Gastric cancer (GC) is a complex disease and a worldwide health burden due to its high prevalence and poor prognosis. A deeper knowledge of the factors involved in the development and progression of GC could help to identify subpopulations at risk that therefore require surveillance or early treatment strategies. Current research is based on the study of genetic variants that confer a higher risk of GC and their interactions with environmental exposure. Recently, meta-analysis has emerged as an important statistical method involving pooling of data from individual association studies to increase statistical power and obtain more conclusive results. Given the importance of chronic inflammation in the process of gastric carcinogenesis, the present article reviews the most recent meta-analyses of the contribution of cytokine gene polymorphisms to GC risk.
El cáncer de estómago es una patología compleja que representa un grave problema sanitario a nivel mundial tanto por su incidencia como por el pobre pronóstico de los pacientes que lo padecen. Un mejor conocimiento de los factores implicados en el desarrollo y evolución del cáncer gástrico (CG) nos ayudará a identificar qué sub-poblaciones de individuos tienen un mayor riesgo de desarrollar CG y precisan, por tanto, un seguimiento más detallado o una actuación terapéutica precoz. En la actualidad, la investigación sobre epidemiología genética de enfermedades complejas está orientada a la identificación de variantes genéticas que aumenten la susceptibilidad y al estudio de su relación con factores ambientales. En los últimos años, el meta-análisis ha surgido como una importante herramienta estadística que permite englobar los datos obtenidos en los estudios de asociación, aumentando el poder estadístico de dichos estudios y obteniendo resultados más concluyentes. Dada la importancia de la inflamación crónica en el proceso de carcinogénesis gástrica, revisaremos en nuestro artículo los meta-análisis realizados en los últimos años sobre polimorfismos en genes que codifican la síntesis de citocinas y su implicación en el desarrollo del CG.
Gastric cancer (GC) represents the fourth most common cancer and the second most frequent cause of cancer deaths worldwide.1 Despite advances in diagnosis and treatment, most patients with GC present with late-stage disease and an overall 5-year survival of approximately 20–30% in Western countries.2,3 GC is well known to be a heterogeneous disease which shows distinct clinical, epidemiological, and molecular features among tumours arising from the cardia or non-cardia within the stomach, and among intestinal and diffuse histological subtypes.4,5 These phenotypic differences are determined by the combined effects of multiple environmental, namely smoking habit and diet,6,7 and genetic risk factors. Among them, Helicobacter pylori (H. pylori) infection has been identified as the single most common cause of GC.8 This organism, which colonizes over half of the world's population, first induces a chronic superficial gastritis in most infected people, initiating a process that in certain individuals may lead to GC. However, it is still a matter of speculation as to why only a minority (<1%) of the population infected with H. pylori develops gastric malignancy, suggesting that factors other than bacterial infection alone participate in the carcinogenesis process.
