In a recent randomized trial of Staphylococcus aureus bacteremia and native valve endocarditis, daptomycin was found not inferior to standard therapy. We summarized findings in the subgroup of patients with endocarditis according to the Duke criteria.
MethodsPatients were randomly assigned to receive daptomycin 6mg/kg/day or standard therapy (vancomycin 1g every 12h or antistaphylococcal penicillin 2g every 4h, both with gentamicin 1mg/kg every 8h for the first 4 days). The primary end point was success in the modified intent-to-treat population 6 weeks after the end of therapy.
ResultsFifty-three patients were included: 35 with right-sided endocarditis (RIE) and 18 with left-sided endocarditis (LIE). The success rates in patients with RIE were similar between daptomycin and the comparator (42% vs 44%). Patients with RIE with septic pulmonary infarcts responded similarly to treatment with daptomycin and standard therapy (60% vs 67%). In the LIE population, treatment success rates were poor in both arms (11% vs 22%).
ConclusionDaptomycin is an efficacious and well-tolerated alternative to standard therapy in the treatment of RIE. Patients with LIE had a poor outcome regardless of the treatment received. Daptomycin is also effective in treating endocarditis with septic pulmonary infarcts.
En un reciente ensayo aleatorizado sobre bacteriemia por Staphylococcus aureus y endocarditis de la válvula natural, daptomicina no resultó inferior a la terapéutica estándar. Resumimos los hallazgos en el subgrupo de pacientes con endocarditis según los criterios de Duke.
MétodosLos pacientes fueron asignados aleatoriamente a recibir daptomicina, 6mg/kg/día, o la terapéutica estándar (vancomicina 1g cada 12h o una penicilina antiestafilocócica 2g cada 4h, ambos con gentamicina 1mg/kg cada 8h, durante los 4 primeros días). La variable principal fue el éxito en la población modificada por intención de tratamiento 6 semanas después del final del tratamiento.
ResultadosEl estudio incluyó a 53 pacientes: 35 con endocarditis infecciosa de las cavidades derechas (RIE) y 18 con endocarditis infecciosa de las cavidades izquierdas (LIE). En los pacientes con RIE, las tasas de éxito con daptomicina y el tratamiento de comparación fueron similares (42% frente a 44%). Los pacientes con RIE e infartos pulmonares sépticos respondieron de forma similar al tratamiento con daptomicina y con la terapéutica estándar (60% frente a 67%). En la población con LIE, las tasas de éxito fueron pobres con ambos brazos (11% frente a 22%).
ConclusiónDaptomicina es una alternativa eficaz y bien tolerada a la terapéutica estándar en el tratamiento de la RIE. Los pacientes con LIE tuvieron mal resultado, con independencia del tratamiento recibido. Daptomicina también es eficaz en el tratamiento de la endocarditis con infartos pulmonares sépticos.
Important changes have occurred in recent years in the epidemiology of infective endocarditis (IE) caused by Staphylococcus aureus.1,2 The observed increase in the incidence of the disease can be ascribed to the increase in frequency and complexity of invasive medical procedures performed on seriously ill patients with significant comorbidities.3,4 Therefore, S. aureus endocarditis is currently a disease of elderly, debilitated, and hospitalized patients, in addition to the traditional, relatively healthy, injection drug abusers.5–8 Also, as part of the global shift in the antimicrobial resistance pattern of S. aureus, more methicillin-resistant S. aureus (MRSA) strains are causing bacteremia and endocarditis, thereby contributing to the challenging nature of the problem.9,10
The use of glycopeptides for the treatment of S. aureus endocarditis has been fraught with problems. Vancomycin-intermediate S. aureus isolates and heteroresistant strains have been increasingly reported.11,12 Treatment failure with vancomycin has been described even when the minimum inhibitory concentrations (MICs) are well within the susceptible range (1–2mg/mL).13,14 Alternative treatment strategies for MRSA are, therefore, needed.
Daptomycin is a lipopeptide agent with rapid bactericidal activity against S. aureus.15 It is approved for the treatment of complicated skin and skin-structure infections at a dose of 4mg/kg/day.16 In an international, prospective, randomized trial of daptomycin vs standard therapy for S. aureus bacteremia (SAB)9 and S. aureus infective endocarditis, daptomycin was effective and less nephrotoxic than the comparator.17 It was subsequently approved for treatment of S. aureus bacteremia (SAB) and right-sided endocarditis (RIE) at a dose of 6mg/kg/day. Here we summarize the experience with IE in that trial.
