Invasive aspergillosis is a disease typically related with prolonged neutropenia or the use of corticosteroids. However, the increased use of new therapeutic modalities such as biologic agents that act by blocking specific immune pathways have put more patients at risk for invasive aspergillosis. Most cases of aspergillosis in patients taking monoclonal antibodies have been associated with the use of tumour necrosis factor (TNF)-alpha blockers. However, many more drugs have been implicated, including interleukin-2 inhibitors, and CD52 and CD20 blockers. In this manuscript we review the pathophysiology associated with an increased risk for aspergillosis in these patients, in addition to diagnostic and therapeutic considerations.
La aspergilosis invasora es una enfermedad estrechamente relacionada con situaciones de neutropenia prolongada o el uso de corticosteroides. Sin embargo, el uso de nuevas estrategias terapéuticas, como los agente biológicos que actuan bloqueando la respuesta inmune específica, ha aumentado el riesgo de los pacientes a sufrir esta infección. La mayor parte de los casos de aspergilosis en pacientes bajo terapia de anticuerpos monoclonales se han asociado al uso de bloqueadores del factor de necrosis tumoral alfa (TNFα). Sin embargo, muchos otros fármacos parecen implicados en estos casos, incluyendo los inhibidores de la interleuquina 2 y bloqueadores de CD52 y CD20. En este trabajo, se hace un repaso de la fisiopatología en este tipo de pacientes como factor de riesgo de la aspergilosis, además de revisar el diagnóstico y el tratamiento de esta enfermedad.
The variety of diseases associated with Aspergillus species depends intimately on the host immune system. Aspergillosis may occur as a life-threatening invasive infection in the severely neutropenic patient and cause allergic diseases in individuals suffering from asthma or cystic fibrosis. Chronic pulmonary infections are also frequently seen in immunocompetent patients with previous structural damage to the lungs.71
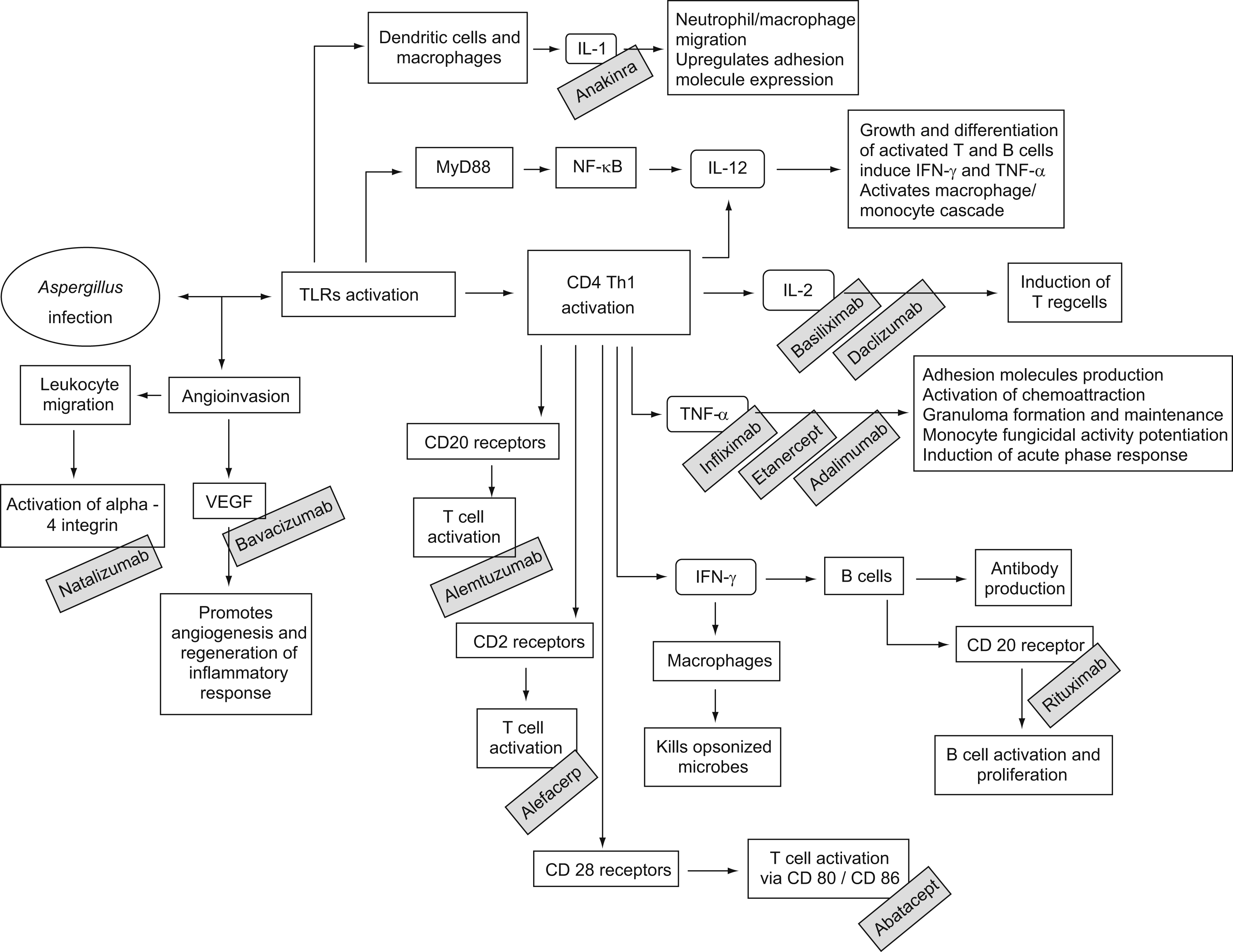
Although prolonged neutropenia is the most important risk factor for invasive aspergillosis (IA), many patients who develop this condition have normal neutrophil counts. Therapy with corticosteroids is possibly the second most important risk factor.2 Moreover, progresses in modern medicine have brought more immunosuppressive drugs to the market, including monoclonal antibodies. These are products of a B cell clone and have revolutionized the treatment of diverse conditions, like cancer, rheumatologic diseases and graft versus host disease (GVHD). For instance, the use of drugs that block natural host immune defence pathways against fungi—such as tumour necrosis factor-alpha (TNF-α)—may render patients at risk for IA (figura 1). In this article we review published cases of aspergillosis in association to the use of monoclonal antibodies.
