Cardiac surgery is a life-saving procedure in patients diagnosed with infective endocarditis (IE). There are several validated risk scores developed to predict early-mortality; nevertheless, long-term survival has been less investigated. The aim of the present study is to analyze the impact of IE-specific risk factors for early and long-term mortality.
MethodsAn observational retrospective study was conducted that included all patients who underwent surgery for IE from 2002 to 2016. Median follow-up time after surgery was 53.2 months (IQI 26.2–106.8 months). In-hospital mortality was analyzed using multiple logistic regression. Long-term survival was analyzed after one, two and five years. Cox proportional hazards regression was employed to identify risk factors related to long-term mortality.
ResultsOf the 180 patients underwent cardiac surgery, 133 were discharged alive (in-hospital mortality was 26.11%). 6 variables were identified as independent factors associated with in-hospital mortality, most of them closely related to the severity of IE: age, multivalvular involvement, critical preoperative status, preoperative mechanical ventilation, abscess and thrombocytopenia.
Long-term survival in patients discharged alive was 89.1%, 87.4% and 77.6% after one, two and five years. Long-term mortality was independent of specific IE factors and 86.51% of deaths were not related to cardiovascular or infectious diseases.
ConclusionDespite the high perioperative mortality rate after surgical treatment for active IE, long-term survival after hospital discharge was acceptable, regardless of the severity of the endocarditis episode. Although in-hospital survival depended mainly on several IE factors, long-term survival was not related to the severity of endocarditis baseline affection.
La cirugía cardíaca es un procedimiento fundamental en pacientes diagnosticados de endocarditis infecciosa (EI). Existen varias escalas de riesgo para predecir la mortalidad temprana; sin embargo, la supervivencia a largo plazo ha sido menos estudiada. El objetivo es analizar el impacto de los factores de riesgo específicos de EI en la mortalidad temprana y a largo plazo.
MétodosEstudio observacional retrospectivo que incluyó a todos los pacientes operados por EI entre 2002 y 2016. La mediana del tiempo de seguimiento fue de 53,2 meses (IQI: 26,2-106,8 meses). La mortalidad intrahospitalaria se analizó mediante regresión logística múltiple. La supervivencia se analizó a uno, 2 y 5 años. Los factores de riesgo de mortalidad tardía se analizaron mediante regresión de Cox.
ResultadosDe los 180 pacientes operados, 133 sobrevivieron al postoperatorio inmediato (26,11% de mortalidad intrahospitalaria). Encontramos 6 factores asociados a la mortalidad hospitalaria: edad, afectación multivalvular, estado preoperatorio crítico, ventilación mecánica preoperatoria, absceso y trombopenia.
La supervivencia a largo plazo fue del 89,1, 87,4 y 77,6% después de uno, 2 y 5 años. La mortalidad a largo plazo fue independiente de factores específicos de la EI, y el 86,51% no se relacionó con enfermedades cardiovasculares o infecciosas.
ConclusiónA pesar de la alta tasa de mortalidad peri-operatoria tras cirugía, la supervivencia a largo plazo fue aceptable, independientemente de la gravedad del episodio de endocarditis. Aunque la supervivencia intrahospitalaria guardó relación con factores específicos de endocarditis, y la supervivencia a largo plazo no se correlacionó con la gravedad de la afectación inicial.
Surgical treatment is one of the essential procedures in patients diagnosed with infective endocarditis (IE) in combination with an appropriate antibiotic therapy. However, IE surgery is associated with remarkably high rates of morbidity and mortality. In spite of the advances in surgical techniques, the shortening of waiting-time to surgery and the improvement of antibiotic treatments, the mortality rate associated with surgical treatment continues to be very high.1
Although the indications for surgery are established in current guidelines,2,3 there is a low level of evidence regarding surgery indications. It is believed that less than half of the patients who have surgical indication do not undergo surgical intervention4 because of their high surgical risk. However, patients who have an indication for surgery and are not operated on, still have a dismal prognosis.5 The main reason for not performing a surgical intervention is the estimated high immediate postoperative mortality rate, nevertheless long-term survival is seldom taken into account.
