Administration of antiretroviral drugs to individuals exposed to, but not infected by, HIV has been shown to reduce the risk of transmission. The efficacy of pre-exposure prophylaxis (PrEP) makes it obligatory to include it in an integral program of prevention of HIV transmission, together with other measures, such as use of the condom, training, counseling, and appropriate treatment of infected individuals. In this document, the AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [SEIMC]) provides its views on this important subject. The available evidence on the usefulness of PrEP in the prevention of transmission of HIV is presented, and the components that should make up a PrEP program and whose development and implementation are feasible in Spain are set out.
Se ha demostrado que la administración de fármacos antirretrovirales a personas expuestas y no infectadas por el VIH puede reducir el riesgo de transmisión. La eficacia de la profilaxis pre-exposición obliga a considerar su inclusión en un programa integral de prevención de la transmisión del VIH, junto con otras medidas como el uso del preservativo, la formación y el consejo asistido y el tratamiento adecuado de las personas infectadas. En este documento, el Grupo de Estudio de SIDA (GeSIDA) de la SEIMC aporta su visión sobre este importante tema. Se presenta la evidencia disponible acerca de la utilidad de la PrEP en la prevención de la transmisión del VIH y se enumeran los elementos que deberían integrar un programa de PrEP, cuyo desarrollo y puesta en marcha sea factible y viable en nuestro medio.
Despite the considerable advances in control of HIV infection, the number of newly infected persons continues to grow. The male condom and other barrier methods, while clearly efficacious, have not had the desired effect for control of the epidemic; therefore, alternative approaches to preventing transmission of HIV infection are necessary.
In this context, pre-exposure prophylaxis (PrEP) has been investigated as an additional prevention strategy. Administration of antiretroviral drugs to individuals exposed to but not infected by HIV has been shown to reduce the risk of transmission. Consequently, these individuals should be included in programs for the prevention of transmission of HIV. Supporters of PrEP do not consider it the only, or even the best, preventive measure, but as a tool that should be used in conjunction with current measures. The cornerstone of the struggle against HIV infection continues to be based on use of the condom, training, counseling, and appropriate treatment of infected individuals.
The AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [SEIMC]) is committed to addressing this important subject and providing its views on it. In this document, we aim to present available evidence on the usefulness of PrEP in the prevention of transmission of HIV. Based on the data supporting this strategy, we set out the components that should make up a PrEP program and whose development and implementation are feasible in Spain. The recommendations are graded based on scientific evidence and expert opinion by a letter indicating the strength of the recommendation (A, recommended, should be followed; B, consider, applicable in most situations; C, optional) and a number indicating the source of the recommendation (I, results of randomized clinical trials, meta-analyses; II, results of nonrandomized clinical trials or cohort studies; III, expert opinion).
The HIV epidemic in Spain: state of the artNew diagnoses (incidence) of HIV infection (2009–2014)In 2014, the Spanish System for Information on New Diagnoses of HIV Infection (Sistema de Información sobre Nuevos Diagnósticos de VIH [SINIVIH]) was notified of 3366 new cases of HIV infection in Spain; of these 85% were men, and the mean age was 35 years. Men who have sex with men (MSM) accounted for 54% of all new diagnoses, heterosexual men and women for 26%, and injection drug users (IDU) for 3.4%.1 The EPIVIH study provides the soundest national estimations of incidence, which stands at 1 case per 100 person-years (95%CI, 0.9–1.1) among the 30679 first-time testers with at least one follow-up test between 2000 and 2009.2 The highest risk of infection was recorded among male commercial sex workers (3.0 per 100 person-years; 95%CI, 2.2–4.1), MSM (2.5 per 100 person-years; 95%CI, 2.3–2.7), and IDU (1.6 per 100 person-years; 95%CI, 1.1–2.2). The incidence was 0.1 cases per 100 person-years in heterosexual men and women and female commercial sex workers.