Gastric cancer and genetic susceptibilityAdditional host genetic risk factors are also likely to contribute in gastric carcinogenesis. In this context, gene polymorphisms have been thought to be attractive biomarkers of GC risk since they may modify the effect of environmental exposures. In fact, individual genetic susceptibility may be critical in several processes relevant to gastric carcinogenesis such as mucosal protection, immune response to H. pylori infection, carcinogen detoxification and antioxidant protection, repair of DNA damage, and cell proliferation ability.9
In the year 2000, El-Omar and co-workers first reported the association of specific gene variants with an increased risk of GC.10 According to the authors, the interleukin-1 beta IL1B-31T (rs1143627) and IL-1 receptor antagonist IL1RN*2/*2 genotypes were associated with an increased risk of both, chronic hypochlorhydria and GC, presumably by altering IL-1β levels in the stomach. Since this first landmark report, numerous studies concerning the association of candidate gene polymorphisms and GC risk have been conducted. However, results are inconsistent among studies.11–16 The reasons for these discrepancies are unclear and differences in ethnicities, methodologies, and dominance of different etiologic factors in different populations have been suggested as possible explanations. Nevertheless, most of the studies reported in the literature are small sized and underpowered to detect a robust association. In order to solve this problem, meta-analysis became an important statistical method involving pooling of data from individual association studies to increase sample size, improve statistical power and draw more reliable conclusions.17
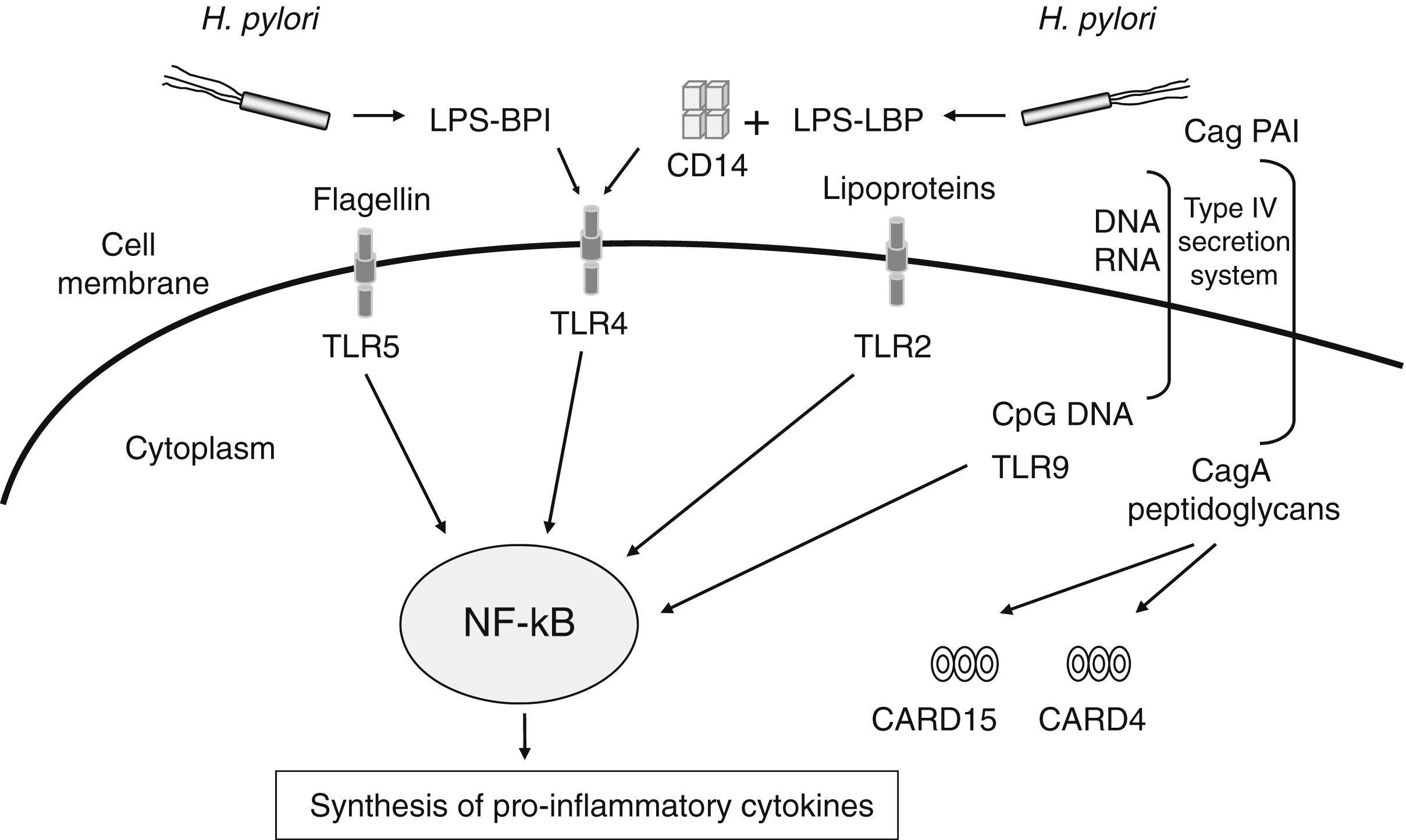
The number of meta-analyses assessing genetic markers of GC risk has dramatically increased in recent years. Given the relevance of H. pylori infection in chronic inflammation and gastric carcinogenesis, one of the more extensively explored pathways has been that related to the inflammatory response to H. pylori infection (Table 1). The innate immune response constitutes the first line of defence against infection by microbes such as H. pylori. This system senses, recognizes, and responds to bacterial components (lipopolysaccharide, peptidoglycan, lipoproteins, flagellin and bacterial DNA) by activating a complex signalling cascade of pattern-recognition receptors (PRRs) located on the surface and cytosol of gastrointestinal epithelial cells, which include CARD15/NOD2, CARD4/NOD1, CD14 and TLRs receptors18 (Fig. 1). Several variants in genes of this arm of the immune system have been shown to affect responsiveness to H. pylori infection, leading to an aberrant activation of the NF-kB pathway and up-regulation of pro-inflammatory molecules. Hold et al. reported the association of the TLR4+896A>G (rs4986790) polymorphism, which results in amino acid substitution (Asp299Gly) affecting TLR4 extracellular domain, with increased risk of noncardia GC and its precursors in H. pylori infected patients.19 This finding has been corroborated by two recent meta-analyses in which the TLR4+896 (rs4986790) G allele was significantly associated with distal GC risk mainly in Western populations.20,21 However, no association between other TLR4 (rs4986791, rs11536889), TLR2 (−196 to −174del), and CD14 (rs2569190) gene polymorphisms extensively studied in several populations were found.
