Higher serum melatonin levels have previously been found in patients with severe sepsis who died within 30 days of diagnosis than in survivors. The objective of our study were to determine whether serum melatonin levels during the first seven days of severe sepsis diagnosis could be associated with sepsis severity and mortality.
MethodsMulticentre study in eight Spanish Intensive Care Units which enrolled 308 patients with severe sepsis. We determined serum levels of melatonin, malondialdehyde (as biomarker of lipid peroxidation) and tumor necrosis factor-alpha at days 1, 4 and 8 of severe sepsis diagnosis. The study's primary endpoint was 30-day mortality.
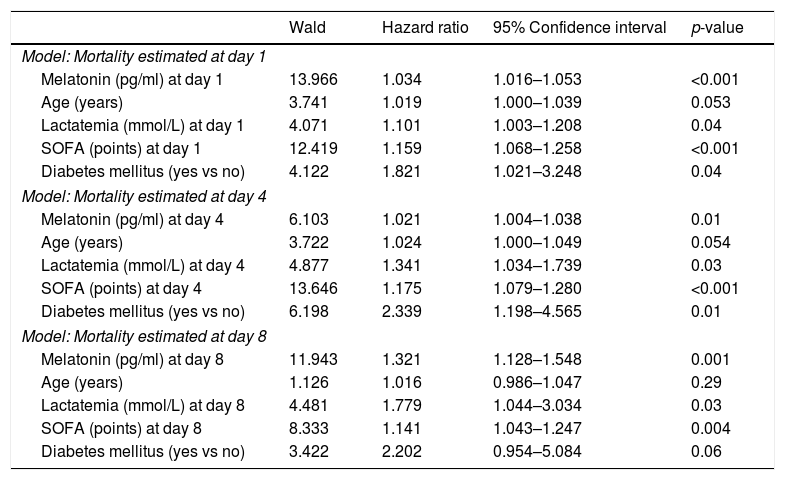
ResultsA total of 103 patients had died and 205 survived at 30 days of severe sepsis diagnosis, with the non-survivors presenting higher serum melatonin levels at days 1 (p<0.001), 4 (p<0.001) and 8 (p<0.001) of severe sepsis diagnosis than the survivor patient group. The multiple logistic regression analysis found that serum melatonin levels at days 1, 4 and 8 of severe sepsis diagnosis (p<0.001, p=0.01 and p=0.001, respectively) were associated with mortality adjusted for age, serum lactic acid, SOFA score and diabetes mellitus.
ConclusionsThe novel and more interesting findings of our study were that serum melatonin levels during the first seven days of severe sepsis diagnosis are associated with sepsis severity and mortality.
Previamente se han encontrado mayores niveles séricos de melatonina en pacientes con sepsis grave que fallecían en los primeros 30 días del diagnóstico de la sepsis grave en comparación con los supervivientes. Los objetivos de nuestro estudio fueron determinar si los niveles séricos de melatonina durante la primera semana del diagnóstico de la sepsis grave están asociados con la gravedad y mortalidad de la sepsis.
MétodosEstudio multicéntrico en 8 Unidades de Cuidados Intensivos españolas con 308 pacientes con sepsis grave. Se determinaron niveles séricos de melatonina, malondialdehído (como biomarcador de peroxidación lipídica) y factor de necrosis tumoral-alfa en los días 1, 4 y 8 del diagnóstico de la sepsis grave. Consideramos la mortalidad a 30 días como la variable resultado principal del estudio.
ResultadosUn total de 103 pacientes estaban fallecidos y 205 vivos a los 30 días del diagnóstico de la sepsis grave, y los fallecidos presentaron superiores niveles séricos de melatonina en los días 1 (p<0.001), 4 (p<0.001), y 8 (p<0.001) del diagnóstico de la sepsis grave que los supervivientes. En el análisis de regresión logística múltiple encontramos que los niveles séricos de melatonina en los días 1, 4 y 8 del diagnóstico de la sepsis grave (p<0.001, p=0.01 and p=0.001, respectively) estaban asociados con la mortalidad controlando por la edad, niveles séricos de ácido lactico, SOFA score y diabetes mellitus.
ConclusionesLos nuevos y más interesantes hallazgos de nuestro estudio son que los niveles séricos de melatonina durante la primera semana del diagnóstico de la sepsis grave están asociados con la gravedad y la mortalidad de la sepsis.
Melatonin is synthesized by the pineal gland (with a circadian rhythm with low values during the day and high values during the night); and also by other organs without circadian rhythm as thymus, retina, bone marrow, gastrointestinal tract, and lymphocytes.1
Melatonin has different effects as sleep regulation,2 and also antioxidant and anti-inflammatory effects, and preservation of mitochondrial function.3–11 Melatonin produces the upregulation of several antioxidant enzymes and is a potent scavenger of reactive oxygen species (ROS). Besides melatonin increases antiinflammatory cytokines as IL-10, and reduces proinflammatory cytokines as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8. Finally, the antioxidant effects of melatonin also protects to mitochondrial respiratory enzyme complex and to mitochondrial deoxyribonucleic acid (mtDNA) from the damage by ROS.