Prevalence of HIV infectionIn 2014, it was estimated that approximately 150000 people were infected with HIV, that is, a prevalence in the general population of 0.4% (95%CI, 0.4%–0.5%). Furthermore, the prevalence of occult HIV infection was estimated to be 0.1%.1 The prevalence of infection varies considerably between the different collectives who engage in risk practices. The EPIVIH study revealed a prevalence of HIV infection of 2.5% (95%CI, 2.4%–2.6%) in 145337 first-time testers during the period 2000–2009.2 By category of transmission and risk situations, the highest prevalence was recorded in transgender women (24.5%; 95%CI, 20.4%–29.0%), male sex workers (19%; 95%CI, 10.5%–24.5%), and IDU (17%; 95%CI, 13.3%–21.2%). Prevalence was 7.6% (95%CI, 7.2%–7.9%) in MSM, 0.9% in heterosexual men and women, and 0.8% (95%CI, 0.5%–1.2%) in female commercial sex workers.2
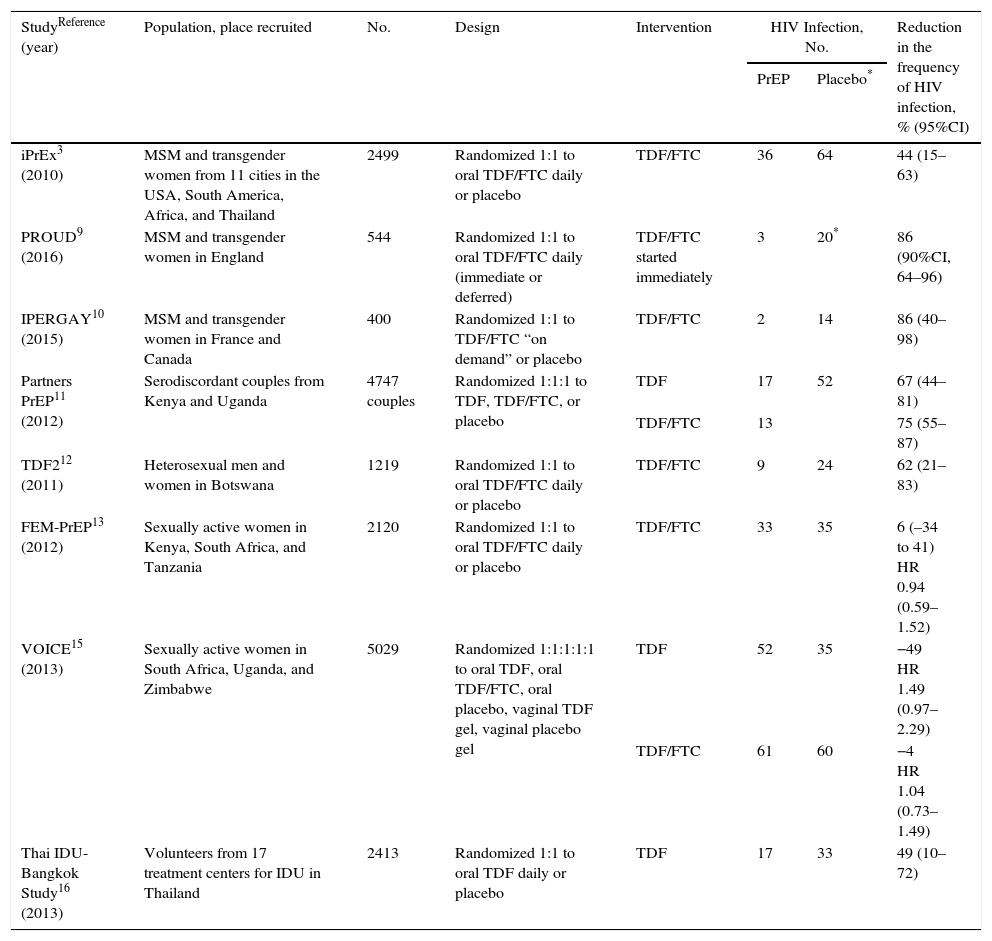
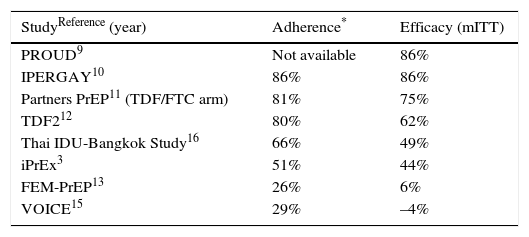
Risks and benefits of PrEPTables 1 and 2 present the results of clinical trials performed to evaluate the efficacy of PrEP. The trials were performed in different groups, including MSM (iPrEx [Iniciativa Profilaxis Pre-exposición],3–8 PROUD,9 and IPERGAY10), heterosexual men and women (Partners-PrEP,11 TDF2,12 FEM-PrEP,13,14 and VOICE [Vaginal and Oral Interventions to Control the Epidemic]15), and IDU (Bangkok Tenofovir Study).16 As can be seen in the tables, efficacy was 44%–86%, except in 2 studies on women (FEM-PrEP13,14 and VOICE15), where PrEP was not efficacious and adherence was very low. Please consult the GeSIDA document on PrEP (http://www.gesida-seimc.org/contenidos/guiasclinicas/2016/gesida-guiasclinicas-2016-profilaxis_pre-exposicionVIH.pdf) for further details on the efficacy of the trials, other follow-up and observational studies, and the risks of PrEP.
Clinical trials with oral PrEP with TDF-based regimens (with[out] FTC).