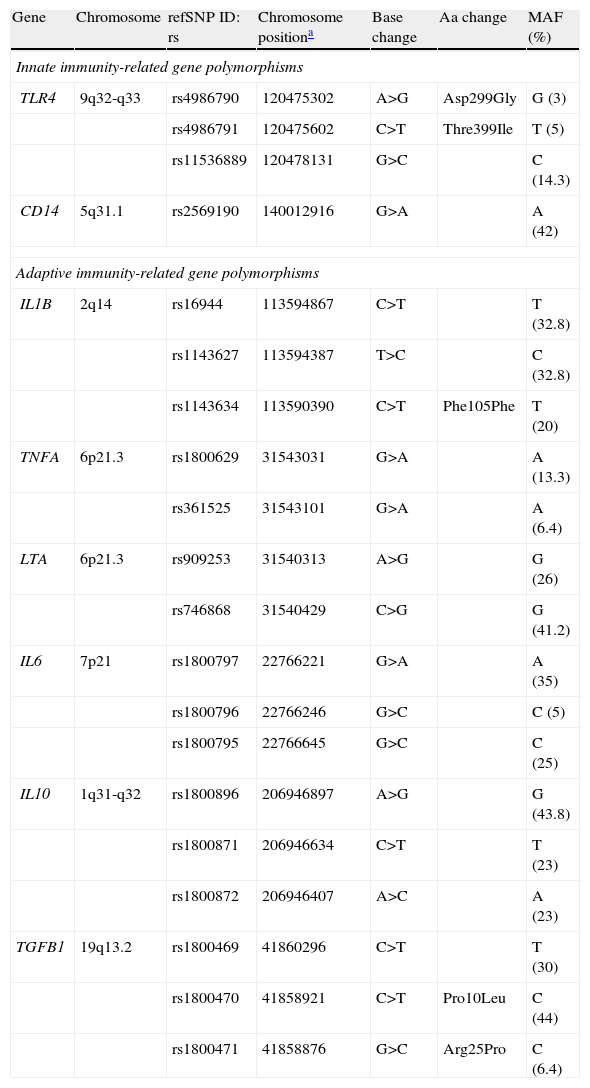
Innate and adaptive immunity-related gene polymorphisms (SNPs) associated with gastric cancer risk.