Previously, there have been found higher serum melatonin levels in non-survivor than in survivor septic patients when severe sepsis was diagnosed, in pediatric12 and adult populations.13 In addition, we found in our previous study with 201 severe septic adult patients that the determination of serum melatonin levels, when severe sepsis was diagnosed, were associated with sepsis severity (assessed by SOFA and lactic acid) and with 30-day mortality.13 The objectives of our current study were to determine whether serum melatonin levels during the first 7 days of severe sepsis diagnosis could be associated with sepsis severity and mortality.
MethodsDesign and subjectsA prospective, multicenter, observational study was carried out with 308 severe septic patients in Intensive Care Units from 8 Spanish hospitals: San Jorge (Huesca), Universitario Dr. Negrín (Las Palmas de Gran Canaria), Quirón Tenerife (Santa Cruz de Tenerife), Clínico Universitario de Valencia (Valencia), General de La Palma (La Palma), Insular (Las Palmas de Gran Canaria), Universitario de Canarias (La Laguna, Tenerife), Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife). The study was approved by the Institutional Ethic Review Boards of the 8 hospitals. Patients or family members signed the informed consent to participate in the study. The inclusion patient period was between 2008 and 2009.
Severe septic patients according to International Sepsis Definitions Conference criteria were included in the study.14 Criteria for patients exclusion were immunosuppressive or radiation or steroid therapy, white blood cell count <1000/μl, human immunodeficiency virus (HIV), solid or hematological tumor, pregnancy, lactation, age <18 years.
Some of those patients were included in previous publications by our team to explore other biomarkers in severe septic patients.15–17 Previously, we determined serum melatonin levels in 201 severe septic adult patients when severe sepsis was diagnosed13; and in the current work were determined serum melatonin levels in 308 severe septic adult patients during the first 7 days of severe sepsis diagnosis.
We considered 30-day mortality as the end-point study. In addition, we recorded the following variables from the patients when severe sepsis was diagnosed: age, sex, bloodstream infection, chronic renal failure (defined as glomerular filtration rate lower than 60ml/min per 1.73m2), chronic obstructive pulmonary disease (COPD), diabetes mellitus, ischemic heart disease, sex, site of infection, microorganism responsible, empiric antimicrobial treatment, Acute Physiology and Chronic Health Evaluation II (APACHE II) score,18 activated partial thromboplastin time (aPTT), bilirubin, creatinine, international normalized ratio (INR), lactatemia, leukocytes, pressure of arterial oxygen/fraction inspired of oxygen (PaO2/FIO2), platelets, Sepsis-related Organ Failure Assessment [SOFA] score.19 Empiric antimicrobial therapy used before knowing the culture results was considered as adequate if the microorganism responsible of sepsis was susceptible at least to one antimicrobial agent used.
Determination of serum concentrations of melatonin, malondialdehyde, and tumor necrosis factor (TNF)-alphaSerum samples were collected and stored at −80¿C. from patients on day 1, 4 and 8 of severe sepsis diagnosis to determine serum concentrations of melatonin, MDA, and TNF-alpha. Blood samples on day 1 was obtained when the diagnosis of severe sepsis was made (thus, in some patients before to ICU admission), and on day 4 and 8 were obtained approximately at 12am.
Serum melatonin was determined by ELISA method using a kit from Immuno Biological Laboratories (IBL Hamburg GmbH, Hamburg, Germany). The detection limit of this assay was 0.13pg/ml; and the intra and inter-assay coefficients of variation (CV) were 6.4% and 11.1%, respectively.
Malondialdehyde is an end-product formed during this lipid peroxidation of cellular membrane phospholipids, which is released into extracellular space and after entry in the blood.20,21 We determined serum malondialdehyde concentrations by thiobarbituric acid-reactive substance (TBARS) assay of to Kikugawa et al.22 The detection limit of this assay was 0.079nmol/mL; and the intra- and inter-assay CV were 1.82% and 4.01%, respectively. Serum melatonin and malondialdehyde determinations were performed in the Physiology Department of the Faculty of Medicine, University of La Laguna (Tenerife, Spain).
Serum concentrations of TNF-alpha were measured by solid-phase chemiluminescent immunometric assays using Immulite® (Siemens Healthcare Diagnostics Products, Llanberis, United Kingdom) in the Laboratory Department of Hospital Universitario de Canarias (La Laguna, Tenerife, Spain). The detection limit of this assay was 1.7pg/mL; and the intra- and inter-assay CV were 3.6% and 6.5%, respectively.
