The micro-elimination of HCV infection in drug users (DU) in our area is a priority in order to achieve the overall elimination of this disease. Coordinated action between specialists in addiction treatment, microbiologists and physicians who treat HCV infection is required to implement infection screening, to achieve universal access to treatment and to prevent new infections and reinfections. The objective of this document was to come to a consensus on the screening, hospital referral, treatment, follow-up and prevention of HCV infection in DU by an expert panel from GEHEP/SEIMC and three scientific societies of addiction treating physicians: SEPD, SOCIDROGALCOHOL and SOMAPA.
La microeliminación de la infección por VHC en pacientes usuarios de drogas (UD) es una prioridad para lograr la eliminación global de esta enfermedad. Se requiere una acción coordinada de especialistas en el tratamiento de adicciones, microbiólogos y médicos que tratan la infección por VHC para realizar el cribado de los pacientes, garantizar el acceso al tratamiento y prevenir nuevas infecciones y reinfecciones. El objetivo de este documento fue consensuar las medidas de cribado, envío a unidades hospitalarias, tratamiento, seguimiento y prevención de la infección por VHC en UD, por parte de un panel de expertos de GEHEP/SEIMC y 3 sociedades científicas implicadas en el tratamiento de las adicciones: SEPD, SOCIDROGALCOHOL y SOMAPA.
The World Health Organization has established the elimination of hepatitis C virus (HCV) infection as a goal to be reached in 2030. Drug users (DU), particularly people who inject drugs (PWID), are the subjects with the highest prevalence of HCV infection in Spain. Because of this, the micro-elimination of this infection in DU in our area is a priority. To accomplish this objective, three conditions are required: (1) all drug users should be screened for HCV; (2) all patients with active infection should be treated, which implies that linkage to care and adherence need to be warranted, and (3) harm reduction programs aimed at preventing new infections and reinfections have to be implemented. Carrying out the above actions demands a coordinated strategy by specialists in addiction treatment and physicians who diagnose and treat HCV infection. These were the reasons why a document on HCV infection management in DU was undertaken. The specific objective of this document was to come to a consensus on the screening, hospital referral, treatment, follow-up and prevention of HCV infection in DU by an expert panel from scientific societies involved in the care of HCV infection in DU.
MethodsThe document deals with six topics: (1) general issues in the management of HCV infected DU; (2) HCV screening in DU; (3) interactions between addiction treatment centers (ATC) and hospital units: linkage to care; (4) drug therapy of HCV infection in DU; (5) drug–drug interactions between direct-anting antiviral agents (DAA) and commonly used medicines or illicit drugs, and (6) harm reduction and prevention of HCV infection. The recommendations included in the full version of the document are displayed in the below sections of this executive summary.
The document was developed by a expert panel including members of the Viral Hepatitis Study Group (GEHEP) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), as the organization promoting the document, the Scientific Spanish Society for Studies on Alcohol, Alcoholism and other addictions (SOCIDROGALCOHOL), the Spanish Society of Dual Disorders (SEPD) and the Andalusian Medical Society of Addictions and Associated Disorders (SOMAPA).
For evidence grading, a system akin to that employed in other SEIMC guidelines was used. Thus, a capital letter and a roman number follow each recommendation. The letter refers to the strength of the recommendation: A: it should be always offered; B: it should be generally offered, and C: it should be optionally offered. The number states the proofs supporting the recommendation: I: one or more clinical trials, metanalysis or integrated analysis of clinical trials; II: one or more observational or case-series studies; III: expert opinion.
General management issues of HCV-infected DUDiet, lifestyle and toxic use- •
A diet and lifestyle aimed at achieving a normal metabolic profile and, when present, to reduce liver steatosis is recommended (AI).
- •
DU with HCV infection should avoid alcohol drinking (AII).
- •
DU with HCV infection should avoid using illicit psychoactive drugs (AIII).
- •
It is recommended not to share pricking or sharp devices, such as razors or toothbrushes (AII).
- •
DU without exposure markers should be vaccinated against hepatitis A virus and hepatitis B virus (AI).
- •
After vaccination, all patients require follow-up to confirm the development of protective titers of anti-HBs (AI).
- •
HCV screening should be conducted in all DU with unknown HCV status (AII).
- •
As in other populations, universal screening is likely to be required to reach HCV elimination in DU (AII).