MethodsStudy design and patientsThis was an open-label, randomized, active-control study conducted between August 28, 2002, and February 16, 2005, in 44 sites in the United States and Western Europe. Patients were considered for enrollment in the study if they were at least 18 years of age and had at least 1 blood culture that was positive for S. aureus within 2 calendar days of initiating the study medication. Exclusion criteria included creatinine clearance less than 30mL/min and known osteomyelitis, polymicrobial bacteremia, or pneumonia. For a full list of the exclusion criteria, please refer to the initial publication.17 In the initial phases of the trial, patients with left-sided endocarditis (LIE) were excluded.
Randomization, treatments, and outcomesEligible patients were randomly assigned to receive either daptomycin (CUBICIN®, Cubist Pharmaceuticals) at 6mg/kg/day or standard therapy, with either 1g vancomycin every 12h (for MRSA isolates) or 2g antistaphylococcal penicillin every 4h (for methicillin-susceptible S. aureus [MSSA] isolates). The duration of treatment was determined by the investigators based on the working diagnosis. All patients who had a high likelihood of LIE at randomization were randomly assigned to receive daptomycin, and all patients randomly assigned to receive standard therapy, also received 1mg/kg gentamicin every 8h for the first 4 days. All patients underwent transesophageal echocardiography (TEE) within 5 days of enrollment, as well as any necessary follow-up echocardiography. Additionally, all patients underwent diagnostic evaluation for metastatic foci of infection.
The primary efficacy measure was the success rate in the modified intent-to-treat (MITT) population 6 weeks after the end of therapy. An independent external adjudication committee blinded to therapy, consisting of 5 infectious diseases experts, reviewed individual patient data to establish final diagnoses and outcomes. Overall success was defined as survival, clinical cure or improvement, and a documented clearance of bacteremia. In addition, patients who did not complete adequate therapy, who received potentially effective non-study antibiotics, or who did not have blood cultures taken 6 weeks after completion of therapy were considered “treatment failures.” In this analysis, we also describe clinical success based on the presence of all of the following criteria: survival, clinical outcome of cure or improvement, and resolution of S. aureus infection 6 weeks after the end of therapy.
Entry and final diagnoses and duration of therapyEntry diagnoses were determined according to the modified Duke University criteria for IE.18 Final diagnoses were based on standard clinical definitions. Uncomplicated RIE (uRIE) was defined as definite or possible MSSA endocarditis in the absence of predisposing abnormalities or active infection of the mitral or aortic valve in a patient who actively uses injection drugs, has a serum creatinine level of less than 2.5mg/dL, and has no evidence of extrapulmonary sites of infection. Minimum duration of therapy for patients with uRIE ranged from 14 to 28 days. Complicated RIE (cRIE) was similarly defined, but patients also had extrapulmonary sites of infection, a serum creatinine level of at least 2.5mg/dL, or MRSA bacteremia, or did not use injection drugs. Patients with cRIE received a minimum of 28–42 days of therapy. Patients with LIE were treated for a minimum 28–42 days.
Molecular analysisIn order to evaluate differences in the genotypic profiles of S. aureus isolates causing infections of variable severity, we compared S. aureus isolates causing LIE (usually the most serious infection) with those causing uncomplicated bacteremia (least serious infection). Pulsed-field gel electrophoresis (PFGE) on these isolates was performed as previously described.19 In addition, and following previously reported methodology,19 DNA extraction and polymerase chain reaction (PCR) assays were used to examine 33 bacterial determinants, including toxins, adhesins, agr groups I–IV, staphylococcal cassette chromosome mec (SCCmec) types I–IV, as well as other virulence genes.
Statistical analysisBecause of the limited number of patients in each arm, only descriptive statistics were performed for this subgroup analysis.
ResultsStudy populationOf the 235 patients included in the MITT population in the original study, 181 were thought to have definite or possible endocarditis on enrollment, according to the modified Duke criteria for IE. A total of 53 patients received a final diagnosis of IE. All 37 patients who fulfilled the Duke criteria for definite endocarditis at enrollment had IE as the final diagnosis. Of the 144 patients with possible endocarditis according to the Duke criteria, 15 (10%) had a final diagnosis of IE. An additional patient was initially classified in the group without endocarditis, but was subsequently found to have IE.
