To describe the epidemiology and risk factors associated with extra-pulmonary tuberculosis (EPTB).
MethodCases of tuberculosis (TB) diagnosed from 1991 to 2008 in a Caucasian population were classified as EPTB or pulmonary TB (PTB). Of all cases, 63.7% were followed up in a specialist TB unit. A standardised protocol for data collection was used, including: gender, age, BCG vaccination, contact with PTB patient, smoking habit, alcohol abuse, diabetes mellitus, immunosuppressive drugs/steroids and HIV-status. These variables were compared between EPTB and PTB groups. Statistical analysis was based on logistic regression. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated.
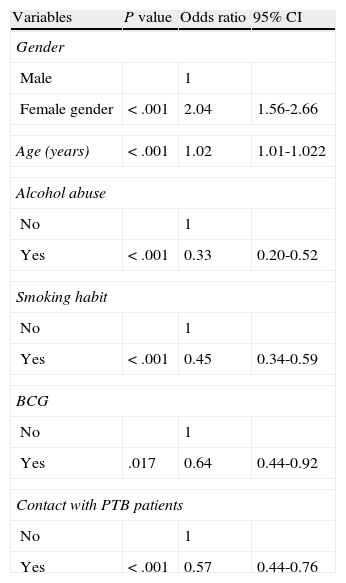
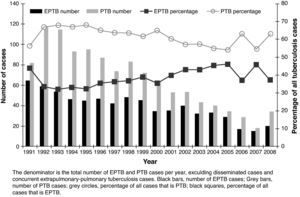
ResultsAmong the 2,161 cases diagnosed, 1,186 were PTB and 705 EPTB. The overall TB incidence had fallen from 79.9/100,000 in 1992 to 27.1/100,000 in 2008, P<.05. The number of EPTB cases decreased more slowly than PTB. EPTB increased from 30.6% of cases in 1991-1996 to 37.6% in 2003-2008 (lymphatic site increased 27%), by trend test P<.05. At multivariate level, being female (OR 2.04; 95% CI: 1.56-2.66) and age (OR 1.02; 95% CI: 1.01-1.022) were associated with EPTB, while alcohol abuse (OR 0.33; 95% CI: 0.20-0.52), smoking habit (OR 0.45; 95%CI: 0.34-0.59), contact with PTB patients (OR 0.57; 95% CI: 0.44-0.76) and BCG vaccination (OR 0.64; 95% CI: 0.44-0.92) had a protective effect. The proportion of female gender and age of patients increased over time, whilst there was a decrease in BCG vaccinated patients.
ConclusionsWhilst there has been a reduction in the overall incidence of TB, the proportion of EPTB increased. The proportional increase in EPTB could be explained by an increase in life expectancy and the predominance of women in the population, and by a decline in BCG vaccinated patients.
Conocer la epidemiología y los factores de riesgo asociados con la tuberculosis extrapulmonar (EPTB).
MétodoLos casos de tuberculosis (TB) diagnosticadas entre 1991-2008 en una población caucásica fueron clasificados como EPTB o TB pulmonar (PTB). De todos los casos, 63,7% fueron seguidos en una consulta monográfica de TB. Se utilizó un protocolo estandarizado para la recogida de los datos, incluyendo: sexo, edad, vacunación con BCG, contacto con algún paciente con PTB, tabaquismo, alcoholismo, diabetes mellitus, corticoides/fármacos inmunosupresores e infección por el VIH. Se compararon las variables entre los grupos de EPTB y de PTB. El análisis estadístico se basó en un estudio de regresión logística. Se calcularon los odds ratio (OR) y sus intervalos de confianza (IC) del 95%.
ResultadosEntre 2.161 casos diagnosticados, 1.186 fueron PTB y 705 EPTB. La incidencia global de TB disminuyó desde 79.9/100.000 en 1992 hasta 27.1/100.000 en 2008, p<0,05. El número de casos de EPTB disminuyó de forma más lenta que el de PTB. La proporción de EPTB aumentó desde 30,6% de los casos en 1991-1996 hasta 37,6% en 2003-2008 (la localización ganglionar aumentó un 27%), p<0,05 en un ji al cuadrado de tendencia. En el estudio multivariante, ser mujer (OR 2,04; IC 95%: 1,56-2,66) y la edad (OR 1,02; IC 95%: 1,01-1,022) se asociaron con EPTB mientras que el alcoholismo (OR 0,33; IC 95%: 0,20-0,52), el tabaquismo (OR 0,45; IC 95%: 0,34-0,59), el contacto con pacientes con PTB (OR 0,57; IC 95%: 0,44-0,76) y la vacunación con BCG (OR 0,64; IC 95%: 0,44-0,92) tuvieron un efecto protector. La proporción de mujeres y la edad de los pacientes aumentaron a lo largo del tiempo, y descendió el número de pacientes vacunados con BCG.
ConclusionesCon la reducción en la incidencia global de TB, la proporción de EPTB aumentó. El incremento proporcional de la EPTB se podría explicar por el aumento de la esperanza de vida y el predominio de la mujer en la población, y por un descenso de los pacientes vacunados con BCG.
There has been a constant decrease in the number of cases of tuberculosis (TB) in industrialised countries due to improvements in social conditions and the availability of effective antituberculosis drugs.1 This decrease halted between the years 1985 and 1992 due to the relaxation of prevention and control programs, the appearance of HIV infection, and the migratory movements of people from countries with a greater incidence of TB.2,3 Although the total number of cases of TB has decreased, the reduction in cases of extra-pulmonary tuberculosis (EPTB) has been smaller, resulting in a proportionate increase in EPTB compared to pulmonary tuberculosis (PTB).4 In the cases of TB reported in the US, for example, EPTB accounted for 15.7% of total cases in 1993, but 21% in 2006.5 The reasons for this smaller reduction are little known and have been attributed, among others causes, to demographic changes in the susceptible population and a decreased uptake of vaccination with BCG.6,7 There are no prospective studies that analyse other possible enhancing factors for EPTB infection.
We present a prospective research study with the following targets:
- 1.
To describe the epidemiology of EPTB in a Caucasian population, comparing the incidence and characteristics of EPTB with those of PTB.
- 2.
To analyse factors contributing to the increased proportion of cases of EPTB.
