and its proportion is limited to 1.5% because higher contents of Bi2O3 tend to decrease maximum densification values (Fig. 1). In addition, the faster heating rate seems to increase densification, but an effect of soaking time was not appreciable.
The main effects and interactions (Table 3), as well as their standard deviation σ, were obtained according to Box et al. [22]. It was considered as significant the effects higher than 3σ. Maximum temperature has the greatest effect on densification followed by heating rate and the interaction T×r. However, densification seems not to be influenced by soaking time and the other interactions.
Calculation of the effect of firing parameters on the densification for each proposed composition (the effects considered not significant are signalled with an asterisk).
| Comp. | Effects and interactions of thermal treatment on densification | σ | ||||||
|---|---|---|---|---|---|---|---|---|
| r | T | t | r×T | r×t | T×t | r×T×t | ||
| A | 4.96 | 38.89 | 1.02* | 4.49 | 0.77* | 0.70* | 0.96* | 0.44 |
| B | 5.14 | 38.70 | −0.48* | 4.58 | 2.16* | −0.70* | 2.16* | 0.94 |
| C | 3.96 | 34.21 | 1.02* | 3.35 | 0.85* | 0.91* | 1.05* | 0.82 |
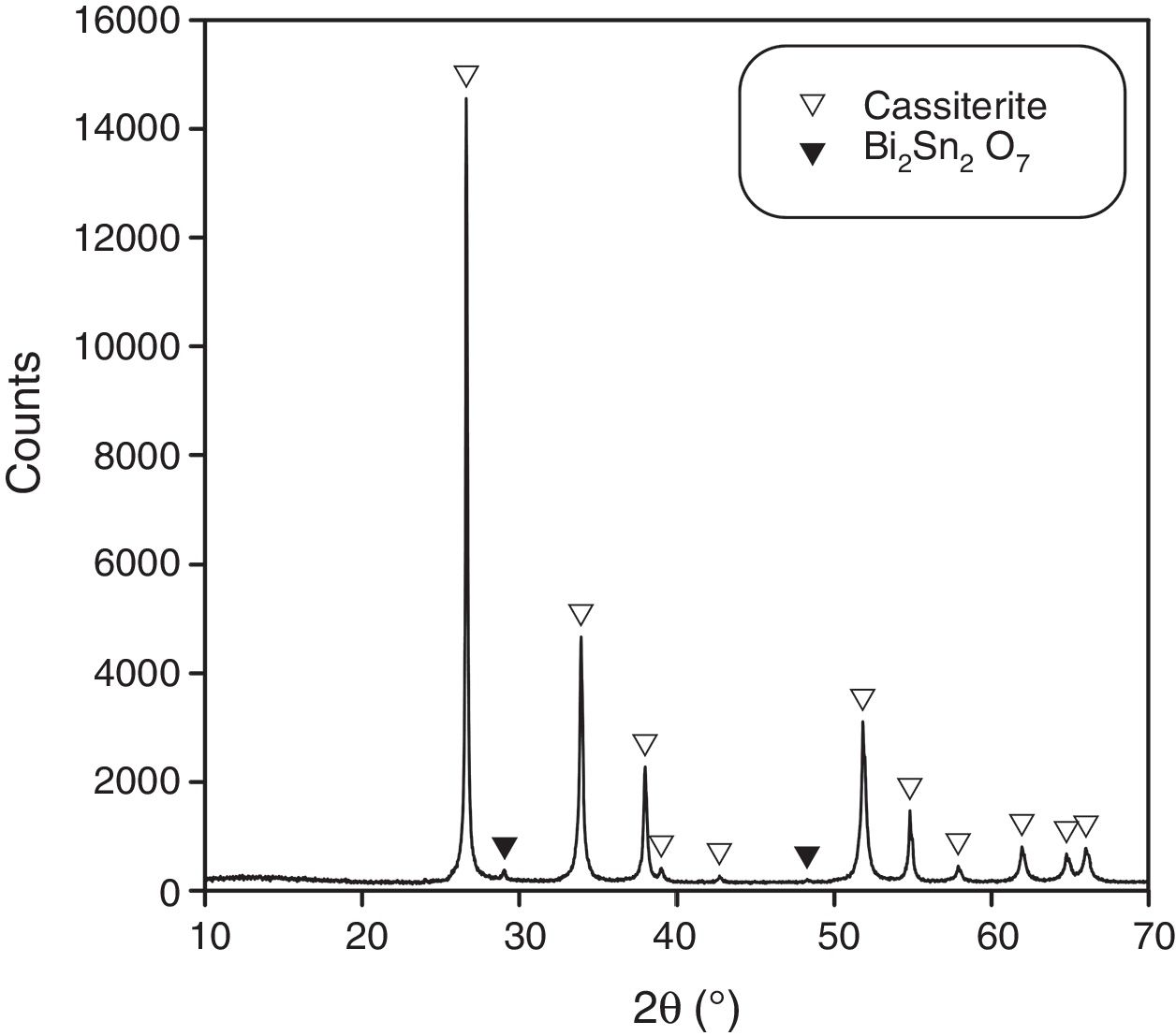
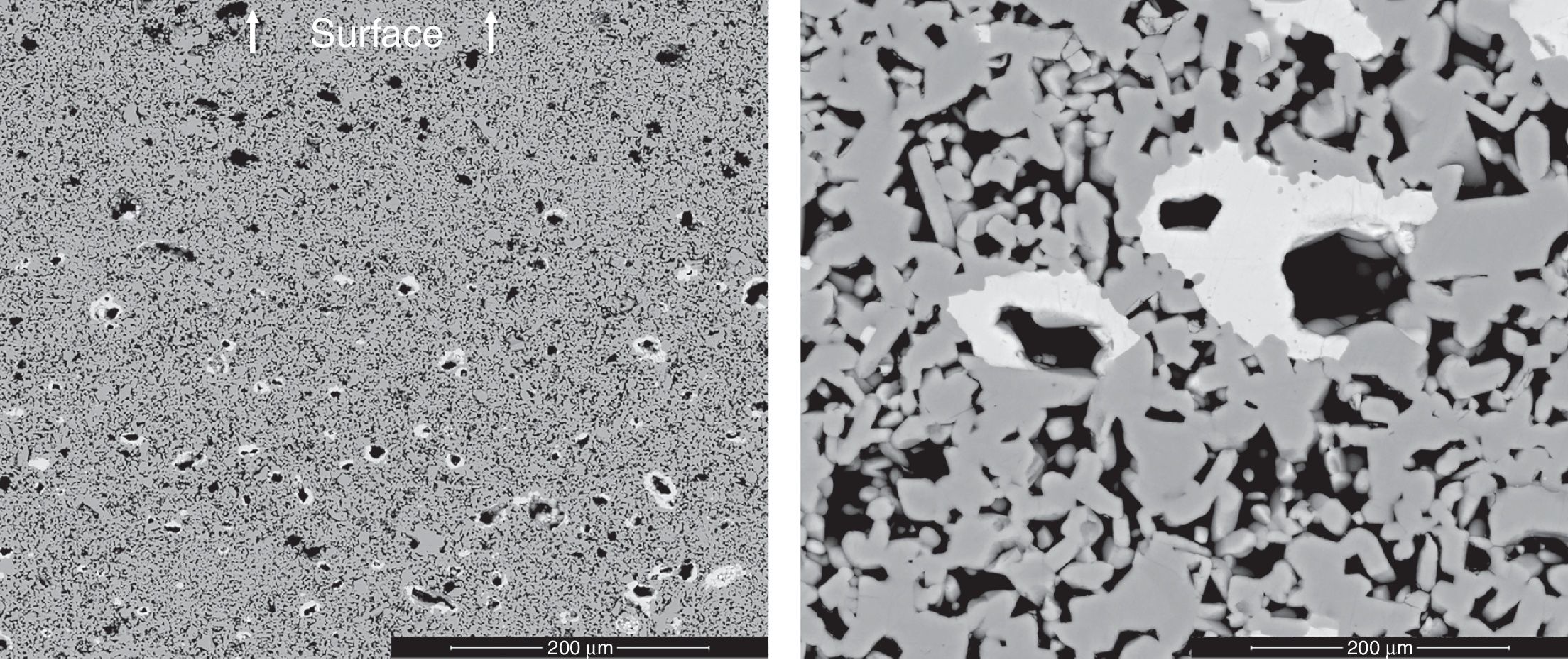
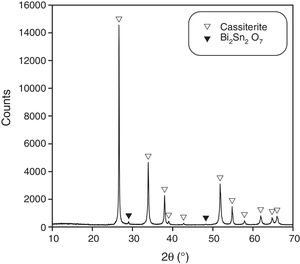
XRD of samples of composition B treated with the 15/1300/4 cycle identified a small proportion of a pyrochlore-type compound Bi2Sn2O7, being cassiterite the main phase (Fig. 2). This pyrochlore showed an inhomogeneous spatial distribution as SEM images demonstrate (Fig. 3). It was concentrated in the centre of the sample and the volume near the surface was practically free of this phase. In consequence, there is a loss of bismuth oxide