| StudyReference (year) | Population, place recruited | No. | Design | Intervention | HIV Infection, No. | Reduction in the frequency of HIV infection, % (95%CI) | |
|---|---|---|---|---|---|---|---|
| PrEP | Placebo* | ||||||
| iPrEx3 (2010) | MSM and transgender women from 11 cities in the USA, South America, Africa, and Thailand | 2499 | Randomized 1:1 to oral TDF/FTC daily or placebo | TDF/FTC | 36 | 64 | 44 (15–63) |
| PROUD9 (2016) | MSM and transgender women in England | 544 | Randomized 1:1 to oral TDF/FTC daily (immediate or deferred) | TDF/FTC started immediately | 3 | 20* | 86 (90%CI, 64–96) |
| IPERGAY10 (2015) | MSM and transgender women in France and Canada | 400 | Randomized 1:1 to TDF/FTC “on demand” or placebo | TDF/FTC | 2 | 14 | 86 (40–98) |
| Partners PrEP11 (2012) | Serodiscordant couples from Kenya and Uganda | 4747 couples | Randomized 1:1:1 to TDF, TDF/FTC, or placebo | TDF | 17 | 52 | 67 (44–81) |
| TDF/FTC | 13 | 75 (55–87) | |||||
| TDF212 (2011) | Heterosexual men and women in Botswana | 1219 | Randomized 1:1 to oral TDF/FTC daily or placebo | TDF/FTC | 9 | 24 | 62 (21–83) |
| FEM-PrEP13 (2012) | Sexually active women in Kenya, South Africa, and Tanzania | 2120 | Randomized 1:1 to oral TDF/FTC daily or placebo | TDF/FTC | 33 | 35 | 6 (–34 to 41) HR 0.94 (0.59–1.52) |
| VOICE15 (2013) | Sexually active women in South Africa, Uganda, and Zimbabwe | 5029 | Randomized 1:1:1:1:1 to oral TDF, oral TDF/FTC, oral placebo, vaginal TDF gel, vaginal placebo gel | TDF | 52 | 35 | −49 HR 1.49 (0.97–2.29) |
| TDF/FTC | 61 | 60 | −4 HR 1.04 (0.73–1.49) | ||||
| Thai IDU-Bangkok Study16 (2013) | Volunteers from 17 treatment centers for IDU in Thailand | 2413 | Randomized 1:1 to oral TDF daily or placebo | TDF | 17 | 33 | 49 (10–72) |
Association between adherence (determined by detection of adequate levels of tenofovir in blood) and efficacy of PrEP based on regimens with oral TDF/FTC.