Despite improvement in surgical treatment, the published in-hospital mortality rate ranges from 6% to 33%.2,6 Perioperative mortality rates vary considerably in affected patients and depend on several factors such as: patient clinical characteristics, preoperative status, intraoperative difficulties and surgical complications. In recent years, many studies have tried to address the predictors for short-term survival after IE surgery. The impact on in-hospital mortality for some IE-specific factors (such as microbiological cultures, abscess formation, sepsis, …) has been analyzed extensively6 and several IE specific scores have been developed for perioperative mortality risk assessment. These specific scores have become an important tool in evaluating results after surgery, and in addition, they are widely used in clinical practice to predict immediate prognostic.7–9
Although in-hospital survival depends mainly on IE-specific factors, the influence of all these specific factors on long-term survival has been less intensively investigated. Therefore, individual long-term life expectancy is difficult to predict.
The aim of the present study is to analyze the impact of IE-specific risk factors for early and long-term mortality.
MethodsAn observational retrospective study was conducted and included all patients who underwent surgery for infective endocarditis from 2002 to 2016. Patients were diagnosed with active left side IE based on the modified Duke criteria.10 The study was done in a tertiary care referral center and patients were identified from hospital database (cardiac surgery department database and infective endocarditis group registry).
All episodes of left side IE affecting native and prosthetic valves and admitted for surgical treatment during the study period were included. Cases of IE exclusively related to cardiac implantable electronic devices and cases of right side IE were excluded. Demographic data, risk factors for endocarditis, clinical variables and outcomes were all evaluated.
Long-term follow up was conducted through analysis of hospital databases and personal telephone interviews. Complete follow-up was possible in 122 of the 133 surviving patients (91.73%). Median follow-up time after surgery was 53.2 months (IQI 26.2–106.8 months).
The primary objective of the study was the analysis of factors associated with decreased survival in two different time frames: the immediate postoperative period and long-term follow-up.
Factors associated with in-hospital mortality were analyzed using multiple logistic regression. In-hospital mortality was defined as death within the first 30 postoperative days. We performed a univariate analysis and subsequently a multivariate analysis. Multivariate logistic regression analysis was performed through the methodology of all possible equations analysis. All those variables associated with a decreased survival in the univariate analysis (p<0.05), and those that were considered potentially clinically relevant based on previously published literature were included in the multivariate analysis. Factors considered relevant were: female sex, urgent surgery, previous cardiac surgery, cardiogenic shock, septic shock, prosthetic valve IE, multivalvular involvement, renal failure, abscess and S. aureus as casual microorganism.9,11–14 The most parsimonious regression model with the lowest Bayesian information criteria (BIC) was selected.
Discrimination (capacity to predict in-hospital mortality) of specific-IE scores was assessed by calculating the area under the Receiver Operating Characteristic (ROC) curve (AUC). IE-specific scores assessed were: STS-IE,7 De Feo-Cotrufo,15 PALSUSE,11 Costa16 and Risk-E score.12
Contrarily, long-term survival in patients who survived to the early post-operative period was analyzed after one, two and five years using the Kaplan–Meier method. The analysis of factors associated with long-term survival was performed using univariate Cox proportional hazards regression, and the results were expressed as Hazard ratios (HR), with their 95% confidence intervals (95% CI). Reliability of the different scores in predicting long-term mortality was assessed by Harrell's concordance index for survival data (‘C index’).
Definitions:
- -
Heart failure was diagnosed on the basis of criteria guidelines.6
- -
Renal insufficiency was defined as the presence of a serum creatinine concentration >2mg/dl.
- -
Severe pulmonary hypertension was defined as pulmonary artery pressure >55mmHg.
- -
Paravalvular abscess was considered as an infection and necrosis with the formation of a purulent cavity with the capacity to invade structures.17
- -
Septic shock was defined as an acute circulatory failure due to sepsis, with persistent systolic pressure <90mmHg, despite adequate volume resuscitation.
- -
Cardiogenic shock was considered as acute circulatory failure due to myocardial dysfunction, with systolic pressure <90mmHg, tissue hypoperfusion and low cardiac index.
- -
Thrombocytopenia was defined as a platelet count of <150,000 platelets/ml.18
- -
Vegetations were defined as oscillating intra-cardiac mass on valve or implanted material in the absence of an alternative anatomic explanation, diagnosed and measured by echocardiography.19
- -
Multivalvular involvement was defined as involvement of the aortic and mitral valves.
- -
Emergency surgery was defined as the surgery performed in the first 24h within the diagnosis.2
Statistical analysis was performed using Stata/IC 14.2 (Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). Continuous variables were expressed as mean and standard deviation (SD) if normally distributed, or as median and interquartile interval (IQI) in the presence of marked asymmetries. Categorical variables were expressed as frequency and proportions. Student T test was used to compare continuous variables, and Chi Square test was employed to compare proportions.
A p-value of less than 0.05 was considered as statistically significant.