during the thermal treatment, which mainly comes from the vicinity of the sample surface. In the other hand, the Bi2Sn2O7 was present in discrete agglomerates, showing interphases with the tin oxide particles which points to a wetting by a liquid phase at high temperature.
It can be proposed that Bi2O3 reacts with SnO2 to generate Bi2Sn2O7 at T>850°C [23], and consequently very small densification is obtained at 1100°C. This fact is due to the absence of any liquid phase and the predominant surface mass transport mechanisms characteristic of pure SnO2 sintering. Surface transport which masks any volumetric mass transport mechanism. By contrast, the presence of a bismuth-rich liquid phase at 1300°C is the way for a volumetric mass transport mechanism which allows densifications around 45%. In parallel, the high oxygen partial pressure generated by Bi-containing compounds reduces the gas-phase transport of SnO2. Accordingly, it is advisable to add bismuth oxide to promote SnO2 densification at 1300°C. By contrast, Bi2O3 effect is negligible at 1100°C.
The effect of heating rate can be related with bismuth oxide losses, because the weight loss of samples treated at 1300°C and the faster heating rate was slightly lower than their counterparts obtained with the slower heating rate (the mean values were 4.18% and 4.00% respectively). By contrast, the weight losses of samples treated at 1100°C were around a mean value of 0.96%, slightly higher to the 0.8% content of PVA, meaning that the bismuth oxide losses were clearly inferior. According with this hypothesis, the lower heating rate allows the diffusion out of the sample of a bigger fraction of the gaseous species generated by Bi2Sn2O7 sublimation. In consequence, the blocking