| StudyReference (year) | Adherence* | Efficacy (mITT) |
|---|---|---|
| PROUD9 | Not available | 86% |
| IPERGAY10 | 86% | 86% |
| Partners PrEP11 (TDF/FTC arm) | 81% | 75% |
| TDF212 | 80% | 62% |
| Thai IDU-Bangkok Study16 | 66% | 49% |
| iPrEx3 | 51% | 44% |
| FEM-PrEP13 | 26% | 6% |
| VOICE15 | 29% | –4% |
mITT: modified intention to treat.
- 1.
Not all persons who engage in risk practices are candidates for PrEP. Some bodies recommend PrEP in groups with an annual risk of infection above a specific threshold. The International AIDS Society sets the threshold at an incidence of 2 cases per 100 person-years,17 and the World Health Organization sets it at 3 cases per 100 person-years.18
- 2.
The safety and efficacy profile of PrEP has not been established for all age groups. To date, clinical trials on PrEP have been performed with persons aged ≥18 years, with no solid evidence on the efficacy of this approach in younger patients or in patients aged ≥50 years.3,9–13,16
- 3.
Adherence to PrEP is key to its efficacy. Verification of the patient's willingness to adhere appropriately to recommendations is justified before prescribing PrEP.
PrEP should be evaluated in individuals who request or agree to the intervention and who fulfill the following criteria:
- 1.
HIV infection must be ruled out. In cases where it is not clear whether or not the patient is infected, PrEP should not be recommended until HIV infection can reasonably ruled out.
- 2.
The patient should be prepared to adhere to recommendations and undergo follow-up.
- 3.
No clinical or laboratory contraindications for TDF or emtricitabine (FTC).
- 4.
Inclusion in one of the target populations for PrEP
- A.
PrEP should be recommended to people with a high risk of HIV infection. A high risk is understood to be inclusion in a group in which the risk is greater than 2 cases per 100 person-years (AI):
- •
MSM and transgender women who have had sexual relations without a condom during the previous 6 months and 1 of the following:
- ∘
Sexual relations with more than 2 partners
- ∘
Diagnosis of ≥1 sexually transmitted infection (STI)
- ∘
Administration of postexposure prophylaxis
- ∘
Use of psychoactive substances during sexual relations
- ∘
- •
- B.
PrEP should be considered in individuals with a high risk or in whom there is some evidence of a benefit, as follows:
- •
Individuals whose partner(s) is infected with HIV and is not undergoing clinical or virological follow-up and who do not use a condom (BI)
- •
Individuals who engage in unprotected and transactional sexual relations (money, drugs, accommodation) (BIII)
- •
IDU who share syringes (BI)
- •
Individuals in situations of social vulnerability who are exposed to unprotected sexual contact with a high risk of HIV infection (CIII)
- •
- A.
PrEP is a medical intervention that should be prescribed and monitored by a physician. The physician must have experience in the care of HIV-infected patients and use of antiretroviral drugs and be an expert in STIs19(AIII).
Where should PrEP be prescribed?- 1.
Centers where PrEP is prescribed should fulfill a series of conditions to ensure that they can appropriately implement PrEP programs: presence of an experienced physician (see above), capacity to rule out HIV and other STIs during the initial workup and during follow-up, and capacity to perform the necessary examinations to evaluate drug toxicity and resistance in the case of infection.
- 2.
The medication used for PrEP can only be dispensed in a hospital; therefore, only hospital units that provide care for HIV-infected patients will be able to prescribe it. It is unlikely that all hospital units have sufficient resources or that all potential users will be able to attend a hospital. Therefore, additional care structures for prescription and dispensing will be necessary.