The Ethical Review Board (ERB) of the Hospital approved the implementation of this study (ERB number 313/2016, approved on November 28, 2016). The requirement for informed written consent was waived. Patient identification was encoded, complying with the requirements of the Organic Law on Data Protection 15/1999.
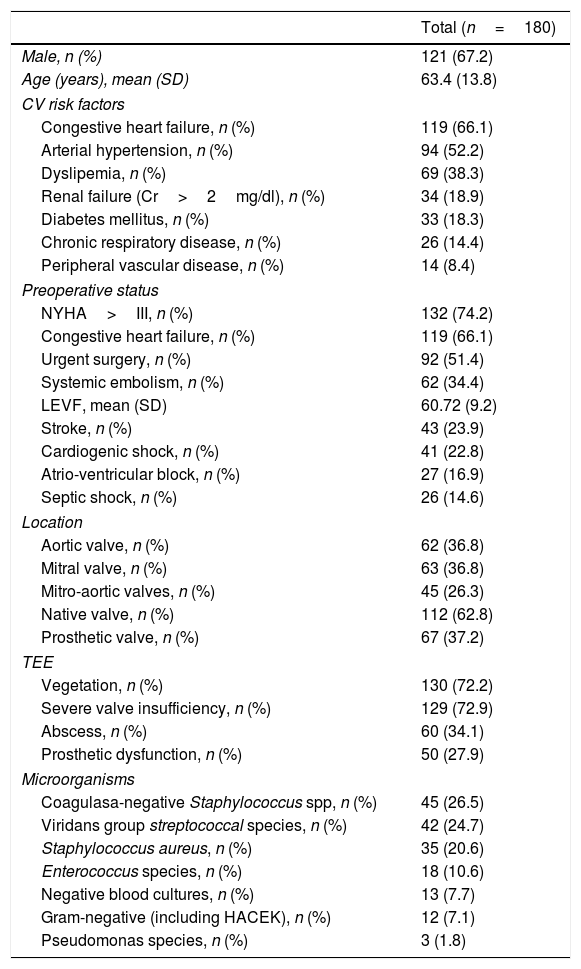
ResultsA total of 180 patients were analyzed during the study period. All patients were on antibiotic treatment before the surgical procedure. Valve replacement was performed in all of them in association with other additional techniques needed to achieve the correct exeresis of all the affected tissues such as: drainage of abscesses, debridement of the necrotic tissues and reconstruction and repair of infected regions. Baseline characteristics of the patients at admission are presented in Table 1.
Baseline patient characteristics.
| Total (n=180) | |
|---|---|
| Male, n (%) | 121 (67.2) |
| Age (years), mean (SD) | 63.4 (13.8) |
| CV risk factors | |
| Congestive heart failure, n (%) | 119 (66.1) |
| Arterial hypertension, n (%) | 94 (52.2) |
| Dyslipemia, n (%) | 69 (38.3) |
| Renal failure (Cr>2mg/dl), n (%) | 34 (18.9) |
| Diabetes mellitus, n (%) | 33 (18.3) |
| Chronic respiratory disease, n (%) | 26 (14.4) |
| Peripheral vascular disease, n (%) | 14 (8.4) |
| Preoperative status | |
| NYHA>III, n (%) | 132 (74.2) |
| Congestive heart failure, n (%) | 119 (66.1) |
| Urgent surgery, n (%) | 92 (51.4) |
| Systemic embolism, n (%) | 62 (34.4) |
| LEVF, mean (SD) | 60.72 (9.2) |
| Stroke, n (%) | 43 (23.9) |
| Cardiogenic shock, n (%) | 41 (22.8) |
| Atrio-ventricular block, n (%) | 27 (16.9) |
| Septic shock, n (%) | 26 (14.6) |
| Location | |
| Aortic valve, n (%) | 62 (36.8) |
| Mitral valve, n (%) | 63 (36.8) |
| Mitro-aortic valves, n (%) | 45 (26.3) |
| Native valve, n (%) | 112 (62.8) |
| Prosthetic valve, n (%) | 67 (37.2) |
| TEE | |
| Vegetation, n (%) | 130 (72.2) |
| Severe valve insufficiency, n (%) | 129 (72.9) |
| Abscess, n (%) | 60 (34.1) |
| Prosthetic dysfunction, n (%) | 50 (27.9) |
| Microorganisms | |
| Coagulasa-negative Staphylococcus spp, n (%) | 45 (26.5) |
| Viridans group streptococcal species, n (%) | 42 (24.7) |
| Staphylococcus aureus, n (%) | 35 (20.6) |
| Enterococcus species, n (%) | 18 (10.6) |
| Negative blood cultures, n (%) | 13 (7.7) |
| Gram-negative (including HACEK), n (%) | 12 (7.1) |
| Pseudomonas species, n (%) | 3 (1.8) |
SD: standard deviation, n (%): number of observation and percentage, NYHA: New York Heart Association, LEVF: left ventricular ejection fraction, Cr: creatinine, TEE: transesophageal echocardiography, HACEK: Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella spp.