A specialised unit for following up patients with TB was opened within our hospital in 1991, and a program for prevention and control of TB began in the North West Spanish region of Galicia in 1995. An active search of all the diagnosed cases of TB was performed from 1991 in our health area with a predominantly Caucasian population. In 1991 the population consisted of 218,749 inhabitants, with a mean age of 38.8 years (16.3% older than 64 y); 51.% women (59.9% older than 64 y); 0.29% were born outside Spain and the AIDS incidence was 6 cases per 100,000.8,9 In 2008 the population decreased to 205,122, with mean age of 44.1 years, (22.9% older than 64 y), with a life expectancy of 77.6 years for men and 84.8 years for women; 51.8% were women, (58.6% older than 64y); 2.1% were immigrants (40.9% from European Union, 3.5% from Eastern Europe, 4.7% from Africa, 2.5% from Asia, 48.3% from Central and South America), and the AIDS incidence was 4.5 cases per 100,000.10,11
We analysed data collected at the time of diagnosis for all cases of TB recorded during an 18 year period (1991-2008). Cases of TB were defined by the presence of positive cultures for M. tuberculosis complex, histological samples showing presence of tuberculous granulomas and by clinical diagnosis with a favourable response to the antituberculous treatment. Five cases with M. bovis were excluded. The cases of TB were classified as either EPTB or PTB. EPTB sites encompassed pleural, lymphatic, genitourinary, bone and/or joint, meningeal, peritoneal, gastrointestinal, cutaneous and unclassified cases.12 EPTB cases that included >1 EPTB disease site were classified according to the major site. Cases of disseminated TB and cases with concurrent EPTB-PTB were excluded from our principal analysis, because they were not distinctly classifiable as either EPTB or PTB. In order to determine the possible ramifications of this definition of EPTB, we performed a separate analysis that compared disseminated and concurrent EPTB-PTB with EPTB only and with PTB only. In addition, we performed a separate analysis in which disseminated and concurrent EPTB were added to our existing EPTB classification.
In order to respond to the first goal of the study, we performed an analysis of the evolution of the EPTB and their different locations for all cases of TB recorded during the 18 year period. A standardised protocol was used to gather data from each patient at the time of diagnosis as regards socio-demographic and clinical information, including sex, age, country of birth, previous diagnosis of TB, sites of disease, methods of diagnosis, antitubercular drugs susceptibility of M. tuberculosis isolates from culture-positive patients, alcohol abuse and human immunodeficiency virus (HIV) status. After obtaining informed consent, HIV testing was performed on patients suspected of having an HIV infection. HIV status was classified as HIV-infected, HIV-negative, or missing or unknown HIV status. Missing or unknown HIV status included patients having indeterminate, unknown, or missing test results, as well as patients refusing or not offered testing.
Of all diagnosed cases in the area, 1,377 (63.7%) [533 with EPTB and 661 with PTB] were prospectively followed up in the specialised TB unit opened in 1991. In order to determine the second of our objectives, we performed an analysis in these cases comparing the characteristics of EPTB patients with those of PTB, and we performed an adjusted analysis to assess the factors that appeared to be associated with the EPTB and influenced their evolution over time.