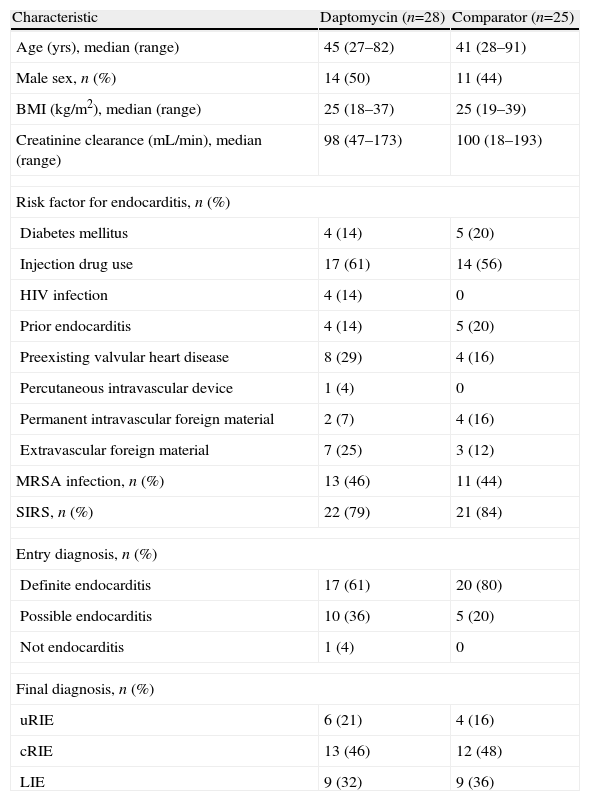
Of the 53 patients with a final diagnosis of endocarditis, 28 were randomly assigned to receive daptomycin and 25 were randomly assigned to standard therapy. There were no major differences between the study arms with regard to baseline patient characteristics, risk factors for endocarditis, entry diagnoses, or final diagnoses (Table 1). The most common type of IE in both groups was cRIE (25 patients, 47%), followed by LIE (18 patients, 34%) and uRIE (10 patients, 19%). The proportion of patients with MRSA IE was similar in both groups (46% for daptomycin vs 44% for the comparator). Clinical evidence of systemic inflammatory response syndrome was present in 22 patients treated with daptomycin (79%) and in 21 patients treated with standard therapy (84%). Sixteen patients randomly assigned to receive daptomycin completed the course of therapy and received a median of 27 days of treatment. In the comparator arm, 14 patients completed the course of therapy and received a median of 30 days of antibiotic treatment. Gentamicin was administered as study medication for 4 days to 1 patient in the daptomycin group and for a median of 5 days to all 25 patients in the comparator group. Twenty-two patients with IE (41%) received a diagnosis of septic pulmonary infarct during the first 5 days of the study (10 in the daptomycin arm and 12 in the comparator arm).
Characteristics of patients with IE in the MITT population based on treatment assignment
| Characteristic | Daptomycin (n=28) | Comparator (n=25) |
| Age (yrs), median (range) | 45 (27–82) | 41 (28–91) |
| Male sex, n (%) | 14 (50) | 11 (44) |
| BMI (kg/m2), median (range) | 25 (18–37) | 25 (19–39) |
| Creatinine clearance (mL/min), median (range) | 98 (47–173) | 100 (18–193) |
| Risk factor for endocarditis, n (%) | ||
| Diabetes mellitus | 4 (14) | 5 (20) |
| Injection drug use | 17 (61) | 14 (56) |
| HIV infection | 4 (14) | 0 |
| Prior endocarditis | 4 (14) | 5 (20) |
| Preexisting valvular heart disease | 8 (29) | 4 (16) |
| Percutaneous intravascular device | 1 (4) | 0 |
| Permanent intravascular foreign material | 2 (7) | 4 (16) |
| Extravascular foreign material | 7 (25) | 3 (12) |
| MRSA infection, n (%) | 13 (46) | 11 (44) |
| SIRS, n (%) | 22 (79) | 21 (84) |
| Entry diagnosis, n (%) | ||
| Definite endocarditis | 17 (61) | 20 (80) |
| Possible endocarditis | 10 (36) | 5 (20) |
| Not endocarditis | 1 (4) | 0 |
| Final diagnosis, n (%) | ||