Figure 1. Monoclonal antibodies may increase the overall risk for invasive aspergillosis by blocking several important components of the host immune system. IFN, interferon; IL, interleukin; NF- κB, nuclear factor kappa B; Th, T helper cells; TLRs, tool-like receptors; TNF, tumour necrosis factor; Treg, T regulatory cells; VEGF, vascular endothelial growth factor.
Search strategyFor the purpose of this review we searched PubMed database using several combinations of the following free terms: Aspergillus, aspergillosis, monoclonal antibodies, rituximab, infliximab, etanercept, adalimumab, anakinra, abatacept, natalizumab, bevacizumab, alemtuzumab, alefacept, efalizumab, basiliximab and daclizumab. References from relevant articles were also reviewed. Conference abstracts were reviewed using the Aspergillus website ( http://www.aspergillus.org.uk ).
Tumour necrosis factor-alpha (TNF-α) inhibitorsThe host defence against Aspergillus infections is mediated by phagocytic cells of the innate immune system—alveolar macrophages ingest and kill Aspergillus conidia, whilst polymorphonuclear leukocytes destroy germinating Aspergillus hyphae that have escaped from macrophages.9,45 Neutrophils participate in the adaptive T helper cell response that in turn modulates antifungal activity, enhancing phagocyte effector cell function.46 T helper 1 lymphocytes (Th1) enhance fungicidal activity by the production of pro-inflammatory cytokines such as TNF-α, interferon-gamma (IFN-γ), and the interleukins (ILs) IL-2 and IL-12. Conversely, T helper 2 lymphocytes (Th2) produce other cytokines (i.e., IL-4, IL-5 and IL-10) that produce the opposite effect, by suppressing macrophage/neutrophil phagocytic activity. In animal models of IA, this shift to a Th2 response is associated with unfavourable outcomes.46 TNF-α inhibition can increase susceptibility to infections through inhibition of IFN-γ production, a decreased expression of pattern-recognition receptors and by promoting leukocyte apoptosis.60 TNF-α has yet a non-chemotactic effect for phagocytic cells, inducing leukocyte and endothelial cell expression of adhesion molecules, influencing phagocytic cell trafficking in the lungs.9 TNF-α is also critical for granuloma formation and maintenance. Granulomas have a role in containing the systemic spread of intracellular pathogens, containing persistent tuberculosis (TB) and preventing its reactivation, for example.56 TNF-α has also a role in mediating macrophage apoptosis72 and potentiating anti-fungicidal activity of human monocytes6. Both TNF-α and IFN-γ potentiate the expression of Toll-like receptor 4 (TLR4) on the cell membrane. TLR4 expression is important for recognition of fungal pathogens such as Candida albicans and Aspergillus fumigatus by host cells (e.g., dendritic cells and macrophages). Patients receiving anti-TNF-α therapy could be at increased risk for severe fungal infections because of an inability to recognise fungal antigens via toll-like receptor signalling.