For cases followed up in the TB unit, data was gathered from each patient as regards socio-demographic and clinical characteristics, previous diagnosis of TB, drug susceptibility test results, and risk factors, including: female gender, age, Bacillus Calmette-Guerin (BCG) vaccination, history of contact with PTB patients, gastrectomy, smoking and alcohol habits, diabetes mellitus, chronic renal failure (CRF), neoplasia, use of immunosuppressive drugs/steroids, albumin level and HIV status. Individuals were considered to be BCG vaccinated if this was recorded in their vaccination records or by the presence of cutaneous scar. Immunosuppressive drugs considered were anti-TNF, chemotherapy and steroids, if more than 15mg prednisone/day were administered during at least 2 weeks. Smoking habit was considered if the patient smoked 5 or more cigarettes/day13 and alcohol abuse if the patient drank more than 60g ethanol/day.14
This study was approved by ethics committee of our hospital. Computerised data-bases for registration of data were created. These did not allow the participants in the study to be identified, and only health professionals had access to the information, maintaining strict confidentiality.
The results of the two series were analysed using the commercially available SPSS software, version 14.0. Variables were compared between EPTB and PTB groups. Variables were also compared between cases followed up and not followed up in TB unit. The 18 years of study duration were divided into three groups of 6 years and the EPTB trend and its different locations were assessed by the Chi-squared test. The age of the patients was divided into groups to compare the distribution of sites and of risk factors for EPTB. Continuous variables were analysed using Student‘s t-test or analysis of variance. Categorical data were analysed using the Chi-squared or Fisher‘s exact test, as appropriate. Statistical significance was set at P<.05. To identify the risk factors for EPTB and to control for possible confounding factors, all variables associated with a level of significance of P<.20 in the univariate analysis were included in a stepwise multiple logistic regression analysis. On analysing risk factors for EPTB, and in order to establish the evolution of EPTB over time, we used the patients with PTB as a reference. Interaction factors were analysed, but not included in the final model, as they were not found to be significant. Odds ratios (OR), 95% confidence intervals (95% CI) and P values were calculated for each potential risk factor.
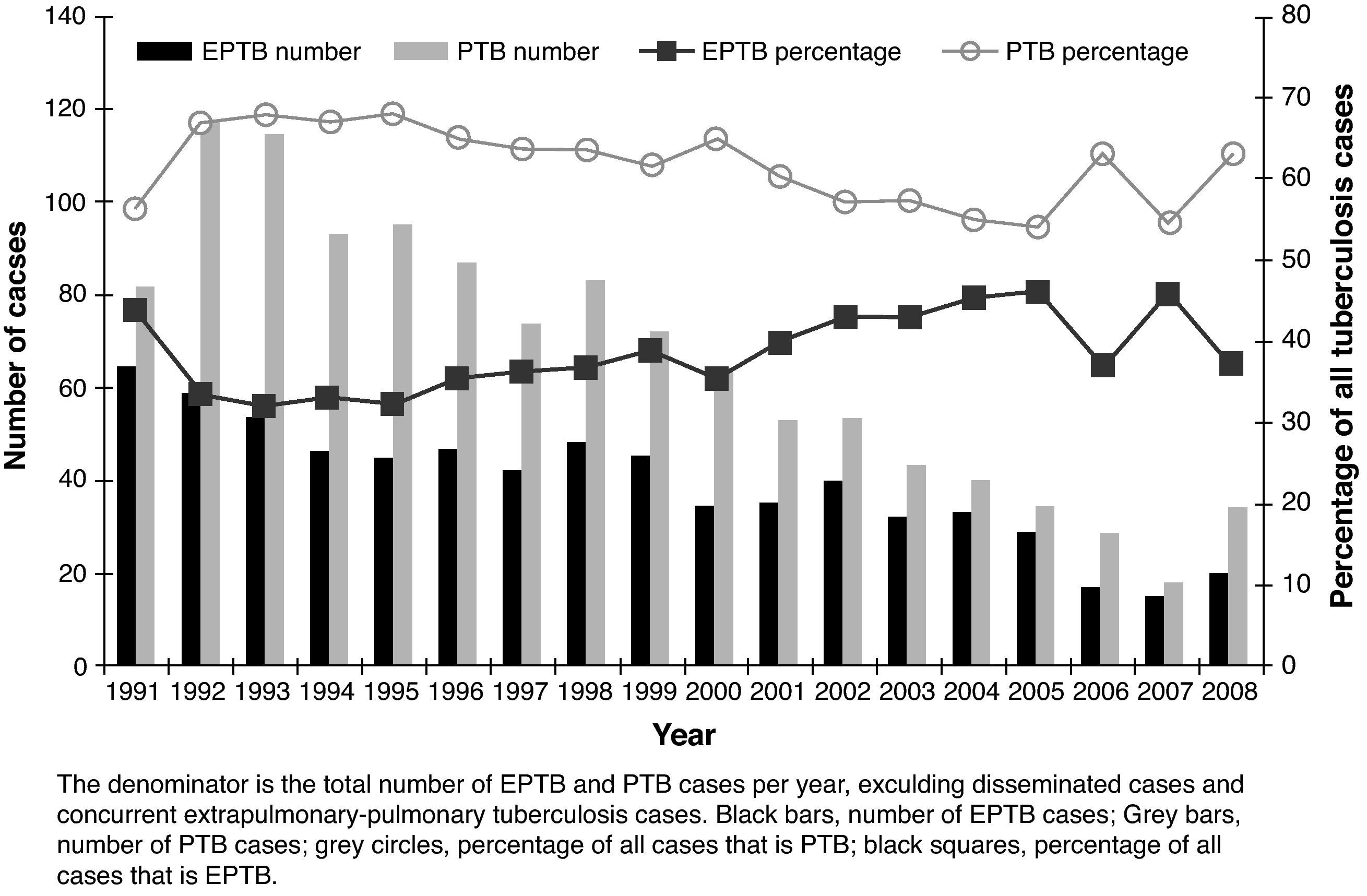
ResultsA total of 2,161 cases of TB were diagnosed. Five (0.23%) cases were in patients born outside Spain. The incidence decreased over time from 79.9 per 100,000 in 1992 (sputum smear-positive 26.3) to 27.1 per 100,000 in 2008 (sputum smear-positive 10). Among the total of 2,161 cases, 705 (32.6%) were EPTB, 1,186 (54.9%) were PTB, 106 (4.9%) were disseminated TB, and 164 (7.6%) were concurrent EPTB-PTB. The number of cases of both EPTB and PTB decreased over time, but EPTB cases decreased more slowly; cases of PTB decreased an average of 4.7% annually, compared with a 2.2% annual decrease for EPTB cases (Fig. 1). As a result of the slower decrease in the number of EPTB cases, the proportion of EPTB increased from 30.6% of all TB cases in the 1991-1996 period to 37.6% in the last six years, P=.01.
A total of 37.6% of EPTB diagnoses were confirmed by Mycobacterium tuberculosis cultures, 177 (25.1%) of cases were diagnosed by the presence of tuberculous granulomas in a histological sample, and 263 (37.3%) cases based on clinical diagnosis and favourable response to treatment. Table 1 shows the evolution of EPTB and PTB patient characteristics. The proportion of cases confirmed by culture was constant over time. Out of the 265 EPTB cases with a Mycobacterium tuberculosis positive culture, drug sensitivity testing was performed in 142 (all cases after 1995), and all were isoniazid and rifampin sensitive.
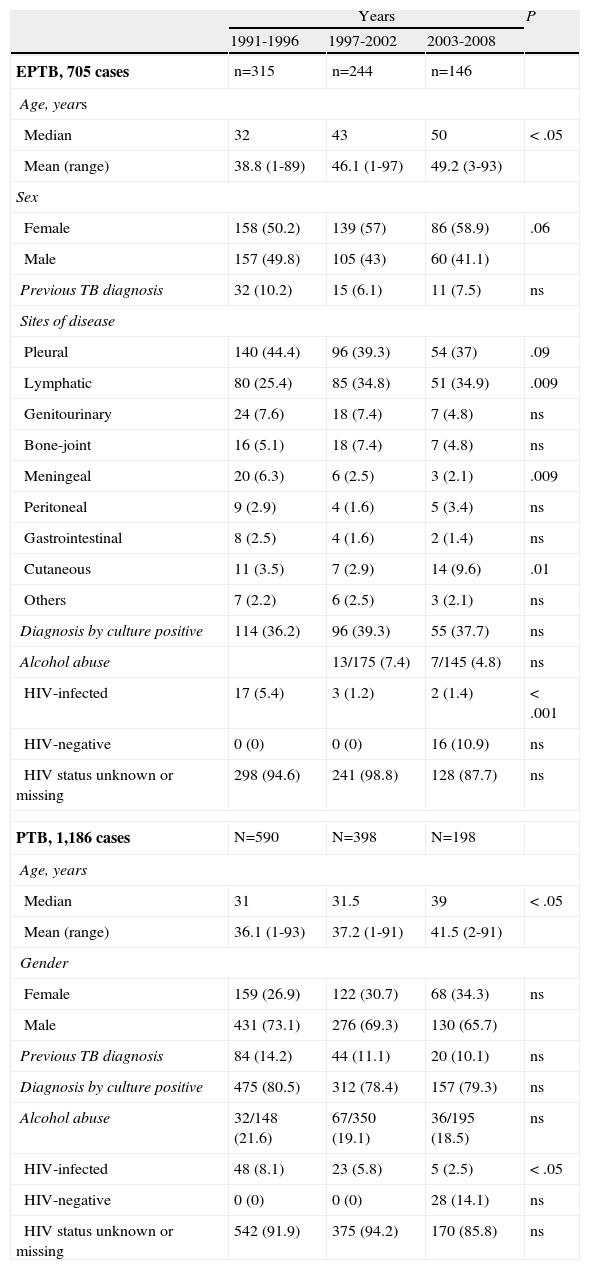
Evolution of extrapulmonary tuberculosis (EPTB) and pulmonary tuberculosis (PTB) patient characteristics in the global series. (n=1,891).