Statistical methodsMedian and interquartile ranges were used to report continuous variables, and were compared between survivor and non-survivor groups by Mann–Whitney U test. Frequencies and percentages were used to report categorical variables, and were compared between survivor and non-survivor groups by with chi-square test. Receiver operation characteristic (ROC) curves using serum melatonin levels, lactatemia, and SOFA score as independent variables, and mortality at 30 days as dependent variable were obtained. Were carried out multiple logistic regression analyses to assess the association between serum melatonin level at days 1, 4 and 8 of severe sepsis diagnosis and 30-day mortality controlling for age, lactatemia, SOFA score and diabetes mellitus; and hazard ratio and 95% confidence intervals were calculated for each variable. We used those variables in multiple logistic regression analyses because those variables showed statistically significant differences in the univariate comparison analysis between survivor and non-survivor patients. We performed a Kaplan–Meier survival analysis introducing serum melatonin levels lower or higher than 11.0pg/mL, and survival at 30 days. Youden J index was used for the selection of 11.0pg/mL as the optimal prognostic cut-off value of serum melatonin level. We determined the association between serum levels of melatonin, acid lactic, malondialdehyde and TNF-alpha, and SOFA score at days 1, 4 and 8 of severe sepsis diagnosis by Spearman's rank correlation coefficient; and Bonferroni correction for multiple comparisons was used. For the statistical analyses, we used the programs NCSS 2000 (Kaysville, Utah) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
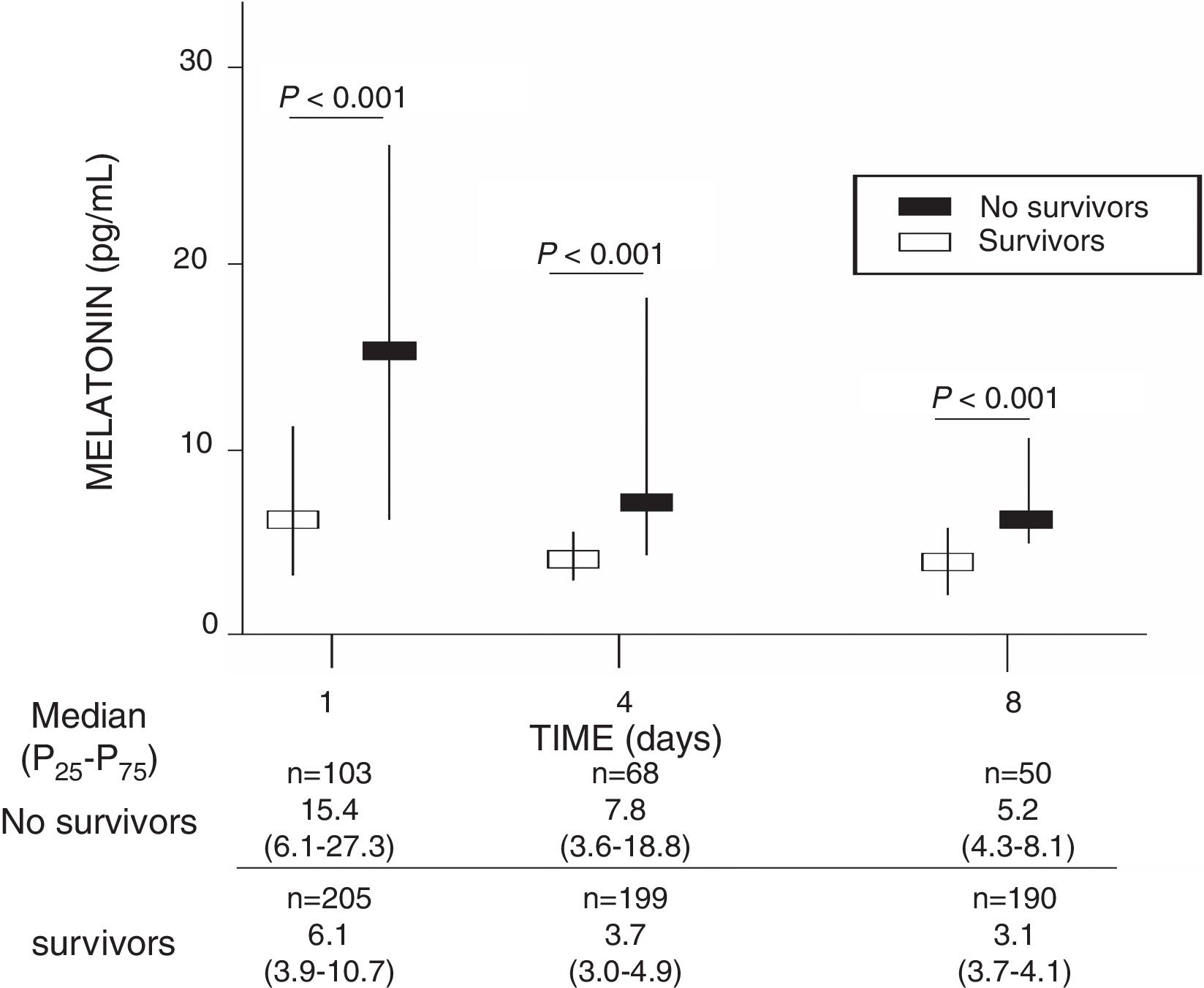
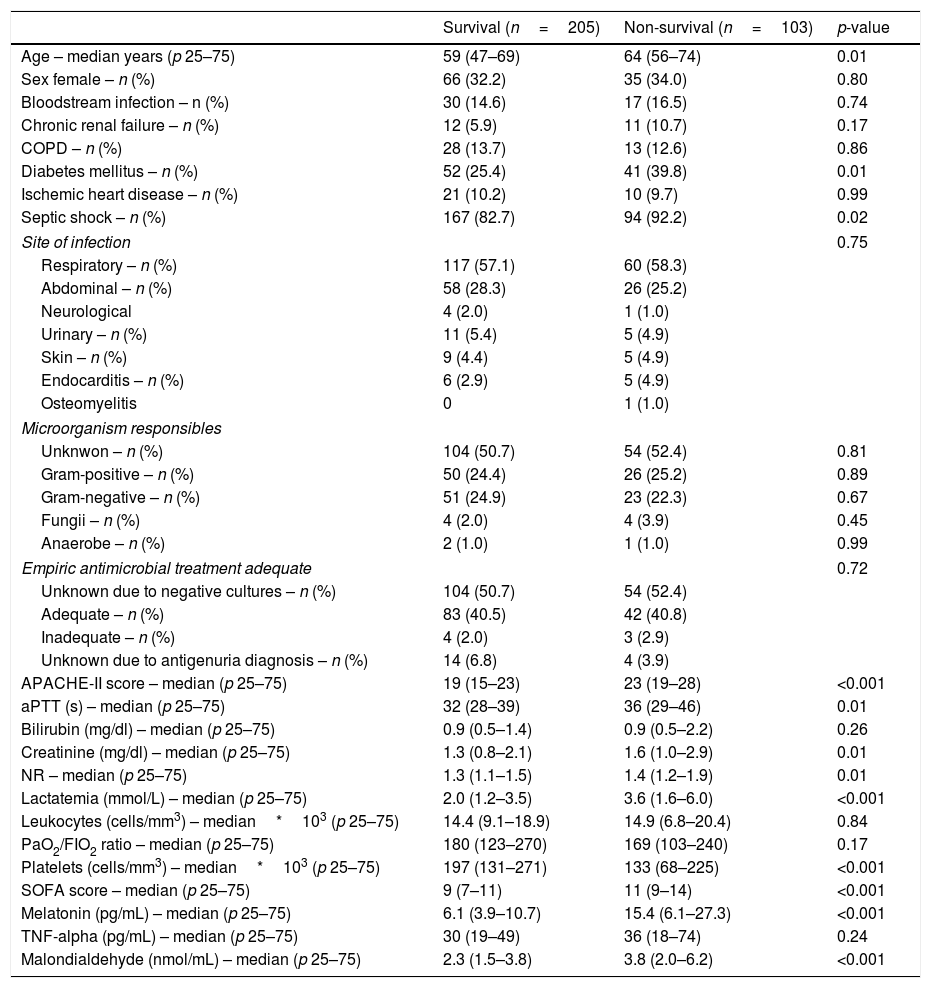
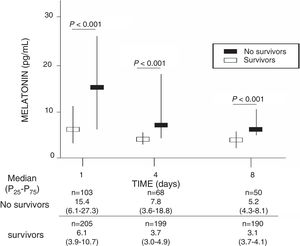
ResultsA total of 103 patients were dead and 205 live at 30 days of severe sepsis diagnosis. We found higher age, higher rate of diabetes mellitus, higher serum levels of melatonin, malondialdehyde, creatinine, and lactatemia, and higher INR, aPTT, SOFA and APACHE-II scores, and lower platelet count in non-survivor patient group than in survivor one when severe sepsis was diagnosed (Table 1). However, we did not found statistically significant differences between both patient groups on bilirubin, leukocytes, PaO2/FIO2, bloodstream infection, chronic renal failure, COPD, ischemic heart disease, sex, site of infection, microorganism responsible, and empiric antimicrobial treatment. In addition, we found that non-survivor showed higher serum melatonin levels at days 1 (p<0.001), 4 (p<0.001), and 8 (p<0.001) of severe sepsis diagnosis than survivor patient group (Fig. 1).