- 3.
Centers where PrEP is prescribed must follow uniform criteria for correct prescription and follow-up. In this sense, centers intending to provide the service should be evaluated and accredited.
- 1.
PrEP programs should be developed in centers (either in a single center or between several centers) that guarantee appropriate fulfillment of all phases of the strategy, including the initial evaluation and follow-up, as well as dispensing. All centers must have the following (AIII):
- -
Physician with experience in the management of HIV infection, antiretroviral drugs, and STIs
- -
Standardized clinical histories
- -
Pharmacy service that stores, supervises, and dispenses medication and provides information on correct follow-up of the regimen prescribed
- -
A laboratory to diagnose HIV infection, measure viral load, and study resistance
- -
A laboratory for evaluation of blood parameters and biochemistry (necessary for follow-up of drug toxicity)
- -
Capacity to evaluate the presence and diagnosis of STIs
- -
Capacity to provide counseling on adherence and sexual health
- -
- 2.
Centers participating in PrEP programs are varied and can adapt to various situations. They include hospital HIV infection units, centers providing care for STIs, sexual and reproductive health centers, harm reduction centers for IDU, and community centers. Specific centers for the development of this strategy could be created for as long as they are necessary (AIII).
- 3.
Protocols or methods should be defined to enable easy and effective access to medication for individuals who have been prescribed PrEP in a nonhospital center (AIII).
- 1.
Daily regimens (preferred option). Daily administration of a tablet combining TDF 300mg and FTC 200mg. Monotherapy with TDF can be prescribed in exceptional cases of intolerance or FTC-induced toxicity11,16(AI).
- 2.
Intermittent regimen (alternative option). The combination of TDF/FTC should be prescribed according to the following regimen: 2 tablets taken together between 24 and 2h before exposure, 1 tablet taken 24h after the first dose, another tablet taken daily for as long as the patient is exposed to risk practices, and a tablet taken 24h after the most recent sexual relationship. This regimen is not indicated if follow-up requires more than 7 tablets per week. The efficacy of this dosing regimen has only been demonstrated in MSM and cannot therefore be recommended in other clinical situations (AI).
- 1.
Clinical evaluation. A complete clinical history should be taken. This will include substance use and addictions, a sexual behavior questionnaire, personal history (specifically STIs), and concomitant medication. The criteria for receiving PrEP should be stressed.
- 2.
Laboratory evaluation. PrEP includes potentially nephrotoxic drugs that are also active against hepatitis B virus (HBV). Before prescription, the laboratory evaluation should fulfill 3 objectives18,20:
- a.
It is necessary to rule out pre-existing HIV infection. Signs and symptoms of acute infection should be evaluated and HIV serology testing ordered. In the case of a clinical picture compatible with acute retroviral syndrome or a well-founded suspicion of recent infection and negative serology results, HIV viral load should be assessed and the initiation of PrEP postponed until infection has been ruled out.21
- b.
HBV serology should be evaluated. Chronic HBV infection does not contraindicate PrEP, although it is important to know the patient's serological status. If chronic HBV infection is not detected, susceptible persons should be vaccinated against this infection.
- c.
Renal function should be evaluated. Since TDF can alter renal function, renal insufficiency should be ruled out before prescribing PrEP. PrEP with TDF/FTC is contraindicated if the eGFR is <60mL/min.
- a.
- 3.
Evaluation of STIs. Previous clinical evaluation of PrEP should include complete screening for STIs including hepatitis C infection, even if symptoms are not present.