| Years | P | |||
| 1991-1996 | 1997-2002 | 2003-2008 | ||
| EPTB, 705 cases | n=315 | n=244 | n=146 | |
| Age, years | ||||
| Median | 32 | 43 | 50 | < .05 |
| Mean (range) | 38.8 (1-89) | 46.1 (1-97) | 49.2 (3-93) | |
| Sex | ||||
| Female | 158 (50.2) | 139 (57) | 86 (58.9) | .06 |
| Male | 157 (49.8) | 105 (43) | 60 (41.1) | |
| Previous TB diagnosis | 32 (10.2) | 15 (6.1) | 11 (7.5) | ns |
| Sites of disease | ||||
| Pleural | 140 (44.4) | 96 (39.3) | 54 (37) | .09 |
| Lymphatic | 80 (25.4) | 85 (34.8) | 51 (34.9) | .009 |
| Genitourinary | 24 (7.6) | 18 (7.4) | 7 (4.8) | ns |
| Bone-joint | 16 (5.1) | 18 (7.4) | 7 (4.8) | ns |
| Meningeal | 20 (6.3) | 6 (2.5) | 3 (2.1) | .009 |
| Peritoneal | 9 (2.9) | 4 (1.6) | 5 (3.4) | ns |
| Gastrointestinal | 8 (2.5) | 4 (1.6) | 2 (1.4) | ns |
| Cutaneous | 11 (3.5) | 7 (2.9) | 14 (9.6) | .01 |
| Others | 7 (2.2) | 6 (2.5) | 3 (2.1) | ns |
| Diagnosis by culture positive | 114 (36.2) | 96 (39.3) | 55 (37.7) | ns |
| Alcohol abuse | 13/175 (7.4) | 7/145 (4.8) | ns | |
| HIV-infected | 17 (5.4) | 3 (1.2) | 2 (1.4) | < .001 |
| HIV-negative | 0 (0) | 0 (0) | 16 (10.9) | ns |
| HIV status unknown or missing | 298 (94.6) | 241 (98.8) | 128 (87.7) | ns |
| PTB, 1,186 cases | N=590 | N=398 | N=198 | |
| Age, years | ||||
| Median | 31 | 31.5 | 39 | < .05 |
| Mean (range) | 36.1 (1-93) | 37.2 (1-91) | 41.5 (2-91) | |
| Gender | ||||
| Female | 159 (26.9) | 122 (30.7) | 68 (34.3) | ns |
| Male | 431 (73.1) | 276 (69.3) | 130 (65.7) | |
| Previous TB diagnosis | 84 (14.2) | 44 (11.1) | 20 (10.1) | ns |
| Diagnosis by culture positive | 475 (80.5) | 312 (78.4) | 157 (79.3) | ns |
| Alcohol abuse | 32/148 (21.6) | 67/350 (19.1) | 36/195 (18.5) | ns |
| HIV-infected | 48 (8.1) | 23 (5.8) | 5 (2.5) | < .05 |
| HIV-negative | 0 (0) | 0 (0) | 28 (14.1) | ns |
| HIV status unknown or missing | 542 (91.9) | 375 (94.2) | 170 (85.8) | ns |
HIV: human immunodeficiency virus.
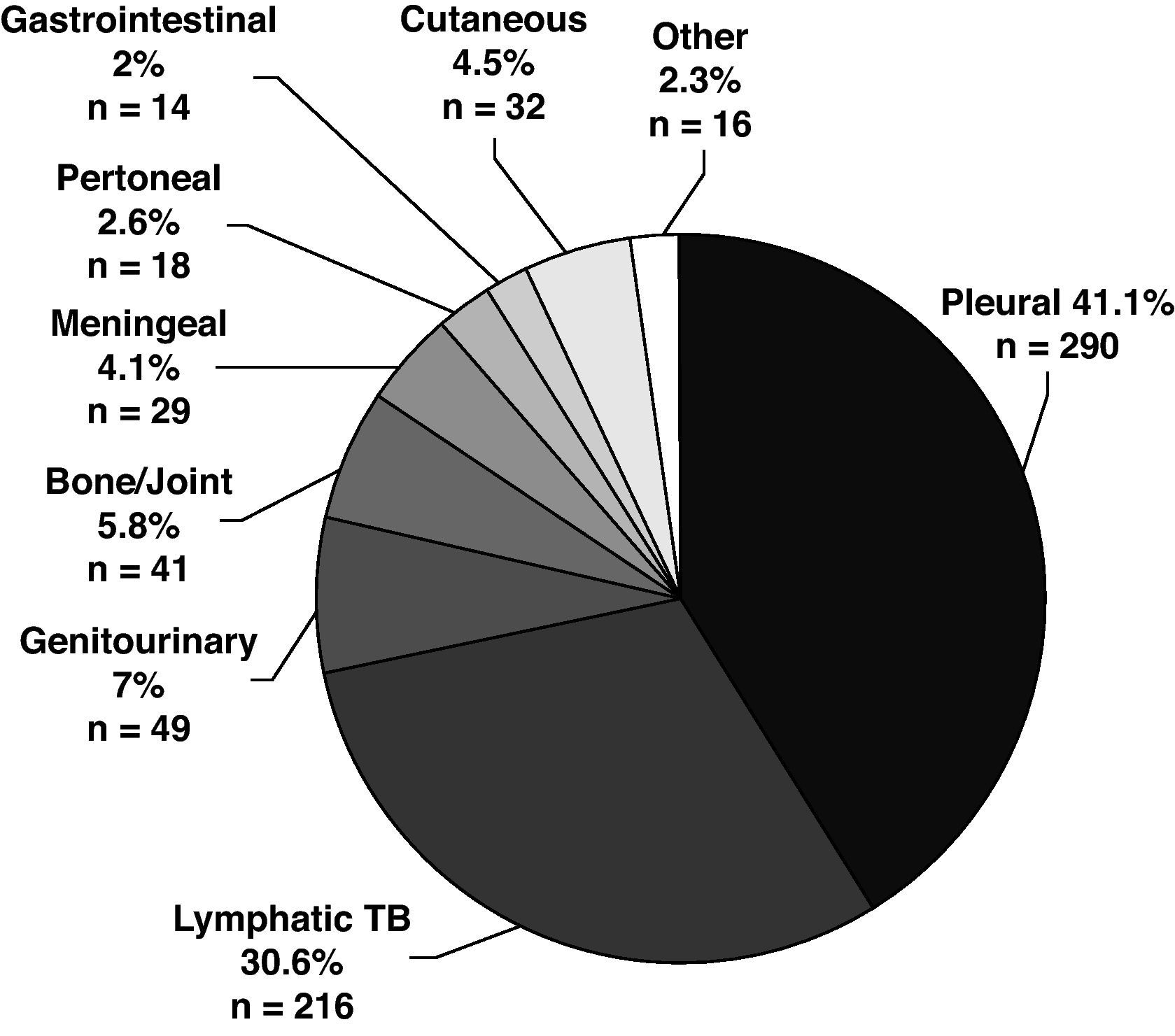
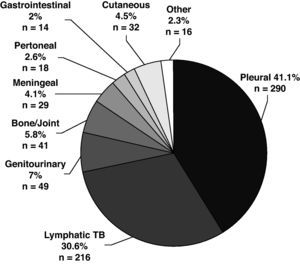
The disease site of the largest proportion of EPTB cases was pleural TB followed by lymphatic TB (Fig. 2). The frequency of the individual TB sites had modified over time. The cases of pleural TB and meningeal TB decreased, whilst cutaneous and lymphatic TB increased.