Comparisons between non-survivor and survivor severe septic patients on demographic and clinical characteristics at moment of severe sepsis diagnosis.
| Survival (n=205) | Non-survival (n=103) | p-value | |
|---|---|---|---|
| Age – median years (p 25–75) | 59 (47–69) | 64 (56–74) | 0.01 |
| Sex female – n (%) | 66 (32.2) | 35 (34.0) | 0.80 |
| Bloodstream infection – n (%) | 30 (14.6) | 17 (16.5) | 0.74 |
| Chronic renal failure – n (%) | 12 (5.9) | 11 (10.7) | 0.17 |
| COPD – n (%) | 28 (13.7) | 13 (12.6) | 0.86 |
| Diabetes mellitus – n (%) | 52 (25.4) | 41 (39.8) | 0.01 |
| Ischemic heart disease – n (%) | 21 (10.2) | 10 (9.7) | 0.99 |
| Septic shock – n (%) | 167 (82.7) | 94 (92.2) | 0.02 |
| Site of infection | 0.75 | ||
| Respiratory – n (%) | 117 (57.1) | 60 (58.3) | |
| Abdominal – n (%) | 58 (28.3) | 26 (25.2) | |
| Neurological | 4 (2.0) | 1 (1.0) | |
| Urinary – n (%) | 11 (5.4) | 5 (4.9) | |
| Skin – n (%) | 9 (4.4) | 5 (4.9) | |
| Endocarditis – n (%) | 6 (2.9) | 5 (4.9) | |
| Osteomyelitis | 0 | 1 (1.0) | |
| Microorganism responsibles | |||
| Unknwon – n (%) | 104 (50.7) | 54 (52.4) | 0.81 |
| Gram-positive – n (%) | 50 (24.4) | 26 (25.2) | 0.89 |
| Gram-negative – n (%) | 51 (24.9) | 23 (22.3) | 0.67 |
| Fungii – n (%) | 4 (2.0) | 4 (3.9) | 0.45 |
| Anaerobe – n (%) | 2 (1.0) | 1 (1.0) | 0.99 |
| Empiric antimicrobial treatment adequate | 0.72 | ||
| Unknown due to negative cultures – n (%) | 104 (50.7) | 54 (52.4) | |
| Adequate – n (%) | 83 (40.5) | 42 (40.8) | |
| Inadequate – n (%) | 4 (2.0) | 3 (2.9) | |
| Unknown due to antigenuria diagnosis – n (%) | 14 (6.8) | 4 (3.9) | |
| APACHE-II score – median (p 25–75) | 19 (15–23) | 23 (19–28) | <0.001 |
| aPTT (s) – median (p 25–75) | 32 (28–39) | 36 (29–46) | 0.01 |
| Bilirubin (mg/dl) – median (p 25–75) | 0.9 (0.5–1.4) | 0.9 (0.5–2.2) | 0.26 |
| Creatinine (mg/dl) – median (p 25–75) | 1.3 (0.8–2.1) | 1.6 (1.0–2.9) | 0.01 |
| NR – median (p 25–75) | 1.3 (1.1–1.5) | 1.4 (1.2–1.9) | 0.01 |
| Lactatemia (mmol/L) – median (p 25–75) | 2.0 (1.2–3.5) | 3.6 (1.6–6.0) | <0.001 |
| Leukocytes (cells/mm3) – median*103 (p 25–75) | 14.4 (9.1–18.9) | 14.9 (6.8–20.4) | 0.84 |
| PaO2/FIO2 ratio – median (p 25–75) | 180 (123–270) | 169 (103–240) | 0.17 |
| Platelets (cells/mm3) – median*103 (p 25–75) | 197 (131–271) | 133 (68–225) | <0.001 |
| SOFA score – median (p 25–75) | 9 (7–11) | 11 (9–14) | <0.001 |
| Melatonin (pg/mL) – median (p 25–75) | 6.1 (3.9–10.7) | 15.4 (6.1–27.3) | <0.001 |
| TNF-alpha (pg/mL) – median (p 25–75) | 30 (19–49) | 36 (18–74) | 0.24 |
| Malondialdehyde (nmol/mL) – median (p 25–75) | 2.3 (1.5–3.8) | 3.8 (2.0–6.2) | <0.001 |
APACHE=Acute Physiology and Chronic Health Evaluation; aPTT=Activated partial thromboplastin time; INR=International normalized ratio; PaO2/FIO2=pressure of arterial oxygen/fraction inspired oxygen; SOFA=Sepsis-related Organ Failure Assessment; COPD=Chronic Obstructive Pulmonary Disease; TNF=tumor necrosis factor (TNF)-α; data are presented as number (percentage) or median (interquartile range).