- •
Reflex diagnosis is recommended to identify DU with active HCV infection (AII).
- •
In patients with HCV viremia, if enough sample is available, HCV genotyping may be directly performed (AII).
- •
If feasible, direct HCV-RNA determination is recommended for PWID screening at ATC (AII).
- •
There should be coordinating physicians for HCV infection care both in hospital units and in ATC. They must have either face to face or telematic meetings and keep a direct communication line for information exchange and solving issues (AIII).
- •
An electronic medical record and a patient register should be shared by ATC, primary care centers and specialized units (AIII).
- •
HCV diagnosis in DU, if possible, should be performed at the ATC (AIII).
- •
A coordination program in health care for HCV-infected DU is recommended (AII).
- •
Peer support, if available, should be offered to attend outpatient clinic appointments (AII).
- •
Telemedicine use should be promoted both for communication between health care providers and for the follow-up of patients for whom attending appointments in specialized units is difficult (BII).
- •
In patients who are likely to be poorly adherent, a directly observed treatment in a health care institution that is able to do it, is recommended (AIII).
For more detailed information on HCV treatment recommendations, consensus documents developed by GEHEP/SEIMC can be accessed online https://seimc.org/grupos-de-estudio/gehep/produccion-cientifica/documentos-consenso
Indications of HCV treatment- •
All DU with active HCV infection should be given DAA therapy.
Recommended combinations in patients without previous DAA treatment
- •
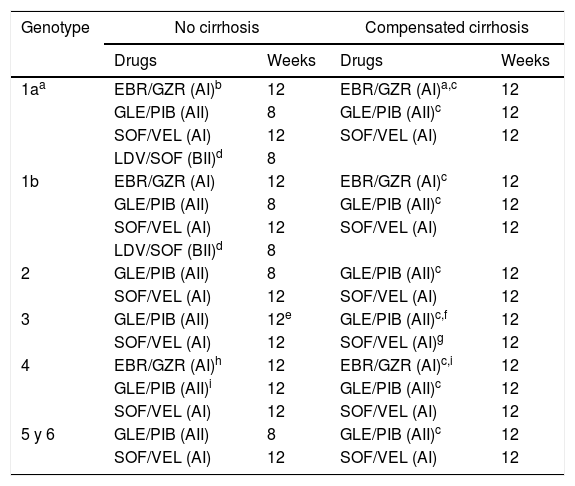
The DAA combinations recommended in patients without prior exposure to DAA are displayed in Table 11.
Table 1.DAA combinations recommended in patients without previous DAA treatment.
Genotype No cirrhosis Compensated cirrhosis Drugs Weeks Drugs Weeks 1aa EBR/GZR (AI)b 12 EBR/GZR (AI)a,c 12 GLE/PIB (AII) 8 GLE/PIB (AII)c 12 SOF/VEL (AI) 12 SOF/VEL (AI) 12 LDV/SOF (BII)d 8 1b EBR/GZR (AI) 12 EBR/GZR (AI)c 12 GLE/PIB (AII) 8 GLE/PIB (AII)c 12 SOF/VEL (AI) 12 SOF/VEL (AI) 12 LDV/SOF (BII)d 8 2 GLE/PIB (AII) 8 GLE/PIB (AII)c 12 SOF/VEL (AI) 12 SOF/VEL (AI) 12 3 GLE/PIB (AII) 12e GLE/PIB (AII)c,f 12 SOF/VEL (AI) 12 SOF/VEL (AI)g 12 4 EBR/GZR (AI)h 12 EBR/GZR (AI)c,i 12 GLE/PIB (AII)i 12 GLE/PIB (AII)c 12 SOF/VEL (AI) 12 SOF/VEL (AI) 12 5 y 6 GLE/PIB (AII) 8 GLE/PIB (AII)c 12 SOF/VEL (AI) 12 SOF/VEL (AI) 12 Abbreviations: EBR/GZR: elbasvir/grazoprevir; GLE/PIB: glecaprevir/pibrentasvir; SOF/VEL: sofosbuvir/velpatasvir; LDV/SOF: ledipasvir/sofosbuvir.
bIf plasma HCV-RNA <800.000IU/mL or, in subjects with ≥800.000UI/ml, if there is no evidence of substitutions associated to resistance (RAS) to EBR.