Median time from the beginning of symptoms to surgery was 15.9 days (IQI 3–21 days) and 17.2% underwent surgery within the first 48h after diagnosis. Emergency surgery was required in 92 patients (51.4%) because of onset of clinical deterioration during hospital admission.
The study population was a very high surgical risk cohort. The mean predicted risk of mortality was: 33.8% (95% CI: 29.7–37.8%) by EuroSCORE I and 14.1% (95% CI: 11.7–16.5%) by EuroSCORE II. STS Score was also calculated whenever possible (in cases with multiple valve endocarditis the calculation of the score is not possible). STS Score was determined using 73.7% of the sample, and its predicted risk of mortality was 10.7% (95% CI: 8.3–13.2).
- a)
In-hospital mortality
The observed in-hospital mortality rate was 26.1% (47 patients). The observed mortality rate did not significantly change during the study period. Dividing the sample into three periods, we found a 23.7% of mortality between 2002 and 2006, 28.2% in 2007–2010 and 28.2% between 2011 and 2016.
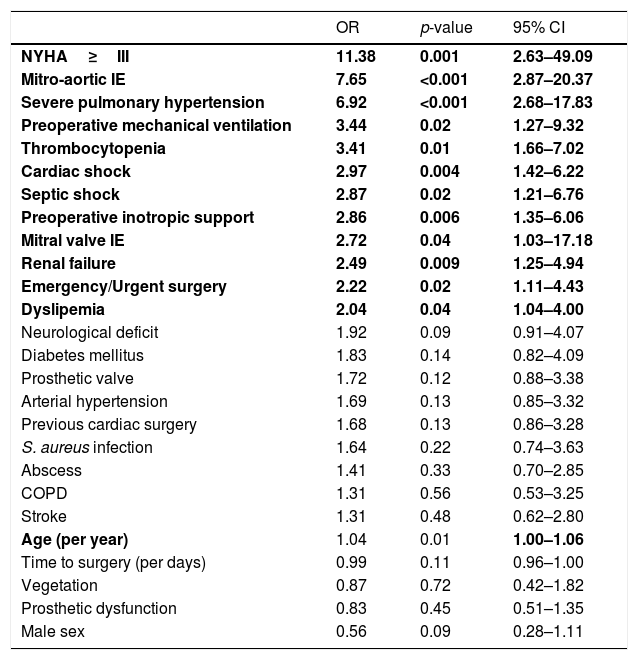
Univariate logistic analysis identified 12 factors related to in-hospital mortality (Table 2): age, multivalvular affection, emergency surgery, dyslipidemia, severe pulmonary hypertension, class New York Heart class (NYHA)≥III, renal failure, cardiac shock, septic shock, preoperative inotropic support, preoperative mechanical ventilation and thrombocytopenia.
Univariate analysis of the risk factors associated with in-hospital mortality in the 180 patients who underwent cardiac surgery for active infective endocarditis.