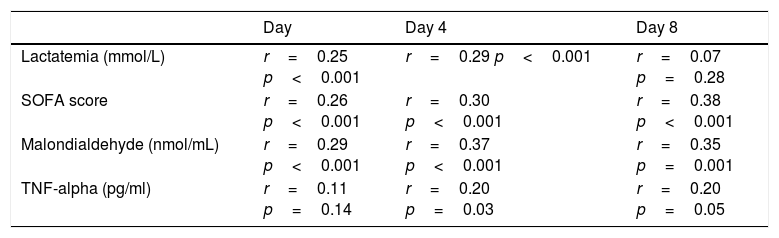
We found, after Bonferroni correction for multiple comparisons, a positive correlation between serum melatonin levels, serum malondialdehyde levels and SOFA score at days 1, 4 and 8 of severe sepsis diagnosis (Table 2). Also, we found a positive correlation between serum levels of melatonin and acid lactic at days 1 and 4 of severe sepsis diagnosis.
Correlations between melatonin, SOFA, lactatemia, TNF-alpha and IL-10 during the first week of severe sepsis.
| Day | Day 4 | Day 8 | |
|---|---|---|---|
| Lactatemia (mmol/L) | r=0.25 p<0.001 | r=0.29 p<0.001 | r=0.07 p=0.28 |
| SOFA score | r=0.26 p<0.001 | r=0.30 p<0.001 | r=0.38 p<0.001 |
| Malondialdehyde (nmol/mL) | r=0.29 p<0.001 | r=0.37 p<0.001 | r=0.35 p=0.001 |
| TNF-alpha (pg/ml) | r=0.11 p=0.14 | r=0.20 p=0.03 | r=0.20 p=0.05 |
SOFA=Sepsis-related Organ Failure Assessment score; TNF=tumor necrosis factor. After Bonferroni correction only p<0.003 are statistically significant.
We found in multiple logistic regression analyses that serum melatonin levels at days 1, 4 and 8 of severe sepsis diagnosis (p<0.001, p=0.01 and p=0.001, respectively) were associated with mortality controlling for age, lactatemia, SOFA score and diabetes mellitus (Table 3).
Multiple logistic regression analyses to predict mortality at 30 days.
| Wald | Hazard ratio | 95% Confidence interval | p-value | |
|---|---|---|---|---|
| Model: Mortality estimated at day 1 | ||||
| Melatonin (pg/ml) at day 1 | 13.966 | 1.034 | 1.016–1.053 | <0.001 |
| Age (years) | 3.741 | 1.019 | 1.000–1.039 | 0.053 |
| Lactatemia (mmol/L) at day 1 | 4.071 | 1.101 | 1.003–1.208 | 0.04 |
| SOFA (points) at day 1 | 12.419 | 1.159 | 1.068–1.258 | <0.001 |
| Diabetes mellitus (yes vs no) | 4.122 | 1.821 | 1.021–3.248 | 0.04 |
| Model: Mortality estimated at day 4 | ||||
| Melatonin (pg/ml) at day 4 | 6.103 | 1.021 | 1.004–1.038 | 0.01 |
| Age (years) | 3.722 | 1.024 | 1.000–1.049 | 0.054 |
| Lactatemia (mmol/L) at day 4 | 4.877 | 1.341 | 1.034–1.739 | 0.03 |
| SOFA (points) at day 4 | 13.646 | 1.175 | 1.079–1.280 | <0.001 |
| Diabetes mellitus (yes vs no) | 6.198 | 2.339 | 1.198–4.565 | 0.01 |
| Model: Mortality estimated at day 8 | ||||
| Melatonin (pg/ml) at day 8 | 11.943 | 1.321 | 1.128–1.548 | 0.001 |
| Age (years) | 1.126 | 1.016 | 0.986–1.047 | 0.29 |
| Lactatemia (mmol/L) at day 8 | 4.481 | 1.779 | 1.044–3.034 | 0.03 |
| SOFA (points) at day 8 | 8.333 | 1.141 | 1.043–1.247 | 0.004 |
| Diabetes mellitus (yes vs no) | 3.422 | 2.202 | 0.954–5.084 | 0.06 |
SOFA=Sepsis-related Organ Failure Assessment score.