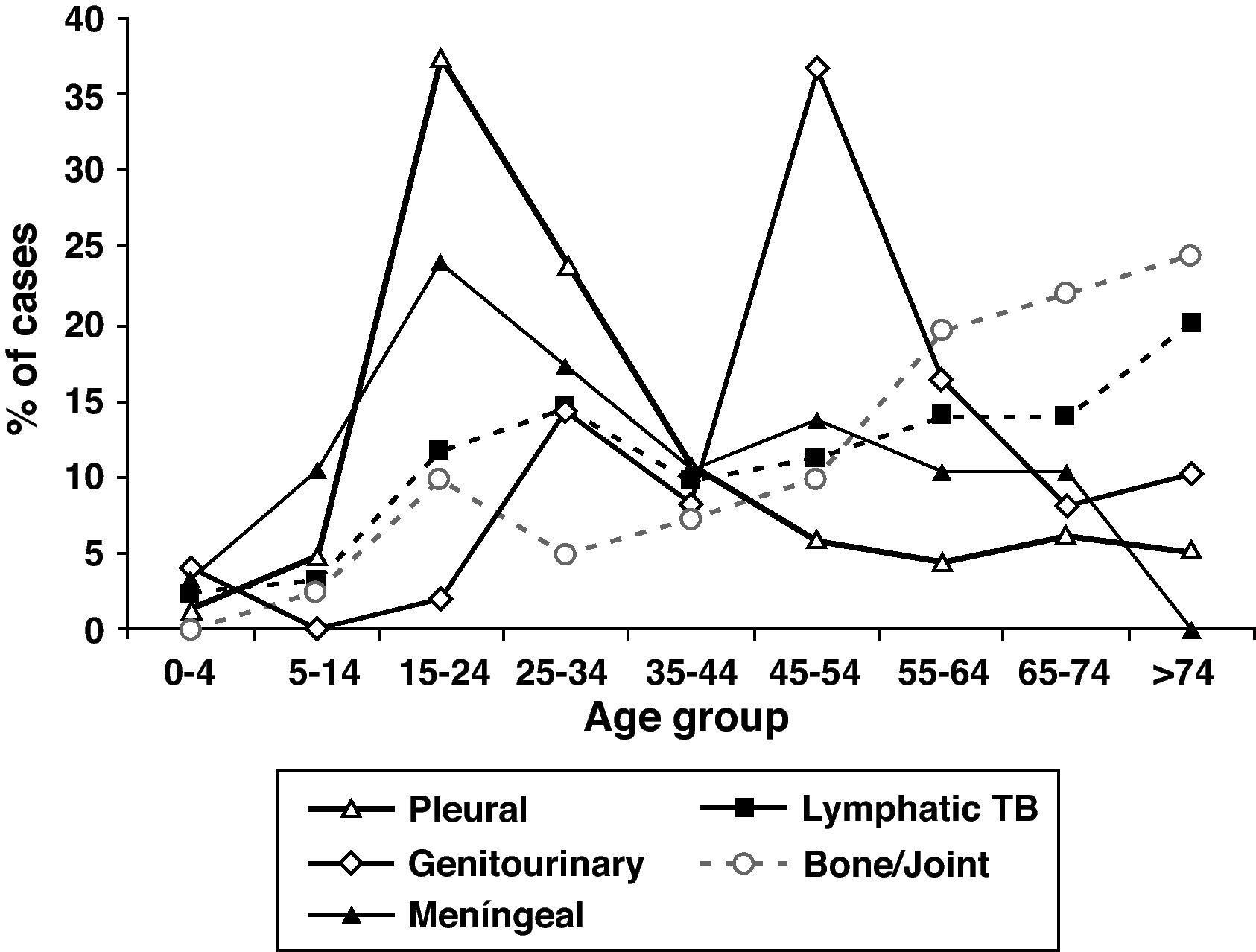
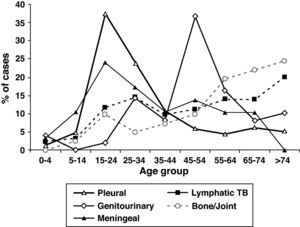
The mean age of patients with EPTB was higher than in patients with PTB and increased over time, P<.001). EPTB cases increased with age, but the anatomical sites involved differed according to age (Fig. 3). The mean age of patients with pleural (33.4y), peritoneal (43.7y) and meningeal TB (35.7y) was lower than the mean age of patients who had lymphatic (50.6y), bone and/or joint (58.6y), genitourinary (52.1y), gastrointestinal (56.1y) and cutaneous TB (52.7y). Although 27.7% of all EPTB patients were > 60 years old, as many as 39.5% of lymphatic TB cases occurred among patients > 60 years old. Children <15 years old were no more likely to have EPTB than patients ≥ 15 years. They accounted for 5.4% of all TB patients, but 13.8% of meningeal TB cases.
Slightly more than half of the EPTB patients were female (OR 2.85, 95% CI: 2.35-3.46) and the proportion of female gender in EPTB patients increased over time. Except for pleural, gastrointestinal and meningeal TB, in which 57.9%, 71.4% and 58.6%, respectively of patients were male, all other extrapulmonary disease sites were slightly more common in female. The largest female proportion occurred among lymphatic (70.8%) and cutaneous TB cases (71.9%).
Alcohol abuse was less frequent in EPTB patients than in patients with PTB (OR 0.25; 95% CI: 0.16-0.42), and the proportion of patients with alcohol abuse decreased over time.
Among the 705 EPTB patients, 22 (3.2%) were HIV-infected. The proportion of EPTB patients becoming infected with HIV descreased over time. The EPTB disease sites associated with the highest proportion of HIV infection were meningeal (20.7%) and the lowest was pleural TB (0.7%), P<.05. HIV-infected patients had lower odds of EPTB compared with non-HIV-infected patients (OR 0.47, 95% CI; 0.29-0.76). To determine whether our EPTB case definition affected odds related to HIV infection and younger age, we compared disseminated TB and concurrent EPTB-PTB cases with PTB-only cases and then repeated the analysis with EPTB-only cases. Patients with HIV infection had greater odds of having concurrent EPTB-PTB or disseminated TB (27.8%) than of having PTB (6.4%) OR 4.3 (95% CI: 3.2-5.88), or EPTB alone (3.1%) OR 8.9 (95% CI: 5.6-14). A separate analysis in which the categories of disseminated and concurrent EPTB-PTB were added to our original EPTB definition and then compared with PTB, showed a change in the association with HIV from an OR of 0.47 (95% CI: 0.29-0.76) for EPTB with our original EPTB definition to OR 1.61 (95% CI: 1.2-2.1). Children <15 years of age had similar odds of concurrent EPTB-PTB or disseminated TB (4.1%) when compared with EPTB (5.4%) and PTB (6.3%).