The objective is to provide integral care for individuals who are going to receive PrEP. This includes the following:
- -
Complete blood count
- -
Serology testing for hepatitis A virus
- -
Pregnancy should be ruled out and the woman questioned about her desire to become pregnant
- -
Evaluation of the patient's commitment to taking PrEP correctly and insistence on the importance of adherence, so that PrEP is successful. Information on possible associated adverse effects18,20
Individuals who are going to receive PrEP should receive clear information on key aspects of this approach:
- -
PrEP is an intervention designed to prevent HIV infection. It should be placed in context with other preventive interventions, such as use of condoms
- -
PrEP does not protect against other STIs
- -
PrEP does not work if adherence is poor and could lead to toxicity if it is not monitored appropriately
Patients should also be counseled on sexual health and adherence should be reinforced. Once it has been decided that PrEP is indicated, and before initiation, the patient should be informed about the visits and analyses that are necessary when taking PrEP.
During follow-upVisit at 2–4 weeksObjective To confirm that the user is not HIV-infected, that he/she fulfills the criteria for PrEP, and that there are no limitations with respect to prescription of PrEP.
Steps:
- •
Evaluation of baseline laboratory results
- •
Counseling
- •
New HIV test in the case of recent infection
- •
Prescription of medication
- •
Arrange an appointment at 4–8 weeks, with a request for a laboratory workup
- •
Vaccination against hepatitis C infection, if necessary
Objective evaluate tolerance, toxicity, and adherence.
Steps
- •
Evaluation of adverse effects, consumption of other medications, and use of recreational substances and drugs
- •
Evaluation of adherence
- •
Arrange appointment at 3 months, with request for laboratory workup
Objective: Evaluate tolerance, toxicity, adherence, HIV infection, and other STIs
Steps
- •
Evaluation of adverse effects, consumption of other medications, and use of recreational drugs or substances
- •
Evaluation of adherence
- •
Analysis with evaluation of renal function
- •
Serology testing for HIV/qualitative PCR assay for HIV
- •
Rule out other STIs
- •
Pregnancy test
- •
Counseling
- •
Prescription of medication
- •
Arrange appointment at 3 months, with request for laboratory workup
PrEP is a promising approach for preventing sexual transmission of HIV infection in women of childbearing age, whether pregnant or not, in areas with a high prevalence of HIV infection.22,23 Acute HIV infection during pregnancy and postpartum in mothers who breastfeed increases the risk of vertical transmission.24,25
Exposure to TDF or TDF/FTC as part of antiretroviral therapy in pregnant women and their newborns has not been associated with malformations or other relevant defects in exposed newborns.26–28
Current therapy guidelines recommend initiating antiretroviral therapy in all HIV-infected individuals. In the case of serodiscordant couples, treatment of the infected person is recommended. Based on available information, unprotected sexual relations at the time of conception when the infected person is receiving antiretroviral therapy and has an undetectable viral load are reasonably safe.29 Therefore, in Spain, PrEP offers no clear advantage to noninfected women whose infected partner is receiving antiretroviral therapy and has a suppressed viral load.
Recommendations- •
PrEP is not recommended in women wishing to become pregnant, regardless of whether they are the partner of an HIV-infected man receiving ART with a viral load that has been suppressed for at least 6 months (BI).
- •
If PrEP is indicated in a pregnant woman or if the woman becomes pregnant during PrEP, she should be informed about the risks and benefits for mother and fetus. The woman should decide how to proceed (AIII).
In the case of HBV-infected individuals for whom PrEP is indicated, TDF or TDF/FTC should be administered initially according to the indications and regimens established for treatment of HBV infection.30 Given that treatment of HIV-infected patients with entecavir can lead to resistance to lamivudine or FTC,31 it should not be started in candidates for PrEP with chronic HBV infection.
In patients with chronic HBV infection for whom treatment of HBV infection is not indicated, PrEP can be considered. In these patients, HBV infection should be monitored specifically according to established guidelines.30
Discontinuing TDF in patients with chronic HBV infection can lead to increased replication of HBV, which, in turn, may be accompanied by severe clinical manifestations.32
Recommendations- •
In patients with chronic HBV infection who are candidates for PrEP, the indication for treatment of HBV infection should be evaluated and, ideally, TDF/FTC should be administered (AI).