| Gene | Chromosome | refSNP ID: rs | Chromosome positiona | Base change | Aa change | MAF (%) |
| Innate immunity-related gene polymorphisms | ||||||
| TLR4 | 9q32-q33 | rs4986790 | 120475302 | A>G | Asp299Gly | G (3) |
| rs4986791 | 120475602 | C>T | Thre399Ile | T (5) | ||
| rs11536889 | 120478131 | G>C | C (14.3) | |||
| CD14 | 5q31.1 | rs2569190 | 140012916 | G>A | A (42) | |
| Adaptive immunity-related gene polymorphisms | ||||||
| IL1B | 2q14 | rs16944 | 113594867 | C>T | T (32.8) | |
| rs1143627 | 113594387 | T>C | C (32.8) | |||
| rs1143634 | 113590390 | C>T | Phe105Phe | T (20) | ||
| TNFA | 6p21.3 | rs1800629 | 31543031 | G>A | A (13.3) | |
| rs361525 | 31543101 | G>A | A (6.4) | |||
| LTA | 6p21.3 | rs909253 | 31540313 | A>G | G (26) | |
| rs746868 | 31540429 | C>G | G (41.2) | |||
| IL6 | 7p21 | rs1800797 | 22766221 | G>A | A (35) | |
| rs1800796 | 22766246 | G>C | C (5) | |||
| rs1800795 | 22766645 | G>C | C (25) | |||
| IL10 | 1q31-q32 | rs1800896 | 206946897 | A>G | G (43.8) | |
| rs1800871 | 206946634 | C>T | T (23) | |||
| rs1800872 | 206946407 | A>C | A (23) | |||
| TGFB1 | 19q13.2 | rs1800469 | 41860296 | C>T | T (30) | |
| rs1800470 | 41858921 | C>T | Pro10Leu | C (44) | ||
| rs1800471 | 41858876 | G>C | Arg25Pro | C (6.4) | ||
MAF: Minor Allele Frequency. Data concerning Caucasian population.
Schematic representation of PRRs (pattern-recognition receptors) and their activating ligands involved in the signalling of the innate immune response against Helicobacter pylori infection. This system recognizes and responds to bacterial components (lipopolysaccharide, peptidoglycan, lipoproteins, flagellin and bacterial DNA) by activating a complex signalling cascade of PRRs located on the surface and cytosol of gastrointestinal epithelial cells (TLRs receptors, CD14, CARD15/NOD2, CARD4/NOD1), leading to the activation of the NF-kB pathway and up-regulation of pro-inflammatory molecules.
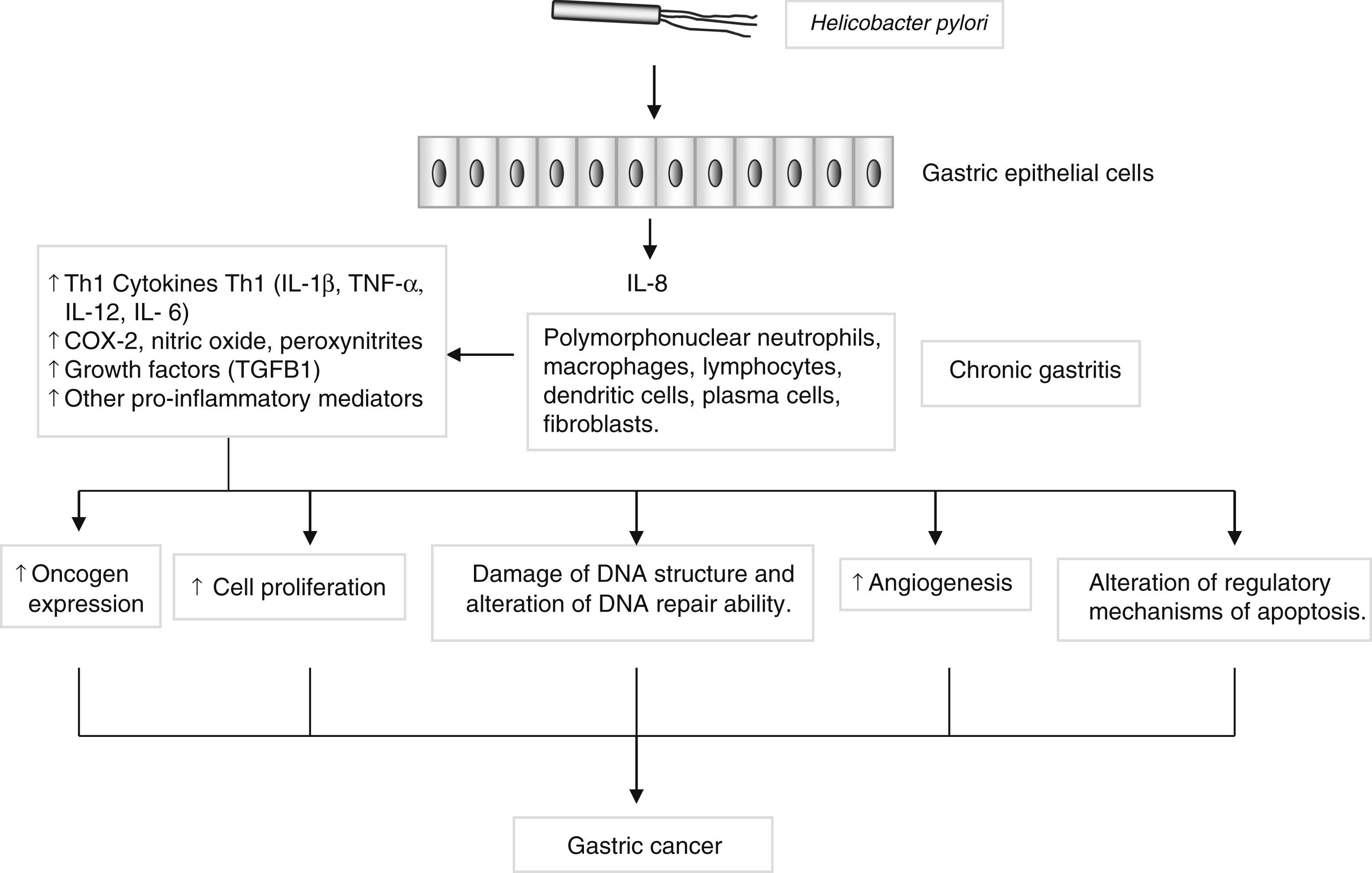
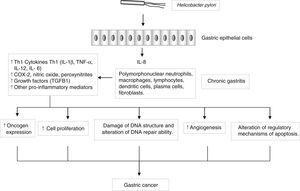
Genes encoding pro- and anti-inflammatory mediators, such as IL1B, TNF, LTA, IL6, IL1RN, IL10 and TGFB, are also of special interest since they are thought to play a key role in GC development and progression22 (Fig. 2). A large number of epidemiological studies have investigated the impact on GC susceptibility of 3 functionally important base transitions at positions −31T>C (rs1143627), −511C>T (rs16944), and +3954C>T (rs1143634) in the IL1B gene, and a variable number of an 86-bp tandem repeat polymorphism (VNTR) in intron 2 of the IL1RN gene. Recently, two meta-analyses performed by Zhang et al.23 and Xu et al.24 