| OR | p-value | 95% CI | |
|---|---|---|---|
| NYHA≥III | 11.38 | 0.001 | 2.63–49.09 |
| Mitro-aortic IE | 7.65 | <0.001 | 2.87–20.37 |
| Severe pulmonary hypertension | 6.92 | <0.001 | 2.68–17.83 |
| Preoperative mechanical ventilation | 3.44 | 0.02 | 1.27–9.32 |
| Thrombocytopenia | 3.41 | 0.01 | 1.66–7.02 |
| Cardiac shock | 2.97 | 0.004 | 1.42–6.22 |
| Septic shock | 2.87 | 0.02 | 1.21–6.76 |
| Preoperative inotropic support | 2.86 | 0.006 | 1.35–6.06 |
| Mitral valve IE | 2.72 | 0.04 | 1.03–17.18 |
| Renal failure | 2.49 | 0.009 | 1.25–4.94 |
| Emergency/Urgent surgery | 2.22 | 0.02 | 1.11–4.43 |
| Dyslipemia | 2.04 | 0.04 | 1.04–4.00 |
| Neurological deficit | 1.92 | 0.09 | 0.91–4.07 |
| Diabetes mellitus | 1.83 | 0.14 | 0.82–4.09 |
| Prosthetic valve | 1.72 | 0.12 | 0.88–3.38 |
| Arterial hypertension | 1.69 | 0.13 | 0.85–3.32 |
| Previous cardiac surgery | 1.68 | 0.13 | 0.86–3.28 |
| S. aureus infection | 1.64 | 0.22 | 0.74–3.63 |
| Abscess | 1.41 | 0.33 | 0.70–2.85 |
| COPD | 1.31 | 0.56 | 0.53–3.25 |
| Stroke | 1.31 | 0.48 | 0.62–2.80 |
| Age (per year) | 1.04 | 0.01 | 1.00–1.06 |
| Time to surgery (per days) | 0.99 | 0.11 | 0.96–1.00 |
| Vegetation | 0.87 | 0.72 | 0.42–1.82 |
| Prosthetic dysfunction | 0.83 | 0.45 | 0.51–1.35 |
| Male sex | 0.56 | 0.09 | 0.28–1.11 |
OR: Odds Ratio, NYHA: New York Heart Association, CI: confident interval, COPD: chronic obstructive pulmonary disease. The variables in bold font are the ones included in the multivariate analysis.
After multivariate regression, six variables were identified as independent factors related to in-hospital mortality factors: age (OR 1.0; 95% CI 1.0–1.1); multivalvular involvement (OR 9.2; 95% CI 2.8–29.6); NYHA class ≥III (OR 7.4; 95% CI 1.5–36.5); preoperative mechanical ventilation (OR 2.7; 95% CI 0.7–10.7), abscess (OR 2.1; 95% CI 0.8–5.6) and thrombocytopenia (OR 3.7; 95% CI 1.5–9.3).