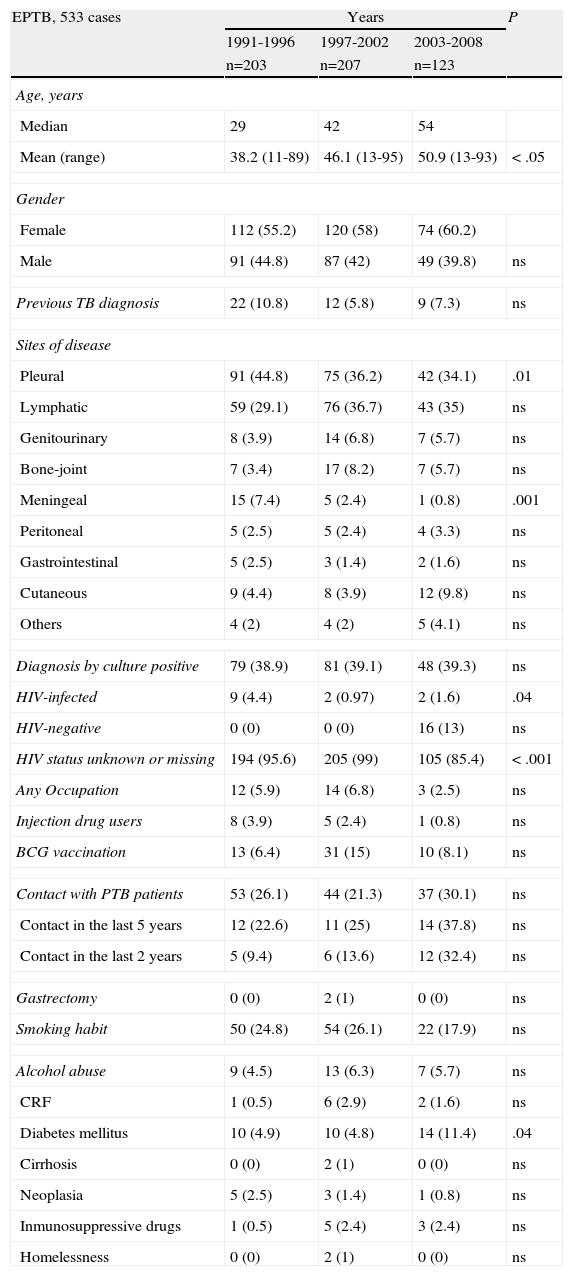
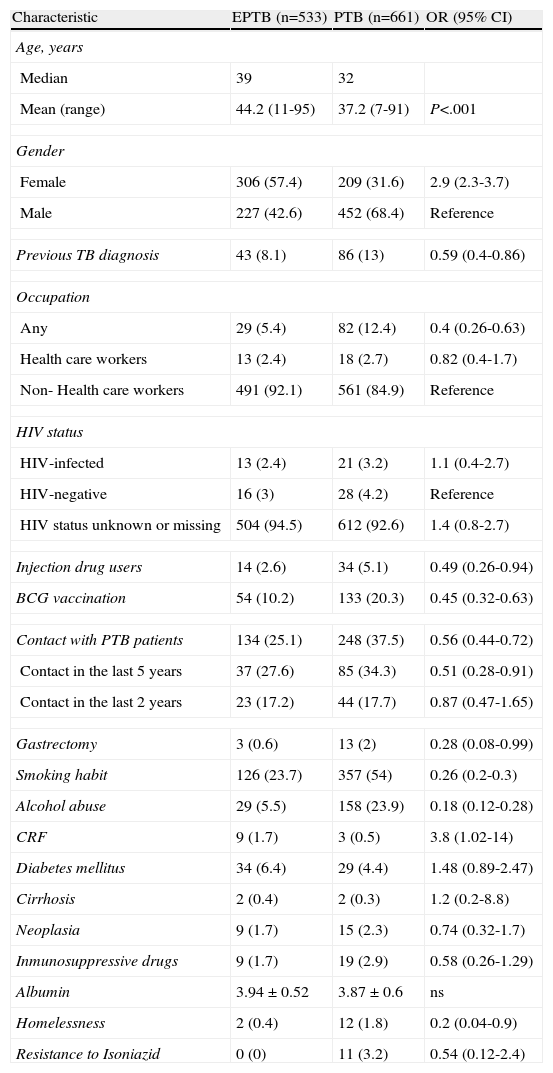
Of all diagnosed cases, 1,377 (63.7%) were prospectively followed up in a specialist TB unit Of the 1,377 cases, 533 (38.7%) were EPTB, 661 (48%) were PTB, 59 (4.3%) were disseminated TB, and 124 (9%) were concurrent EPTB-PTB. Table 2 shows the evolution of EPTB patient characteristics followed up. Table 3 shows the patient characteristics of EPTB compared with those of PTB patients. In the multivariate analysis, being female and older age were associated with EPTB, while alcohol abuse, smoking habit, contact with PTB patients, and BCG vaccination had a protective effect (Table 4). The odds ratios were somewhat different for EPTB locations when each were analysed separately. The likelihood of developing EPTB was more than two-fold higher among females than in males, and it was greater for lymphatic (OR 5.23; 95% CI: 3.3-8.3) and genitourinary locations (OR 3.8; 95% CI: 1.3-11.5), whereas when analysing a pleural location separately as a specific form of EPTB, being female (OR 1.3; 95% CI: 0.9-1.8), age (OR 0.99; 95% CI: 0.98-1) and vaccination with BCG (OR 0.69; 95% CI: 0.4-1.1) were not associated with this form of EPTB.
Evolution of extrapulmonary tuberculosis (EPTB) patient characteristics followed by tuberculosis unit.
| EPTB, 533 cases | Years | P | ||
| 1991-1996 | 1997-2002 | 2003-2008 | ||
| n=203 | n=207 | n=123 | ||
| Age, years | ||||
| Median | 29 | 42 | 54 | |
| Mean (range) | 38.2 (11-89) | 46.1 (13-95) | 50.9 (13-93) | < .05 |
| Gender | ||||
| Female | 112 (55.2) | 120 (58) | 74 (60.2) | |
| Male | 91 (44.8) | 87 (42) | 49 (39.8) | ns |
| Previous TB diagnosis | 22 (10.8) | 12 (5.8) | 9 (7.3) | ns |
| Sites of disease | ||||
| Pleural | 91 (44.8) | 75 (36.2) | 42 (34.1) | .01 |
| Lymphatic | 59 (29.1) | 76 (36.7) | 43 (35) | ns |
| Genitourinary | 8 (3.9) | 14 (6.8) | 7 (5.7) | ns |
| Bone-joint | 7 (3.4) | 17 (8.2) | 7 (5.7) | ns |
| Meningeal | 15 (7.4) | 5 (2.4) | 1 (0.8) | .001 |
| Peritoneal | 5 (2.5) | 5 (2.4) | 4 (3.3) | ns |
| Gastrointestinal | 5 (2.5) | 3 (1.4) | 2 (1.6) | ns |
| Cutaneous | 9 (4.4) | 8 (3.9) | 12 (9.8) | ns |
| Others | 4 (2) | 4 (2) | 5 (4.1) | ns |
| Diagnosis by culture positive | 79 (38.9) | 81 (39.1) | 48 (39.3) | ns |
| HIV-infected | 9 (4.4) | 2 (0.97) | 2 (1.6) | .04 |
| HIV-negative | 0 (0) | 0 (0) | 16 (13) | ns |
| HIV status unknown or missing | 194 (95.6) | 205 (99) | 105 (85.4) | < .001 |
| Any Occupation | 12 (5.9) | 14 (6.8) | 3 (2.5) | ns |
| Injection drug users | 8 (3.9) | 5 (2.4) | 1 (0.8) | ns |
| BCG vaccination | 13 (6.4) | 31 (15) | 10 (8.1) | ns |
| Contact with PTB patients | 53 (26.1) | 44 (21.3) | 37 (30.1) | ns |
| Contact in the last 5 years | 12 (22.6) | 11 (25) | 14 (37.8) | ns |
| Contact in the last 2 years | 5 (9.4) | 6 (13.6) | 12 (32.4) | ns |
| Gastrectomy | 0 (0) | 2 (1) | 0 (0) | ns |
| Smoking habit | 50 (24.8) | 54 (26.1) | 22 (17.9) | ns |
| Alcohol abuse | 9 (4.5) | 13 (6.3) | 7 (5.7) | ns |
| CRF | 1 (0.5) | 6 (2.9) | 2 (1.6) | ns |
| Diabetes mellitus | 10 (4.9) | 10 (4.8) | 14 (11.4) | .04 |
| Cirrhosis | 0 (0) | 2 (1) | 0 (0) | ns |
| Neoplasia | 5 (2.5) | 3 (1.4) | 1 (0.8) | ns |
| Inmunosuppressive drugs | 1 (0.5) | 5 (2.4) | 3 (2.4) | ns |
| Homelessness | 0 (0) | 2 (1) | 0 (0) | ns |
HIV: human immunodeficiency virus; BCG: Bacillus Calmette-Guerin; CRF: chronic renal failure.