report the association of IL1RN2 and ILIB −511T alleles with an increased risk of distal GC with less conclusive results for the IL1B+3954 variant. Interestingly, the IL1RN2 allele has been associated with high circulating IL-1β levels.25 The relevance of this finding derives from the biological properties of IL-1β as a potent pro-inflammatory cytokine and a powerful inhibitor of acid secretion. As suggested by El-Omar et al.10 those genotypes that enhanced IL-1β production may favour the initiation of a set of events leading to chronic hypochlorhydria, corpus atrophy and increased risk of GC.
Helicobacter pylori induces a chronic superficial gastritis characterized by an infiltration of polymorphonuclear neutrophils, lymphocytes, macrophages and plasma cells. The main biological mediators in this inflammatory response are cytokines (IL-1, IL-6, IL-12, TNF-α, IFN-γ), low-molecular-weight peptide molecules produced by a large variety of cells that possess a broad range of physiological functions. However, persistent inflammation with the subsequent release of pro-inflammatory mediators may induce changes in the epithelium, initiating a process that in certain individuals may lead to the development of gastric cancer.
Similar to IL-1β, tumour necrosis factor-α (TNF-α) and lymphotoxin-α (LT-α, formerly known as TNF-β) are extremely pleiotropic cytokines which play a key role in controlling the inflammatory response in the gastrointestinal mucosa. Although not as potent as IL-1β, TNF-α is also capable of inhibiting gastric acid secretion.26 Among many polymorphisms identified in the TNFA and LTA genes, two G>A nucleotide substitutions at positions −308 (rs1800629) and −238 (rs361525) in the promoter region of the TNFA gene and two restriction fragment length polymorphisms (RFLP) (NcoI +252 G>A rs909253, and AspHI +318 G>C rs746868) in the first intron of the LTA gene, affecting TNF-α and LT-α production, have been extensively studied in relation to GC. To date, two published meta-analyses27,28 on this issue have suggested that the TNFA −308 A allele is associated with GC in Caucasians, but not in Asian populations. However, a recent large two-stage case-control study performed by Hong et al.29 reported the association of the TNFA −308 A variant with GC risk and progression in a Chinese population. Moreover, another meta-analysis by Lu et al.30 showed the association of the LTA rs909253 G>A polymorphism with a higher risk of GC in H. pylori infected patients, specific for the Asian population. However, no association between GC risk and either the TNFA -238 or the LTA rs746868 polymorphism was found.