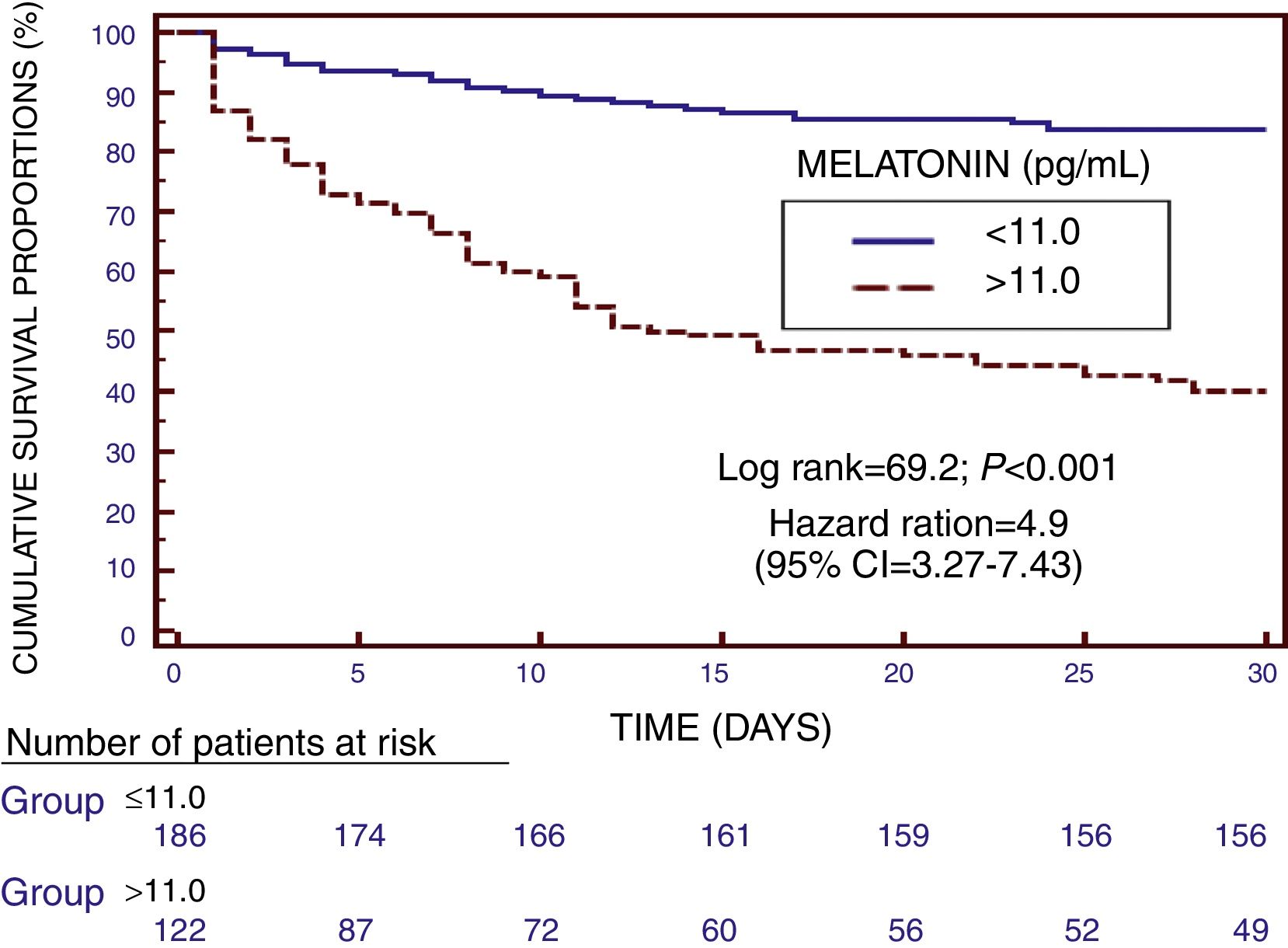
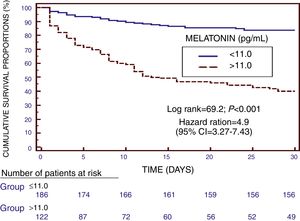
We found in Kaplan–Meier survival analysis a higher risk of death at 30 days of severe sepsis diagnosis in patient group with serum melatonin levels higher than 11.0pg/mL than in patient group with lower levels (Hazard Ratio=4.9; 95% CI=3.27–7.43; p<0.001) (Fig. 2).
We found that the area under the curve (AUC) for each 30-day mortality prediction were of 0.71 (95% CI=0.66–0.76) for serum melatonin levels, of 0.70 (95% CI=0.64–0.75) for SOFA score, and of 0.67 (95% CI=0.61–0.72) for lactatemia; and there were not statistically significant differences between serum melatonin levels and SOFA score AUCs (p=0.67), between serum melatonin levels and lactatemia AUCs (p=0.26), and between lactatemia and SOFA score AUCs (p=0.41).