- •
Patients with chronic HBV infection for whom treatment is not indicated can start PrEP. HBV infection should continue to be monitored, especially if PrEP is suspended (AIII).
- •
Regimens based on intermittent PrEP should not be administered to patients with chronic HBV infection (AIII).
Chronic HCV infection does not contraindicate administration of PrEP, although the possibility of interactions between PrEP and the medication used to treat HCV infection should be taken into account. At present, the only significant interaction is the increase in TDF levels in patients who simultaneously receive sofosbuvir and ledipasvir, which can increase the risk of TDF-induced nephrotoxicity in these patients.
Recommendation- •
Chronic HCV infection does not contraindicate administration of PrEP (AIII).
Diagnosis and treatment of STIs do not contraindicate administration of PrEP. Its presence is a criterion for the administration of PrEP. Acute HIV infection and infection by Neisseria gonorrhoeae, Chlamydia trachomatis, and Treponema pallidum can progress without symptoms; therefore, when a patient is diagnosed with an STI, acute HIV infection and other STIs should be ruled out.
Recommendations- •
The presence of an STI does not contraindicate administration of PrEP (AIII).
- •
In the case of a diagnosis of an STI, acute HIV infection and the presence of other STIs should be ruled out (AIII).
In HIV-infected patients, continued long-term therapy with TDF can lead to renal toxicity, which usually manifests as proximal tubular dysfunction and, less frequently, as reduced glomerular filtration rate.33
Clinical trials with PrEP performed to date have not revealed differences in the onset of renal adverse effects between individuals who received TDF-based PrEP and those who received placebo; however, follow-up of participants was limited in time and in some studies, a significant—but not clinically relevant—decrease in estimated glomerular filtration rate was observed in participants who received TDF.6,10,15,34
No evidence is currently available on TDF-based PrEP in individuals with eGFR <60ml/min.
Recommendation- •
TDF-based PrEP should not be administered to individuals with eGFR <60ml/min (AI).
In 2015, GeSIDA published a consensus document that addressed the use of nonoccupational postexposure prophylaxis in adults and children.35 The document reviewed evidence supporting the use of postexposure prophylaxis and provided clear guidelines on whether it should be started or not. When initiation of postexposure prophylaxis is being evaluated, it is important to take into account the possible indication of PrEP as part of an integral approach for the prevention of STIs.
Recommendation- •
The indication for PrEP should be evaluated in individuals who are evaluated for postexposure prophylaxis (AIII).
HIV infection should be ruled out before starting PrEP. Although the efficacy of PrEP is very high, it is not 100% safe; therefore, HIV infection must be ruled out every 3 months at follow-up visits. A clinical history aimed at recording symptoms and signs of acute retroviral syndrome must be taken, and HIV serology testing with fourth-generation ELISA must be performed.21
If HIV infection is detected, the patient must be sent to specialist centers for evaluation of initiation of treatment and follow-up.
Recommendation- •
Individuals who receive PrEP must be evaluated every 3 months to check for signs and symptoms of acute HIV infection and to rule out infection using serology testing/PCR (AI).
Our knowledge of PrEP is limited by our scant experience of long-term administration. The follow-up period in studies is usually around 1 year.3,9,10,12 Implementation of long-term PrEP and PrEP in larger groups could provide information on long-term toxicity. Data from these cohorts would no doubt affect the definition and limits of PrEP.
At present, PrEP should be discontinued in the following cases:
- 1.
HIV infection
- 2.
HBV infection requiring treatment
- 3.
Patient withdrawal from follow-up
- 4.
Toxicity:
- a.
Renal toxicity
- b.
Bone toxicity
- c.
Any other type of toxicity
- a.
- •
PrEP should be interrupted in any of the following cases:
- -
Patient withdrawal from follow-up (A-III)
- -
HIV infection (A-I)
- -
Onset of limiting toxicity (A-II)
- -
Poor adherence (A-III)
- -
There has been no funding from private institutions for the preparation of this document.