Regarding in-hospital mortality prediction by IE specific scores (20.21), AUC was 0.76 (95% CI 0.68–0.82) for STS-IE Score; 0.68 (95% CI 0.58–0.76) for De Feo-Cotrufo; 0.73 (95% CI 0.66–0.79) for PALSUSE; 0.65 (95% CI 0.57–0.72) for Costa Score and 0.76 (95% IC 0.69–0.82) for Risk-E score.
- b)
Long-term survival
During the study period, 133 patients were discharged alive. Eleven patients were lost in the follow-up period, therefore follow-up was only completed in the remaining 122 patients (91.7%). Observed long-term mortality in patients who discharged alive was 30.3% (37 patients) during a maximum follow-time of 175.3 months.
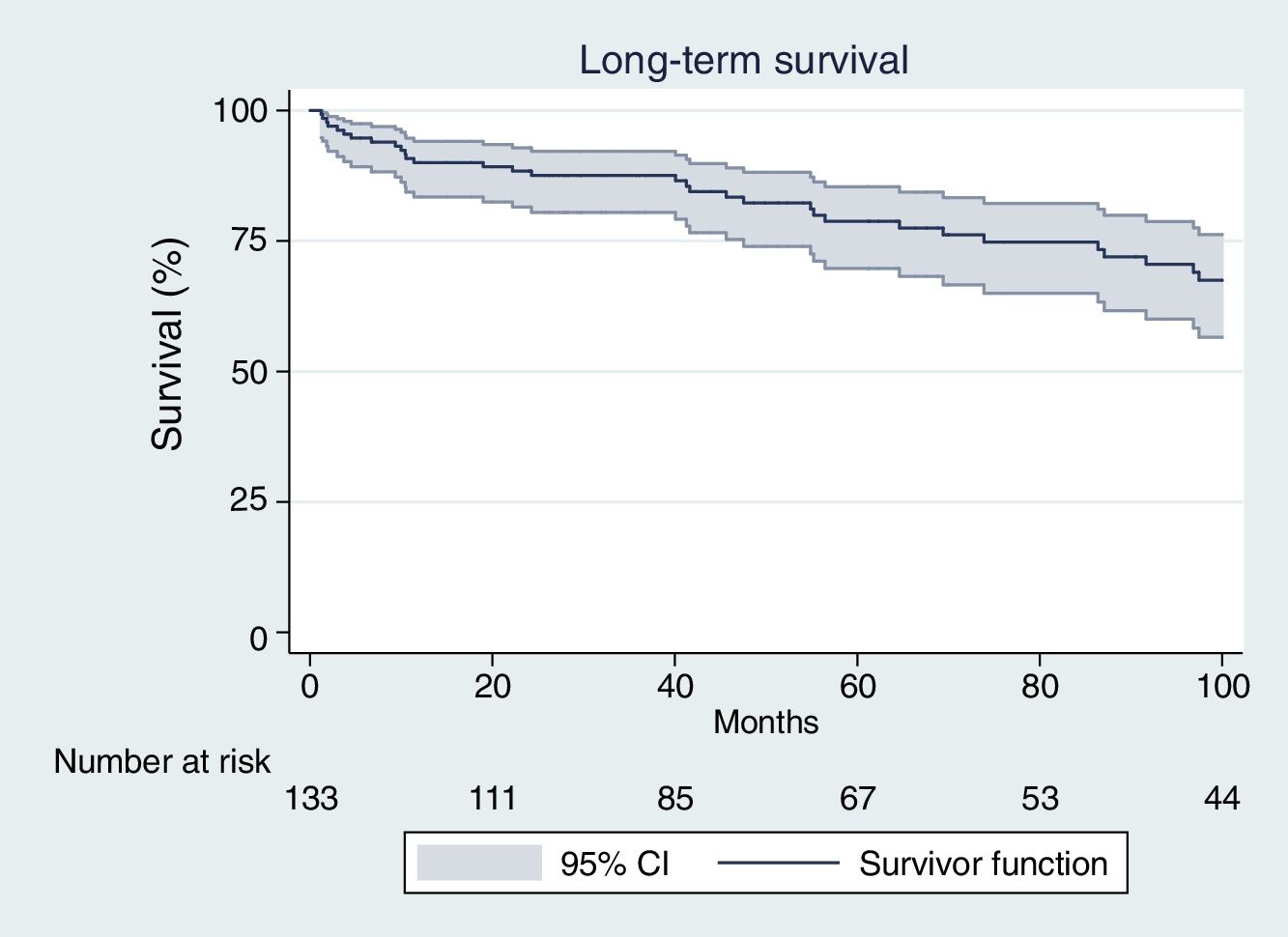
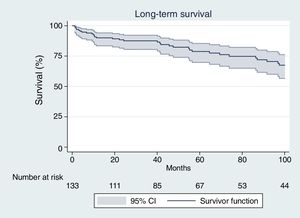
Patients who survived the early post-operative period had a late survival rate of 89.1%, 87.4% and 77.6% after one, two and five years, respectively (Fig. 1).
The median time of survival after hospital discharge (follow up time when half of the patients have died) was 171.0 months after the surgical procedure.
The main causes for late mortality during the follow-up were: cardiovascular complications in 10 patients (7.9%), infectious complications in 7 patients (5.6%) and other pathologies not related to cardiovascular disease in 109 patients (86.5%).
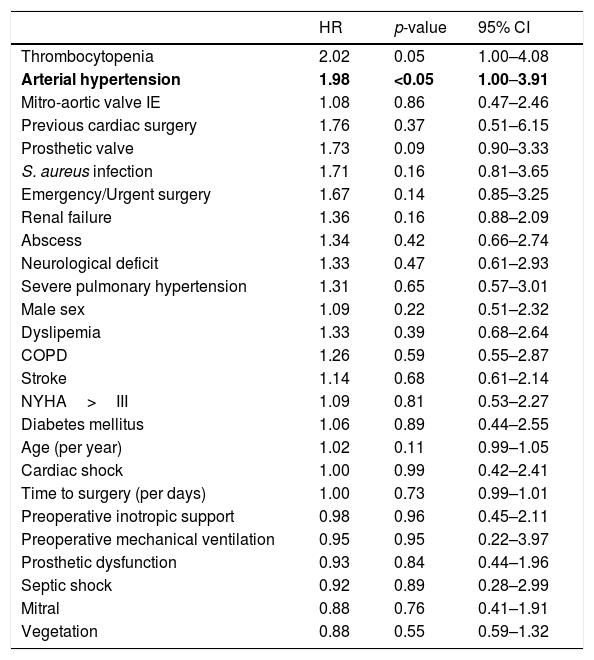
Analysis of long-term survival showed that the only factor significantly associated with long-term mortality was arterial hypertension (HR 1.9; 95% CI 1.0–3.9; p<0.05). All other analyzed IE specific variables did not have any impact on long-term survival (Table 3).