effect over the gas-phase transport of SnO2 is less intense. On the other hand, the loss of bismuth could also be related to the lack of a measurable effect of soaking time over densification. As a bigger volume fraction of the sample loses the bismuth, the densification mechanism is stopped in those zones, and the effect of larger soaking times is lower. Probably with shorter soaking times the significance of this effect could be evaluated. Further research is needed to confirm this point.
ConclusionsBismuth oxide promotes tin oxide densification combined with an adequate thermal cycle. The experimental design has shown that the highest densifications (around 47%) are obtained with proportions of Bi2O3 between 1.0 and 1.5% molar combined with a fast heating rate (15°Cmin−1) and a maximum temperature of 1300°C. Heating rate, maximum temperature and their interaction are the parameters with a significant effect over densification. However, soaking time has no significant effect over densification, at least in the range of values investigated.
Bi2O3 reacts to generate Bi2Sn2O7, which is found in the central volume of the most densified samples but not near their surfaces. In addition, higher densifications are linked with higher mass losses during the thermal treatment due to bismuth compounds volatilization.
The evolution of densification in the presence of bismuth oxide has been interpreted considering the thermochemical behaviour of Bi-containing compounds, and their effect over the mass transport mechanisms.
The authors thank to Ministerio de Economia y Competitividad and Fondo Europeo de Desarrollo Regional for the support to this research [Plan Nacional de I+D, project Ref. CTQ2015-65202-C2-2-R (MINECO/FEDER)].