Characteristics of patients with extrapulmonary tuberculosis (EPTB), compared with patients with pulmonary tuberculosis (PTB).
| Characteristic | EPTB (n=533) | PTB (n=661) | OR (95% CI) |
| Age, years | |||
| Median | 39 | 32 | |
| Mean (range) | 44.2 (11-95) | 37.2 (7-91) | P<.001 |
| Gender | |||
| Female | 306 (57.4) | 209 (31.6) | 2.9 (2.3-3.7) |
| Male | 227 (42.6) | 452 (68.4) | Reference |
| Previous TB diagnosis | 43 (8.1) | 86 (13) | 0.59 (0.4-0.86) |
| Occupation | |||
| Any | 29 (5.4) | 82 (12.4) | 0.4 (0.26-0.63) |
| Health care workers | 13 (2.4) | 18 (2.7) | 0.82 (0.4-1.7) |
| Non- Health care workers | 491 (92.1) | 561 (84.9) | Reference |
| HIV status | |||
| HIV-infected | 13 (2.4) | 21 (3.2) | 1.1 (0.4-2.7) |
| HIV-negative | 16 (3) | 28 (4.2) | Reference |
| HIV status unknown or missing | 504 (94.5) | 612 (92.6) | 1.4 (0.8-2.7) |
| Injection drug users | 14 (2.6) | 34 (5.1) | 0.49 (0.26-0.94) |
| BCG vaccination | 54 (10.2) | 133 (20.3) | 0.45 (0.32-0.63) |
| Contact with PTB patients | 134 (25.1) | 248 (37.5) | 0.56 (0.44-0.72) |
| Contact in the last 5 years | 37 (27.6) | 85 (34.3) | 0.51 (0.28-0.91) |
| Contact in the last 2 years | 23 (17.2) | 44 (17.7) | 0.87 (0.47-1.65) |
| Gastrectomy | 3 (0.6) | 13 (2) | 0.28 (0.08-0.99) |
| Smoking habit | 126 (23.7) | 357 (54) | 0.26 (0.2-0.3) |
| Alcohol abuse | 29 (5.5) | 158 (23.9) | 0.18 (0.12-0.28) |
| CRF | 9 (1.7) | 3 (0.5) | 3.8 (1.02-14) |
| Diabetes mellitus | 34 (6.4) | 29 (4.4) | 1.48 (0.89-2.47) |
| Cirrhosis | 2 (0.4) | 2 (0.3) | 1.2 (0.2-8.8) |
| Neoplasia | 9 (1.7) | 15 (2.3) | 0.74 (0.32-1.7) |
| Inmunosuppressive drugs | 9 (1.7) | 19 (2.9) | 0.58 (0.26-1.29) |
| Albumin | 3.94±0.52 | 3.87±0.6 | ns |
| Homelessness | 2 (0.4) | 12 (1.8) | 0.2 (0.04-0.9) |
| Resistance to Isoniazid | 0 (0) | 11 (3.2) | 0.54 (0.12-2.4) |
HIV: human immunodeficiency virus. BCG: Bacillus Calmette-Guerin. CRF: chronic renal failure.
Multi-variate logistic regression model determining the independent risk factors for developing extrapulmonary tuberculosis relative to pulmonary tuberculosis.
| Variables | P value | Odds ratio | 95% CI |
| Gender | |||
| Male | 1 | ||
| Female gender | < .001 | 2.04 | 1.56-2.66 |
| Age (years) | < .001 | 1.02 | 1.01-1.022 |
| Alcohol abuse | |||
| No | 1 | ||
| Yes | < .001 | 0.33 | 0.20-0.52 |
| Smoking habit | |||
| No | 1 | ||
| Yes | < .001 | 0.45 | 0.34-0.59 |
| BCG | |||
| No | 1 | ||
| Yes | .017 | 0.64 | 0.44-0.92 |
| Contact with PTB patients | |||
| No | 1 | ||
| Yes | < .001 | 0.57 | 0.44-0.76 |
When analysing the distribution of the variables by age groups, the proportion of female gender and of risk factors was different according to the age groups. Female smokers (26.3% versus 51.6%) and alcohol abusers (1.8% versus 27.3%) were lower in number compared to men. Female gender was greater in the 1-22 years group (44.3%) and in >52 years old patients (44.4%). Fifty-nine percent of patients with ages between 23-52 years, and 21.5% in >52 years old, were smokers. Alcohol abuse was greater in the 33-52 years group (34.7%) and decreased in >52 years old patients (16.2%). Two hundred and twelve patients were BCG vaccinated, 85 in the first, 94 in the second, and 33 in the third, time period (P<.05). The percentage of BCG vaccinations varied between 8.1% in patients less than 22 years to 32.5% in those between 33-52 years, and decreased to 2.5% in those older than 52 years. In BCG vaccinated patients, there was one case of meningitis (0.5%) compared to 22 cases (1.8%) in 1,143 non-BCG vaccinated patients. The percentage of patients with diabetes mellitus, CRF and on immunosuppressive treatemnt increased progressively with age, reaching 13.3%, 2.4% and 6.9%, respectively in patients greater than 52 years old.
The frequency of recent contact with active PTB was different according to EPTB sites. In cases of pleural TB there had been preceding TB contact in 57/207 (27.5%) [47.4% within the last 5 years, 29.8% in the last 2 years] and in cases of lymphatic TB there had been preceding contact in 45/176 (25.6%) [11.1% within the last 5 years, 4.4% in the last 2 years].