Bronchoalveolar macrophages secrete TNF-α in response to exposure to Aspergillus conidia6. It has been demonstrated that challenging mice intrathecally with Aspergillus conidia results in a time-dependent increase in lung TNF-α levels, with monoclonal antibody neutralization of TNF-α resulting in a decrease in lung neutrophil recruitment, increase in fungal burden and higher mortality rate.54 Blocking TNF-α results in reduced influx of neutrophils into the lungs and delayed fungal clearance.82 In mice, TNF-α prevents Aspergillus dissemination from lungs to other organs.66 TNF-α also acts in late immunological defence by means of superoxide anion production, which results in hyphal damage and destruction.75,77 TNF-α knockout mice show altered IgG and IgE antibody reactions, antibody responses are impaired, and antigen presentation is inconsistent; in addition, granuloma formation is also impaired.44
The pro-inflammatory cytokine TNF-α has also been implicated in a majority of autoimmune diseases, including rheumatoid arthritis and Crohn's disease.44 In rheumatoid arthritis, overexpression of TNF-α leads to articular inflammation and to destruction to adjacent bone and cartilage, with TNF-α being expressed in diseased joints.22 In addition, TNF-α is central in the pathophysiology of GVHD following haematopoietic stem cell transplantation (HSCT).15 Based on TNF-α role in acute and chronic inflammation, anti-TNF-α agents have been used in the treatment of conditions such as Crohn's disease,69 ulcerative colitis,90 rheumatoid arthritis,17,28,64 psoriatic arthritis,12,68 ankylosing spondylitis,7,8,18,33 juvenile rheumatoid arthritis,74 Behçet disease,55 sarcoidosis,83 Wegener's granulomatosis48 and GVHD.15,35,36
Several biologic agents targeting TNF-α have been developed during the past decade. Three of these drugs have been cleared by the Food and Drug Administration (FDA, USA): etanercept (Enbrel®), infliximab (Remicade®) and adalimumab (Humira®).20,36,77 TNF-α-blocking agents differ in composition, mechanism of action, pharmacokinetics, biopharmaceutical properties, in vitro cell lysis capabilities and immunomodulatory effects.28 Etanercept is a soluble p75 TNF receptor fusion protein that consists of two p75 TNF receptors bound to the Fc portion of IgG, binding both TNF-α and lymphotoxin-α—it is administered once or twice weekly via subcutaneous injection. Infliximab is a chimeric monoclonal antibody comprised of 75% human and 25% mice protein directed against TNF-α. It binds to TNF-α and inhibits its ability to bind to receptors in lamina propria cells. Infliximab is administered intravenously every 6–8 weeks—it neutralizes TNF-α in a dose-dependent manner with higher doses providing better disease control and longer therapeutic responses compared with lower dose regimens. Adalimumab is a humanized monoclonal antibody that is administered subcutaneously. Different regimens may be used—from daily to twice monthly administration.92 Infliximab and adalimumab have a higher affinity to the TNF receptor than etanercept,81 acting in both transmembrane and soluble TNF receptors.
The use of biologics agents such as anti-TNF-α drugs is associated with an increased risk of infection, which is somehow expected based on their mechanism of action.77,95 No significant difference has been detected amongst the three TNF-α blockers in terms of infection rates,28 although some reports have suggested that infections are more frequently associated with infliximab than etanercept. However, the small number of reported cases in these series limited the statistical power of such observations.83 A recent meta-analysis8 showed an odds ratio (OR) for serious infection of 2.0 (95% confidence interval, CI 1.3–3.1) in patients using rituximab or adalimumab for rheumatoid arthritis, with a number need to harm of 59 (95% CI 39–125). Tuberculosis and other granulomatous infections are major problems in these patients, with many patients manifesting extrapulmonary TB.32,43 TB attack rate has been deemed high enough to lead to a recommendation that a tuberculin test should be obtained before biologic therapy is started and every 12 months.15 In addition, a chest radiography before or within 3 months of initiation of treatment should be obtained.101 Combination therapy with infliximab and methotrexate may cause a particularly severe form of immunosuppression with multiple opportunistic infections due a combination of prolonged leukocytopenia and depressed cellular immunity.93