| uRIE | 6 (21) | 4 (16) |
| cRIE | 13 (46) | 12 (48) |
| LIE | 9 (32) | 9 (36) |
BMI, body mass index; cRIE, complicated right-sided infective endocarditis; IE, infective endocarditis; LIE, left-sided infective endocarditis; MITT, modified intent-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; SIRS, systemic inflammatory response syndrome; uRIE, uncomplicated right-sided infective endocarditis
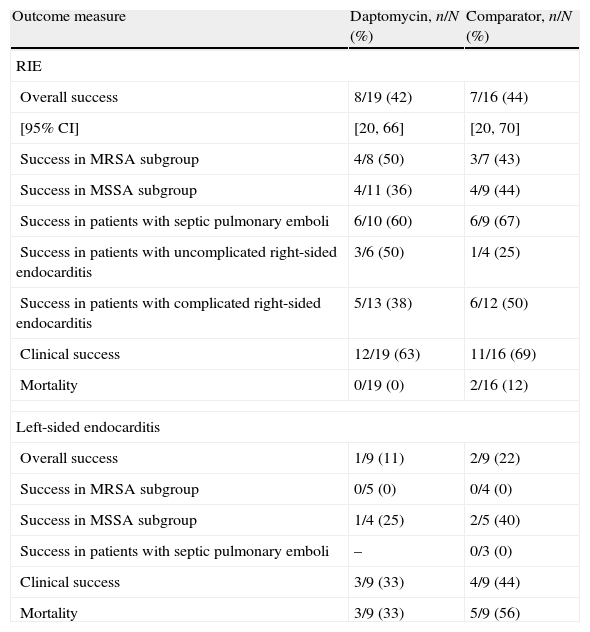
In the MITT population, the overall success rates for patients with RIE treated with daptomycin were similar to those of the comparator MITT population (42% vs 44%; Table 2). Clinical success rates in this group of patients were higher in both arms (63% vs 69%). No difference in outcome was observed between the treatment groups of patients with MRSA or MSSA infection, or in patients with septic pulmonary infarct. Mortality rates were comparable for daptomycin and standard therapy (0% vs 12%).
Outcome 6 weeks after completion of study medication in patients in the MITT population
| Outcome measure | Daptomycin, n/N (%) | Comparator, n/N (%) |
| RIE | ||
| Overall success | 8/19 (42) | 7/16 (44) |
| [95% CI] | [20, 66] | [20, 70] |
| Success in MRSA subgroup | 4/8 (50) | 3/7 (43) |
| Success in MSSA subgroup | 4/11 (36) | 4/9 (44) |
| Success in patients with septic pulmonary emboli | 6/10 (60) | 6/9 (67) |
| Success in patients with uncomplicated right-sided endocarditis | 3/6 (50) | 1/4 (25) |
| Success in patients with complicated right-sided endocarditis | 5/13 (38) | 6/12 (50) |
| Clinical success | 12/19 (63) | 11/16 (69) |
| Mortality | 0/19 (0) | 2/16 (12) |
| Left-sided endocarditis | ||
| Overall success | 1/9 (11) | 2/9 (22) |
| Success in MRSA subgroup | 0/5 (0) | 0/4 (0) |
| Success in MSSA subgroup | 1/4 (25) | 2/5 (40) |
| Success in patients with septic pulmonary emboli | – | 0/3 (0) |
| Clinical success | 3/9 (33) | 4/9 (44) |
| Mortality | 3/9 (33) | 5/9 (56) |
CI, confidence interval; MITT, modified intent-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; RIE, right-sided infective endocarditis.
The overall success rates with LIE were poor in both arms (11% vs 22%; Table 2). None of the 9 patients with LIE with MRSA infection had a successful outcome. Mortality rates were 33% with daptomycin treatment and 56% with standard therapy. Only 3 patients with LIE underwent valve surgery (2 in the daptomycin arm, 1 in the comparator arm): 2 after treatment and 1 during treatment for IE.