Recommended combinations in patients with prior failure to DAA
- •
Patients with prior failure to DAA should be assessed in units with wide experience managing these cases (AIII).
- •
In patients previously treated with DAA, salvage therapy based on a resistance study is always recommended (AII).
- •
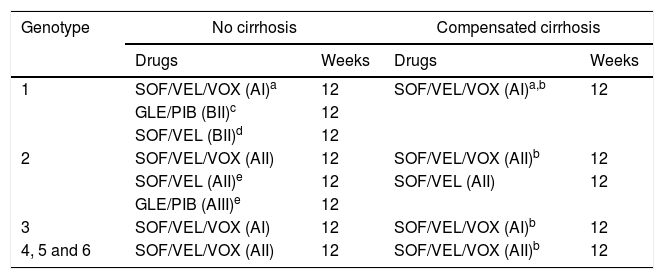
Recommended combinations in patients with prior failure to DAA when a resistance test is not available are shown in Table 2.
Table 2.DAA combinations recommended in patients with previous failure to DAA therapy when a resistance test is not available.
Genotype No cirrhosis Compensated cirrhosis Drugs Weeks Drugs Weeks 1 SOF/VEL/VOX (AI)a 12 SOF/VEL/VOX (AI)a,b 12 GLE/PIB (BII)c 12 SOF/VEL (BII)d 12 2 SOF/VEL/VOX (AII) 12 SOF/VEL/VOX (AII)b 12 SOF/VEL (AII)e 12 SOF/VEL (AII) 12 GLE/PIB (AIII)e 12 3 SOF/VEL/VOX (AI) 12 SOF/VEL/VOX (AI)b 12 4, 5 and 6 SOF/VEL/VOX (AII) 12 SOF/VEL/VOX (AII)b 12 Abbreviations: SOF/VEL/VOX: sofosbuvir/velpatasvir/voxilaprevir; GLE/PIB: glecaprevir/pibrentasvir; SOF/VEL: sofosbuvir/velpatasvir;
aPreferred pangenotypic combination, regardless of the previously used treatment, particularly after failure to a NS5A inhibitor.
cAfter failure to pegylated interferon plus RBV plus NS3 inhibitor or SOF with or without NS3 inhibitors, both in genotype 1a and 1b.
Recommended combinations in patients with decompensated cirrhosis
- •
Patients with decompensated cirrhosis are better managed in units that have wide experience with these patients (AIII).
- •
Sofosbuvir 400mg/velpatasvir 100mg+RBV 12 weeks is the combination recommended for DAA therapy in patients with decompensated cirrhosis with HCV genotype 1–4 (AI) and genotype 5–6 (AII) infections. If RBV cannot be used, sofosbuvir 400mg/velpatasvir 100mg should be given for 24 weeks (AI). In patients with prior failure to sofosbuvir+NS5A, sofosbuvir 400mg/velpatasvir 100mg+RBV for 24 weeks is recommended (CIII).
A detailed exposure of all drug–drug interactions between DAA and other commonly used medicines or illicit drugs can be found in the full version of the consensus document. Also, the website of the University of Liverpool2 shows an updated compilation of these interactions.
- •
Drug–drug interactions between DAA and the remaining medicines taken by the patient should be always checked before choosing the DAA combination to be used (AI).
- •
Hospital pharmacists should be involved in surveillance and prevention of DAA interactions (AII).
- •
Drug–drug interactions have to be particularly suspected in elderly patients, as well as in those with hepatic insufficiency and in those receiving polypharmacy (AI).
- •
Opiate users, particularly those who are PWID, should engage in opiate substitution therapy programs (AI).
- •
PWIDs should access needle-syringe exchange programs (AII). If such programs are not available, they should be instructed on syringe cleaning (AIII).
- •
Inhaled drug users should be informed of the risks of sharing smoking or snorting devices, and advised against sharing them.
- •
Men who have sex with men should be informed of the risks of group sex with drug use (AII). Systematic condom use and avoiding sex practices associated with bleeding should be recommended (AII). Unsterile substance intravenous injection must be avoided (AII).
- •
The above recommendations on harm reduction are also applicable in patients with cleared HCV infection, either spontaneously or after therapy, provided that they continue to be engaged in risk behaviors (AII).