Univariate Cox analysis of the risk factors associated with long-term mortality in the 122 patients who survived the postoperative period after cardiac surgery for active infective endocarditis.
| HR | p-value | 95% CI | |
|---|---|---|---|
| Thrombocytopenia | 2.02 | 0.05 | 1.00–4.08 |
| Arterial hypertension | 1.98 | <0.05 | 1.00–3.91 |
| Mitro-aortic valve IE | 1.08 | 0.86 | 0.47–2.46 |
| Previous cardiac surgery | 1.76 | 0.37 | 0.51–6.15 |
| Prosthetic valve | 1.73 | 0.09 | 0.90–3.33 |
| S. aureus infection | 1.71 | 0.16 | 0.81–3.65 |
| Emergency/Urgent surgery | 1.67 | 0.14 | 0.85–3.25 |
| Renal failure | 1.36 | 0.16 | 0.88–2.09 |
| Abscess | 1.34 | 0.42 | 0.66–2.74 |
| Neurological deficit | 1.33 | 0.47 | 0.61–2.93 |
| Severe pulmonary hypertension | 1.31 | 0.65 | 0.57–3.01 |
| Male sex | 1.09 | 0.22 | 0.51–2.32 |
| Dyslipemia | 1.33 | 0.39 | 0.68–2.64 |
| COPD | 1.26 | 0.59 | 0.55–2.87 |
| Stroke | 1.14 | 0.68 | 0.61–2.14 |
| NYHA>III | 1.09 | 0.81 | 0.53–2.27 |
| Diabetes mellitus | 1.06 | 0.89 | 0.44–2.55 |
| Age (per year) | 1.02 | 0.11 | 0.99–1.05 |
| Cardiac shock | 1.00 | 0.99 | 0.42–2.41 |
| Time to surgery (per days) | 1.00 | 0.73 | 0.99–1.01 |
| Preoperative inotropic support | 0.98 | 0.96 | 0.45–2.11 |
| Preoperative mechanical ventilation | 0.95 | 0.95 | 0.22–3.97 |
| Prosthetic dysfunction | 0.93 | 0.84 | 0.44–1.96 |
| Septic shock | 0.92 | 0.89 | 0.28–2.99 |
| Mitral | 0.88 | 0.76 | 0.41–1.91 |
| Vegetation | 0.88 | 0.55 | 0.59–1.32 |
HR: Hazard Ratio, NYHA: New York Heart Association, CI: confident interval, COPD: chronic obstructive pulmonary disease.
Bold: the factors statistically significant (p<0.05).
Regarding late mortality prediction by endocarditis specific scores we found: STS-IE score7 (HR 1.0; 95% CI 0.9–1.0 p=0.79), Feo-Cotrufo score15 (HR 1.0; 95% CI 0.9–1.1 p=0.32), Costa score16 (HR 1.0; 95% CI 0.9–1.1 p=0.18), PALSUSE11 (1.2; 95% CI 0.9–1.5 p=0.21) and Risk-E score12,20 (HR 1.0; 95% CI 1.0–1.1 p=0.03). The accuracy in mortality risk prediction by C index was: 0.54 for STS-IE score, 0.56 for De Feo-Cotrufo score, 0.59 for Costa score, 0.58 for PALSUSE and 0.64 for Risk-E. In contrast, EuroSCORE II showed HR 1.0; 95% CI 1.0–1.1 p=0.01 in assessment of late survival with C index: 0.59.
DiscussionThe main finding of our analysis is that long term survival after hospital discharge in patients who survive an episode of left side active infective endocarditis is acceptable,21 and the severity of the IE affectation does not affect long-term survival.
First, it has been well established that in-hospital mortality is related to several factors like IE-specific factors (microbiological cultures, abscess formation, sepsis, …),7,12 preoperative status and surgical complications. New specific risk scores showed improved risk assessment in IE surgery because they included IE specific factors that can impact early mortality and are not addressed by classical risk scores.20,22,23 Accordingly in our sample, we found that 6 variables were independent risk factors for in-hospital mortality, three of them were specific IE factors: multivalvular involvement (OR 9.2; 95% CI 2.8–29.6), abscess (OR 2.1; 95% CI 0.8–5.6) and thrombocytopenia (OR 3.7; 95% CI 1.5–9.3). This finding is consistent with previously published scores.7,11,12,14
Second, since long-term prognosis after discharge has been much less investigated, independent factors related to long-term mortality are still unknown. In the present report we analyzed late prognosis in a surgical cohort of patients operated on with active IE and also variables that could impact long-term survival. Despite the fact that mortality in the immediate postoperative period was high, late survival of patients who were discharged alive was acceptable after five years of follow up (77.6%) (Fig. 1). The reported late survival rate was better than in some previously published studies,8,24,25 although the majority of them included medically treated patients. Our study exclusively included patients undergoing surgery, similar to the David et al.26 27-year period study. The authors of that study reported 12% operative mortality and 23% late mortality, higher than other previously published mortality rates and more similar to our own results.