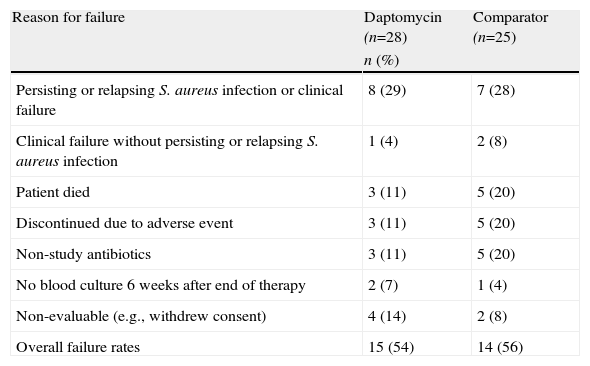
Treatment failureThe overall failure rates of daptomycin and standard therapy were comparable (54% vs 56%; Table 3). The most common reason for failure in both study groups was persisting or relapsing S. aureus infection or clinical failure (29% vs 28%). Patients receiving the comparator were more likely than those receiving daptomycin to experience treatment failure resulting from death, adverse events (AEs), or receipt of potentially effective non-study antibiotics.
Reasons for treatment failure according to the adjudication committee*
| Reason for failure | Daptomycin (n=28) | Comparator (n=25) |
| n (%) | ||
| Persisting or relapsing S. aureus infection or clinical failure | 8 (29) | 7 (28) |
| Clinical failure without persisting or relapsing S. aureus infection | 1 (4) | 2 (8) |
| Patient died | 3 (11) | 5 (20) |
| Discontinued due to adverse event | 3 (11) | 5 (20) |
| Non-study antibiotics | 3 (11) | 5 (20) |
| No blood culture 6 weeks after end of therapy | 2 (7) | 1 (4) |
| Non-evaluable (e.g., withdrew consent) | 4 (14) | 2 (8) |
| Overall failure rates | 15 (54) | 14 (56) |
S. aureus, Staphylococcus aureus.
Daptomycin was well tolerated. The overall incidence of drug-related AEs was lower in the daptomycin arm (32% vs 50%), but the difference was not statistically significant (P=0.27). The proportion of patients in whom treatment was discontinued because of AEs was slightly lower with daptomycin (21%) than with standard therapy (31%; P=0.54). Increases in creatine kinase levels (>500IU/L) were noted in 12% of patients taking daptomycin and in 4% of those who received standard therapy (P=0.61). Conversely, renal toxicity occurred in 21% of patients in the comparator arm and in 15% of patients in the daptomycin arm (P=0.73).
Microbiological and molecular analysisThe minimum inhibitory concentrations (MICs) of vancomycin and daptomycin were determined at baseline. In both treatment arms, the vancomycin MICs ranged between 0.5 and 0.1μg/mL while the daptomycin MICs ranged between 0.12 and 0.5μg/mL.
Regarding molecular analysis, no significant differences were seen in the distribution of virulence genes between the 2 groups of isolates (those causing LIE and those causing uncomplicated bacteremia), including toxins, adhesins, and other genes, such as the agr gene complex. In addition, the S. aureus isolates were similar with respect to SCCmec types, PFGE types, and antimicrobial susceptibility patterns.
DiscussionIn a large cohort of 2212 patients enrolled in the International Collaboration on Endocarditis-Merged Database (ICE-MD), S. aureus was the most common pathogen in native-valve IE, affecting 34% of all patients with definite endocarditis.20 More recent results from the ICE-Prospective Cohort Study (ICE-PCS) confirm that IE is most frequently caused by S. aureus (31%; of which 27% are MRSA). As health care-associated MRSA infection rates are rapidly rising,1 there is growing concern about the continued use of vancomycin to treat serious and often life-threatening infections. Because of increasing MICs, identification of heteroresistant and vancomycin-intermediate S. aureus isolates, and clinical failures,12,14 clinicians are searching for a new antistaphylococcal antibiotic. The results of this subgroup analysis show that daptomycin is an effective alternative to standard therapy for treatment of RIE caused by S. aureus.
We found that the success rates among patients with septic pulmonary emboli who received daptomycin were promising. Although daptomycin is not indicated for the treatment of pneumonia due to drug inactivation by pulmonary surfactant,21 the findings in this study constitute important evidence that daptomycin is effective in treating septic pulmonary emboli concomitant with IE. This can be explained because septic pulmonary emboli are, by definition, hematogenously spread and result in tissue infarction. Therefore, inactivation of daptomycin by surfactant should be less relevant than with lower respiratory tract infections.21
The current analysis provides other important results that will be helpful in conducting future studies. First, the modified Duke criteria for definite endocarditis seemed to underestimate the presence of IE in this study. These findings highlight the difficulty in predicting IE in patients presenting with SAB using the Duke criteria alone. Careful and frequent clinical examination, follow-up blood cultures, and repeat TEE are additional measures that can be used to better define IE in patients at high risk for IE.