IL-6 is also a multifunctional cytokine that plays a major role in the inflammatory response. Production of IL-6 by GC cell lines31 and high levels of IL-6 in serum and tumour tissue in GC patients have been reported.32 Three single nucleotide polymorphisms (SNPs) at positions −174G>C (rs1800795), −572G>C (rs1800796) and −597G>A (rs1800797) in the promoter region of the IL6 gene, influencing IL-6 gene transcription and serum levels,33 have been investigated for GC association in several studies. El-Omar et al.11 first evaluated the association of the IL6-174G>C polymorphism and GC risk in Caucasians and no association was found. Similar results have been reported in two recent meta-analyses by Wang et al.34 and Yin et al.35 in which no associations between IL6-174, -572, and -597 variants and GC risk were observed. However, and as point out by the authors, these results should be interpreted with caution due to the limited number of subjects included in the analyses and the selection bias detected in some studies.
On the other side of the balance, the anti-inflammatory cytokine IL-10 and the transforming growth factor β1 (TGF-β1) are potent suppressors of the Th1-type immune response resulting in down-regulation of the production of pro-inflammatory mediators by macrophages and other immune cells.36,37 Three SNPs in the IL10 promoter at positions −1082G>A (rs1800896), −819C>T (rs1800871), and −592C>A (rs1800872) have been found to be biologically relevant.38 Correlated with variations in IL-10 levels, these functional polymorphisms were suspected to influence GC susceptibility by altered inflammatory responses at the gastrointestinal mucosa. However, data reported in the literature showed very inconsistent results. Four meta-analyses exploring the role of IL10-1082,39,40 -81941 and -59242 gene polymorphisms in GC risk have been published during the year 2012. In summary, the results suggest that IL10-819 TT and IL10-592 AA genotypes may be protective factors for GC in Asians,41,42 while the IL10-1082 A allele was found to be a protective allele for GC risk in the overall population.39,40 Concerning TGFB1, several polymorphisms have been reported to affect TGF-β1 expression and plasma levels.43,44 Among them, a SNP at position -509C>T (rs1800469) in the promoter region and two SNPs at positions +869T>C (rs1982073) and +915G>C (rs1800471) in the signal protein sequence of the gene, which change codon 10 (Leu/Pro) and codon 25 (Arg/Pro), respectively, have been extensively studied in relation to H. pylori infection-related diseases, including GC. However, three recent meta-analyses45–47 found no significant association between the TGFB1 +869T>C and +915G>C polymorphisms and risk of GC with less conclusive results for the TGFB1 −509C>T variant.
In summary, current research is based on the study of genetic variants that confer a higher risk of GC and their interactions with environmental exposure, namely H. pylori infection. In spite of some limitations (only published studies are included, heterogeneity between studies, source of controls, representativeness of cases or quality of studies), meta-analysis is emerging as a promised statistical tool to evaluate the contribution of host genetic factors in GC risk. Our review points out the relevance of well-designed meta-analyses assessing the role of candidate cytokine gene polymorphisms in GC susceptibility, and suggests the association between specific variants of the IL1RN VNTR, IL1B-511 (rs16944), TNFA-308 (rs1800629), and IL10 -1082 (rs1800896) gene polymorphisms and GC risk. A deeper knowledge of factors involved in CG development and progression may allow identification of those individuals at risk and may provide useful predictive information for subgroups of patients who need surveillance or early treatment strategies.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by the Spanish “Fondo de Investigaciones Sanitarias” (grant number PI09/00213) and Instituto de Salud Carlos III (CIBER de enfermedades hepáticas y digestivas).