Out of the 2,161 diagnosed cases, 784 (36.3%) were not followed up in our TB unit but followed up by other physicians. The number of these patients decreased over time (52.1% in 1991-1996, 31.9% in 1997-2002 and 16% in the 2003-2008 time period). These cases had a lower proportion of female gender (32.4% versus 41%), lower EPTB frequency (27.7% vs 43.9%) [pleural site 9.9% vs 15.8% and lymphatic site 5.8% vs 12.9%] and greater proportion of HIV infected patients (12.3% vs 5.1%), with no difference in mean age, alcohol abuse and all other variables analysed.
DiscussionOur data confirms a lower reduction in EPTB compared with the reduction in PTB, in an area with intermediate incidence of TB, a good program for prevention and control of the TB, and predominantly Caucasian population where life expectancy and the proportion of women in the population are increasing. All the diagnosed cases of TB have been reported and the lower reduction in EPTB cases is not due to an increase in the reporting of cases or as a result of over-diagnosis, since the percentage of cases confirmed by culture remained stable over time. In addition, the numbers were not influenced by the migratory movements of patients originating from countries where TB is endemic.15,16
Our results are consistent with those of other studies conducted in geographic zones with low incidence of TB.4–7,15 This evolution of EPTB and PTB was already described before the HIV-driven TB increase.1,17 Understanding the reasons for the slower decrease in EPTB is important in the goal of TB elimination.18 We did not find an association between EPTB and HIV status, compared with PTB, although HIV infection has been suggested as a contributing factor to the increasing EPTB proportion relative to PTB.19,20 This may be attributable to differences with other studies in the definition of EPTB. Studies reporting a positive association between EPTB and HIV include miliary and concurrent EPTB-PTB in their EPTB definition, as in the results of our separate analysis.5
Among EPTB disease sites, and in agreement with other studies, we found variations according to sex, age group and HIV status.19,21 The reasons for the differences are unexplained, but these differences suggest that the natural history of TB infection may be influenced by, the location of the tuberculous bacilli in the body, by age at the time of infection, by time elapsed since infection, by sex, and by genetic factors. The variations by gender and age may be due to the differences in exposure opportunities to active PTB, such as the more extensive social activity of males, alcohol and smoking habits or even unexplained genetic differences.17,18,20,22
Our analysis of prospectively followed patients suggests possible reasons for the slower decrease of EPTB compared to PTB. The recent transmission of TB seems to be associated with a greater frequency of pleural TB, like PTB, and a smaller frequency of lymphatic TB.23 This can be explained partly by the fact that pleural TB predominates as the most frequent form of EPTB in areas with intermediate or high incidence of disease,24 unlike in countries with low incidence of TB, where lymphatic TB predominates.5–7,15–17 As is reflected in our data, as the overall incidence of sputum smear-positive patients descreases there is possibly an associated increase in the proportion of EPTB cases and a change in EPTB sites.
As in others studies, smoking and alcohol habits were independent risk factors for PTB.5,22,25,26 The association between tobacco, alcohol use and TB appears to be causal, but there is not enough evidence to support an association between smoking and alcohol habits with greater severity of disease.27,28 In our patients with EPTB, smoking is lower and alcohol abuse greater than in the general population in our geographical area (35.4% and 3.8%), but similar in the distribution by gender and age groups.29 Women had a lower tendency to smoke and drink compared to men. The higher consumption of tobacco and alcohol appears in middle-aged people and then falls in patients older than 55 years, as happened during the period of our study. The higher frequency of EPTB with the increasing age of patients, female gender, as well as a lower association with tobacco and alcohol use than PTB makes us suspect that demographic changes and a reduction in use of tobacco and alcohol in the population can be contributory factors to the smaller reduction in EPTB compared to PTB. This is seen mainly in developed countries, such as our geographical area, where life expectancy and the predominance of women in the population are increasing, and the use of tobacco and alcohol is falling. On the other hand, it is possible that the increase in patients with other risk factors for developing TB (e.g. Diabetes Mellitus, CRF, neoplasia) and the increasing use of immunosuppressive therapies may modify how TB presents in the future.18.30,31
The number of our BCG vaccinated patients decreased in the last period of the study and we have found a non-significant protective effect of BCG vaccine for meningitis, due to the reduced number of cases, but its effectiveness is accepted in the prevention of miliary disease and meningitis.32 The policies of BCG vaccination and the proportion of population vaccinated differ between countries, and can be another factor that determines differences in the site of TB disease. In the Peto HM et al series,5 the 93% of children <1 year old with meningeal disease were US born, whereas in Turkey, where BCG vaccination is routinely performed, no meningitis or miliary TB occurred in vaccinated children.33
Other factors, such as the virulence of the M. tuberculosis strains, the mode of transmission of mycobacterium and the innate immunity of the host may contribute to differences in the risk of acquiring EPTB.17 The genotypes of the mycobacterium, the polymorphisms of genes determining innate host immunity and environmental factors have a very heterogeneous geographic distribution, but in our area, with a stable population, we do not believe that these variables influenced the results.34,35 We excluded patients with M. bovis cultures, because of its greater propensity to present as EPTB. This aspect is not mentioned in other studies, but it does not seem to be an important problem currently in industrialised countries.5,7,36
Within the limitations of our study we have not been able to analyse the risk factors in all diagnosed cases, but it does not appear that the risk factors that have prevented EPTB cases from declining at the same rate as TB cases were any different from cases not followed up.15,37 We could not study the effect of the amount and duration of smoking and alcohol use on EPTB, and if there is a direct relationship between the level of use and the risk of one or other site of TB. Assessment of previous BCG vaccination can be limited as it is possible that some BCG vaccinated cases did not have a cutaneous scar or that some scars were not due to the administration of the vaccine. However, these cases of erroneous interpretation of the vaccination state appear in 7%-11%,33 and we considered that there were no differences in the registered vaccination data between EPTB and PTB cases.
Another limitation is the low number of patients studied with CRF and cirrhosis, and that HIV-status data was missing or unknown in the majority of the patients. However, in areas with low rates of coinfection, screening of patients at higher risk for HIV infection could be of greater yield than the screening of all patients.38 Furthermore, the impact of HIV-status on comparisons of EPTB and PTB is expected to be small, as the percentage of missing or unknown HIV status data is similar for EPTB and PTB cases.
In conclusion, more information is needed on disease site–specific risk factors in populations not traditionally at risk for TB, as well as having a greater clinical suspicion for EPTB. Since the proportion of EPTB cases is increasing and seems to have a greater association with delayed reactivation of infection,15,39 the measures for prevention and treatment of latent TB infection in the goal of elimination of tuberculosis need to be reinforced.
Conflict of interestsThe authors declare no conflicts of interest related to this study.
The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
We would like to thank Ana Del Rio, Consultant Paediatrician, Royal Blackburn Hospital, East Lancashire, United Kingdom, for revision of the translation to English.