When, we made a subanalysis including only patients with septic shock the findings were similar. We found that non-survivor septic shock patients showed higher serum melatonin levels at days 1 (p<0.001), 4 (p<0.001), and 8 (p<0.001) of diagnosis than septic shock survivor patients; and that serum melatonin levels at days 1 (p=0.001), 4 (p=0.02) and 8 of diagnosis (p<0.001) were associated with mortality of septic shock patients controlling for age, lactatemia, SOFA score and diabetes mellitus in multiple logistic regression analyses (data not showed).
DiscussionWe believed that our current study is the largest providing data on serum levels of melatonin from patients with severe sepsis, and that the new finds more interesting are that serum melatonin during the first 7 days of severe sepsis diagnosis are associated with sepsis severity and mortality.
Previously were reported higher circulating melatonin concentrations in non-survivor than in survivor severe septic patients when severe sepsis was diagnosed12,13; then the higher serum levels of melatonin at days 1, 4 and 8 of severe sepsis diagnosis in non-survivor than in survivor patient group found in our current study is a new finding not previously reported.
In our previous work was found a positive association serum melatonin levels, serum malondialdehyde levels and SOFA score at moment of severe sepsis diagnosis13; then the positive association between serum melatonin levels, serum malondialdehyde levels and SOFA score at days 1, 4 and 8 of severe sepsis diagnosis found in our current study is another new finding not previously reported.
Besides, in our previous work was found an association between serum melatonin levels at moment of severe sepsis diagnosis and mortality13; then the association between serum melatonin levels at days 1, 4 and 8 of severe sepsis diagnosis and mortality found in our current study is another new finding not previously reported.
We have not found statistically significant differences in the comparison of AUC of ROC curves of serum melatonin levels, lactatemia and SOFA score for 30-day mortality prediction. However, serum melatonin levels is the variable with higher Wald in regression analyses to predict mortality at 30 days including lactatemia and SOFA score. Thus, we think that serum melatonin levels could help in mortality prediction of septic patients.
There has been found in septic rats that the administration of melatonin has been found to increase the levels of different antioxidant compounds, reduce malondialdehyde levels, improve mitochondrial function, decrease TNF-alpha and interleukin-6 levels, decrease nitric oxide (NO), reduce biochemical markers of organ dysfunction and increase survival rates.23–31 In addition, in a randomized clinical trial with 20 septic newborns, the use of melatonin reduced malondialdehyde levels; and at 72h after the diagnosis of sepsis, 3 of 10 non-melatonin treated children had died, while all the melatonin-treated newborns survived.32 Besides, the administration of melatonin have been associated with lower circulating malondialdehyde levels and mortality in a randomized clinical trial with 20 asphyxiated newborns,33 and with lower circulating concentrations of IL-6, IL-8, TNF-alpha and nitrite/nitrate in a randomized clinical trial with 24 respiratory distress syndrome newborns.34 We believed that the positive association between serum melatonin levels, serum malondialdehyde levels and SOFA score during the 7 first days of severe sepsis diagnosis, and the association between serum melatonin levels during the 7 first days and mortality at 30 days found in our current study may be due to that non-survivors patients showed a higher oxidative state and sepsis severity than survivor patients, and that the higher serum melatonin levels in non-survivors patients is due to an attempt to avoid dangerous situation; however, those higher serum melatonin levels in non-survivors patients are not enough to compensate the situation finally dead. Thus, the new findings of our study and those findings of previous studies with the administration of melatonin can make us think to investigate more about this issue in septic patients.
However, our study has certain limitations. First, melatonin secretion has a circadian rhythm with high blood levels during the night (between 75 and 150pg/mL) and low values during the day35,36; however, we have not taken blood samples at different moment of day for each patient, and the first blood sample of each patient was obtained when the diagnosis of severe sepsis was made (thus, that sample was not recollected for all patients at the same time of day). Although, we would argue that we have not found a great light intensity difference between light and dark periods in our ICU measures (2.8 vs 0.2lux, ratio approximately of 14) in comparison to light and dark periods in hospital outside (1000 vs 0.1lux, ratio approximately of 10000); thus, the possible influence of light intensity in the determinations of first blood samples is lower. In addition, blood samples obtained at days 4 and 8 were obtained at the same moment of day (approximately at 12am), and at those moments also was found the association between serum melatonin levels and sepsis mortality. Second, the number of patients that were excluded or that declined the participation in the study was not recorded. Third, there is a new sepsis definition37; however, the inclusion patient period was previous to that new definition. Fourth, in 48.7% of our cases was not isolated the microorganism responsible of sepsis; although, in other series that rate is between 40–60%.38,39
ConclusionsThe new finds more interesting of our study were that serum melatonin during the first 7 days of severe sepsis diagnosis are associated with sepsis severity and mortality.
FundingThis study was supported by grants from Instituto de Salud Carlos III (PI14/00220 and INT16/00165) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflicts of interestNone.