Relatively low overall success rates in our patient population were also shown. Success rates in patients with RIE 6 weeks after the end of therapy were comparable in both study arms (42% for daptomycin and 44% for comparator) but were lower than previously reported.22,23 These low rates can be partially attributed to the strict criteria used to define treatment success. For example, when considering only patients in whom treatment was ineffective (i.e., patients who died or experienced clinical or microbiological failure), clinical success rates were higher (63% for daptomycin and 69% for the comparator) and consistent with the rates reported in the literature.22
Patients with LIE fared much worse than patients with RIE, regardless of the treatment received. A final diagnosis of LIE was associated with poor overall success, low rates of clinical success, and lower survival in both treatment groups. Although RIE is usually associated with a favorable outcome,24,25 LIE is a disease of high morbidity and mortality rates. Patients with S. aureus LIE are older and have more comorbidities than patients with S. aureus RIE. They also have a higher likelihood of heart failure, embolization to the central nervous system, and in-hospital death.20 Patients with LIE in this trial had serious complications, including stroke, osteomyelitis, valve perforation, and intracardiac abscesses. Only 1 of 18 patients with LIE in this trial underwent valve surgery while undergoing therapy; it is likely this contributed to the low success rates. In a retrospective study of 513 adult patients with complicated native-valve LIE, medical therapy alone was associated with a 2.12-fold risk for death at 6 months, compared with medical therapy with adjunctive valve surgery.26 A recent prospective analysis in patients with IE identified surgical therapy as a factor associated with a significant reduction in mortality rate (adjusted hazard ratio 0.27, 95% confidence interval [CI] 0.13–0.55).27
S. aureus is an important cause of bloodstream infections with different clinical courses and outcomes, ranging from uncomplicated bacteremia to potentially life-threatening LIE. Molecular analysis of isolates causing infections at the ends of the spectrum of disease severity (LIE vs uncomplicated bacteremia) did not reveal any differences in the virulence genes that promote the infection.
This analysis has several limitations. First, it is a subgroup analysis, in which potential for overinterpretation exists.28 However, the results have been presented with no inference of statistical significance between the study arms. Second, the sample size is small. Therefore, additional studies are necessary, especially in patients with LIE, who were not originally considered for enrollment. Finally, the open-label nature of the trial constitutes a source of bias because investigators were allowed to initiate and discontinue treatment according to their clinical judgment. However, the potential for bias was minimized by using independent echocardiographers and an independent adjudication committee, all of whom were blind to treatment assignment.
In conclusion, our results suggest that daptomycin at 6mg/kg/day is an efficacious and well tolerated treatment option in patients with S. aureus native-valve RIE.
Conflict of interest statementKanafani: no competing interest declared.
Boucher: advisor consultant to Basilea, Cubist, Johnson & Johnson, Merck, Novartis, Pfizer, Targanta, Astellas/Theravance; speaker for Cubist, Novartis; owns shares of Cubist and Pfizer.
Fowler: grant/research support from Cubist, Cerexa, Merck, Theravance, Inhibitex, Nabi, NIH; paid consultant to Cubist, Inhibitex, Leo Pharm, Merck, Johnson & Johnson; on the speaker's bureau for Cubist; received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Theravance, Ortho-McNeil; member on advisory committee of Cubist.
Cabell: no competing interest declared.
Hoen: no competing interest declared.
Miro: medical school grants/honoraria for speaking or advisory boards of BMS, Cubist, Novartis, Theravance, Merck, Gilead, GSK.
Lalani: no competing interest declared.
Vigliani: employee of Cubist during conduct of study and analysis of results.
Campion: paid consultant to Cubist; owns stocks in Cubist.
Corey: consultant for Cubist.
Levine: on the speaker's bureau for Cubist; research support from Cubist.
This work was funded by Cubist Pharmaceuticals. The funding agency was involved in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
This work was approved by the individual Institutional Review Boards at the participating study sites.