- •
In patients with cleared HCV infection with ongoing risk factors, plasma HCV-RNA should be tested at least yearly (AII).
No private or public institution has provided financial support for the elaboration of the herein document.
Conflict of interest- •
Juan A. Pineda has received research grants from Abbvie, Janssen, Gilead, MSD y ViiV, talk honoraria from Abbvie, Janssen, Gilead, MSD and ViiV, financial support to attend educational activities from Janssen and Gilead and has been an advisor of Abbvie, Janssen, Gilead and MSD.
- •
Benjamín Climent has received research grants from Pfizer and talk honoraria from Lundbeck, Pfizer and Reckitt.
- •
Federico García has received research grants from Gilead and Roche, talk honoraria from ViiV, Gilead, Abbvie, MSD, Janssen, Roche, Biomerieux, Hologic, Qiagen, financial support to attend educational activities from ViiV, Gilead, Abbvie, MSD, Janssen, Roche, Biomerieux, Hologic, Qiagen and has been an advisor of ViiV, Gilead, Abbvie, Roche, and Hologic.
- •
Miguel García Deltoro has received research grants from Janssen, ViiV, Gilead and MSD, talk honoraria from Janssen, ViiV, Gilead, Abbvie, MSD, financial support to attend educational activities from MSD and Gilead, and has been an advisor of Janssen, ViiV and Gilead.
- •
Rafael Granados has received talk honoraria from Abbvie, Janssen, Gilead and MSD, financial support to attend educational activities from Janssen, Gilead, Abbvie and MSD, and has been an advisor of Abbvie and Janssen.
- •
Fernanda Gómez has received research grants from Schering-Plough, talk honoraria from Reckitt-Benckiser, and financial support to attend educational activities from Reckitt-Benckiser/Indivior, Lilly, Lundbeck, Pfizer, Janssen and Gilead.
- •
Juan Macías has received research grants from Abbvie, Gilead, Janssen, MSD y ViiV, talk honoraria from Gilead and MSD, financial support to attend educational activities from Janssen and Gilead and has been an advisor of Abbvie, Gilead and MSD.
- •
Álvaro Mena has received research grants from Gilead, talk honoraria from Abbvie, Janssen, Gilead, MSD and ViiV, financial support to attend educational activities from Abbvie, Gilead, Janssen, MSD and ViiV, and has been an advisor of Janssen.
- •
Nicolás Merchante has received talk honoraria from Gilead and MSD, financial support to attend educational activities from Janssen Cilag, MSD, Gilead Sciences and ViiV Healthcare and has been an advisor of Gilead.
- •
Enriqueta Ochoa has received research grants from Lundbeck, talk honoraria from Indivior, Lundbeck and Otsuka, financial support to attend educational activities from Janssen and Gilead and has been an advisor of Janssen-Cilag, Reckitt-Benckiser/Indivior, Lundbeck, Otsuka, Servier, Shire, Lilly and Gilead.
- •
Carlos Roncero has received research grants from Gilead and Indivior, talk honoraria from Janssen-Cilag, Ferrer-Brainfarma, Pfizer, Indivior, Lundbeck, Otsuka, Servier, GSK, Rovi, Astra, Gilead, Sanofi, Exceltis and MSD, and has been an advisor of Janssen-Cilag, Lundbeck, Gilead, MSD, Mundipharma, Indivior, Exceltis and Martindole.
- •
Juan Jesús Ruiz has received talk honoraria from Janssen-Cilag, Reckitt-Benckiser/Indivior, Lundbeck, Pfizer, Gilead and MSD, and financial support to attend educational activities from Janssen-Cilag, Reckitt-Benckiser/Indivior, Lundbeck, Pfizer, Gilead, MSD and Servier.
- •
Francisco Téllez has received financial support to attend educational activities from Gilead, Abbvie and Merck.
- •
Luis Morano has received talk honoraria from Abbvie, Janssen, Gilead and MSD, financial support to attend educational activities from Janssen and Gilead and has been an advisor of Abbvie, Janssen, Gilead and MSD.
The entire version of the document can be found online at. https://seimc.org/contenidos/gruposdeestudio/gehep/dcientificos/documentos/gehep-dc-2018-Manejo_de_la_infeccion_por_VHC_en_UD_v1.pdf and as supplementary material in the journal official website (Appendix 1).