Analysis of factors associated with long-term mortality in previously published studies is difficult to review because the majority of studies include in-hospital mortality and long-term mortality mixed together in the same analysis. Netzer et al.,27 in a study in which both periods were separated, found that prediction factors for long term mortality were: age, congestive heart failure and few clinical symptoms. Mihaljevic and Cols28 published long-term results for multiple IE valve surgery that showed no relationship between active valve IE and late mortality. Marushchak et al.29 described long-term IE survival to be similar to survival rates after valve replacements for non infectious indications matched by age, sex, and valve. Similar to all these studies, we found that long-term survival was independent of all analyzed IE factors.
As expected, Cox regression showed that long-term mortality was ineffectively predicted with risk scores. The accuracy in mortality risk prediction by Harrell's concordance index for survival data (‘C index’) showed suboptimal prognostic ability with all of the analyzed scores. Among the scores, Risk-E score and EuroSCORE II showed a better mortality prediction (C index of 0.65 and 0.59) than STS-IE, PALSUSE, Costa or De Feo-Cotrufo score. EuroSCORE II, which showed the best long-term mortality prediction (HR 1.0; 95% CI 1.0–1.1 p=0.01), is also a marker of increased comorbidities, since late survival was significantly associated with increasing values of EuroSCORE II. Although in-hospital mortality risk would be more accurately estimated by specific scores,22,23 long-term mortality after discharge seemed to be independent of IE specific factors. Instead, long-term mortality was related to patient baseline characteristics and the presence of cardiovascular risk factors. EuroSCORE II, as it has been previously described, is related with long-term mortality after any type of cardiac surgery.30,31
Therefore, although cardiac surgery is an essential procedure in the treatment of IE, it is also associated with remarkably high in-hospital mortality that could lead to discrepancies between the existence of surgical indications and the actual realization of the surgical procedure.1,4 Our results confirmed a high surgical risk for patients undergoing cardiac surgery in our center that could be adequately estimated by specific-IE risk scores.22 However, in patients who survived the early postoperative period, long-term survival was good and it was comparable to expected survival after a standard cardiac surgery procedure regarding previously published literature.32,33 In contrast, the outcome of patients with IE who have indication for surgery, but who do not undergo surgery is disastrous.5,34 In that context, multidisciplinary evaluation of surgical risk is crucial for determining whether to perform surgery.35 Late survival was not affected by the severity of the affectation, local destruction or microbiological findings. As a consequence, once a patient with left-sided IE discharged alive after cardiac surgery, clinical follow-up should be performed similarly to any other patient with prosthesis valve.
The main limitation of our study was the small sample size with only 180 patients that may be a source of bias in the statistical analysis of the results. In addition, it was a single-center retrospective observational study, and therefore a potential inherent bias with observational studies cannot be discarded. The study incorporated patients from 2002 to 2016, therefore the observation time frame is quite large and it could hamper generalizability of the findings. In addition, the relatively long period had a potential risk for changes in surgical techniques and advances in diagnostic and therapeutic tools. This may have modified the observed results. However, there was not any particular trend in the year-to-year observed mortality rate during the study period. Our study may also have a potential referral bias because it was developed in a tertiary center. We only included patients who underwent surgery for active IE, and as a consequence our results are not applicable to medically treated patients. Endocarditis in implantable cardiac devices was not included in our study, therefore our results could not be extrapolated to this population. Regarding missing data, we lost the information of four patients during the follow-up period, but complete follow-up was possible for 91.7% of surviving patients.
In conclusion, despite the high perioperative mortality rate after surgical treatment for active IE, long-term survival after hospital discharge was acceptable, regardless of the severity of the endocarditis episode. Although in-hospital survival depended mainly on several IE factors, long-term survival was not related to the severity of endocarditis baseline affection.
Ethical approvalThe Ethical Review Board (ERB) of the Hospital approved the implementation of this study (ERB number 313/2016, approved on November 28, 2016).
Informed consentThe requirement for informed written consent was waived. Patient identification was encoded, complying with the requirements of the Organic Law on Data Protection 15/1999.
FundingThis research received no specific grant from any funding agency, commercial or non-profit.
Conflict of interestThere was no conflict of interest.
The present authors sincerely thank Kevin Boltuch for his contribution to language editing of the article.