The risk for invasive fungal diseases (IFDs) may also be increased in patients taking TNF-α blockers. A review of 251 cases of IFD associated with TNF-α inhibitors92 found that 86% of cases were associated with infliximab, 14% with etanercept and none with adalimumab. The use of at least one other immunosuppressant medication, typically a systemic corticosteroid, was reported during the course of the fungal infection in 99% of patients.92 The time to onset of IFD after TNF-α blockage varied between a median of 55 days (range 15–140 days) for infliximab to 144 days (46–240 days) for etanercept. A cohort study of allogeneic HSCT recipients with severe GVHD58 showed that the incidence of IFD in patients receiving infliximab was 6.8 cases/1000 GVHD patient-days, in comparison with 0.5 cases/1000 GVHD patient-days in those not treated with this drug. The adjusted IFD hazard ratio of infliximab exposure was 13.6 (95% CI 2.29–80.2). Most IFDs in this study were caused by Aspergillus species. The cumulative probability of IFD was significantly higher and the median time for infection was shorter in infliximab recipients than in non-recipients (67 versus 164 days).52 It has been suggested that prophylaxis against filamentous fungi is justified in this scenario.15 The most prevalent fungal infections after TNF-α blockade therapy in allogeneic HSCT recipients are histoplasmosis, invasive candidosis, cryptococcosis, coccidioidomycosis and aspergillosis.92
Numerous reports and case series have described aspergillosis following use of biologic agents.1,10,14,15,16,20,26,29,41,43,50,52,55,56,58,59,76,86,89,94,96,98,99,100tabla 1,tabla 2 describe the main drugs associated with cases of IA, as well as concomitant risk factors described for these patients. The first reported case was published just 8 years ago in a 25-year old man with fistulizing Crohn's disease that was not on adjunctive therapy with corticosteroids or other immunosuppressive agents.98 IA following anti-TNF-α therapy usually occurs as a lung infection, which is similar to IA affecting other patients at risk. The most frequent underlying diseases in these patients are corticosteroid-refractory GVHD, rheumatoid arthritis and Crohn's disease. In a retrospective cohort of patients treated with infliximab for acute GVHD16, 48% of patients developed fungal infections. These were caused by species of Candida (n=10) and Aspergillus (n=7). Busca et al. reported their experience with the use of etanercept for acute refractory GVHD in 21 patients.10 Cytomegalovirus (CMV) reactivation occurred in 48% of patients, bacterial infections in 14%, and fungal infections in 19%. Three patients developed an Aspergillus infection, which included cases of pulmonary IA, pulmonary aspergillosis in combination with pneumocystosis and cerebral aspergillosis. Cases of breakthrough aspergillosis associated with infliximab therapy have also been reported in HSCT patients on prophylaxis with caspofungin.12 Only in a few occasions have aspergillosis been described in association with adalimumab use.62
Table 1. Monoclonal antibodies and risk for invasive aspergillosis.
| Risk definitively increase | Risk may be increased | Risk is increased when associated with another immunosupressors | Theoretical risk but no conclusive evidence |
| Infliximab | Rituximab | Basiliximab | Anakinra |
| Etanercept | Adalimumab | Daclizumab | Alefacept |
| Alemtuzumab | Abatacept | Natalizumab |
| Efalizumab |
| Bevacizumab |
Table 2. Additional risk factors for invasive aspergillosis in patients treated with monoclonal antibodies.
| Underlying haematological malignancy |
| Chemotherapy-induced neutropenia |
| Therapy with corticosteroids for GVHD |
| Receipt of other immunosupressive drugs following transplantation |
GVHD—graft versus host disease.
In a retrospective study97 analysing the FDA Adverse Events Reporting System for the rate of granulomatous diseases in patients receiving infliximab and etanercept, the risk for aspergillosis was defined as 12.4 and 8.8 per 100,000 patients, respectively. Most patients in the study were also concomitantly receiving therapy with other immunosuppressive agents, including corticosteroids (infliximab group, 41%; etanercept, 66%) and methotrexate (43% and 41%, respectively). Adalimumab was not included because it was not FDA approved at the time of that study. The FDA has recently published online a warning to health care professionals regarding an increase risk for IFD in patients taking TNF-α blockers.35
IL-2 inhibitorsBasiliximabBasiliximab (Simulect®) is a chimeric (human/murine) monoclonal antibody that selectively binds to the α-subunit (CD25) of IL-2r receptors on the surface of activated T lymphocyte. This specific IL-2r binding competitively inhibits IL-2-mediated lymphocyte activation, a crucial phase in cellular immune response of allograft rejection.70 Basiliximab is highly effective in the prevention of acute organ rejection in adult and paediatric renal transplant recipients, in combination with other immunosuppressive agents.75 This agent may be used as induction therapy during solid transplantation,63 to treat acute or chronic GVHD in association with infliximab,78 and also in situations such as leukaemia and lymphoma,96 and multiple sclerosis.56 Basiliximab significantly reduced acute rejection compared with placebo in renal transplant recipients receiving dual- (cyclosporine and corticosteroids) or triple-immunotherapy (azathioprine or mycophenolate mofetil based). The incidence of adverse events was similar in basiliximab and placebo recipients, with no apparent increase in the overall incidence of infections.11,71
A few cases of aspergillosis have been reported in patients treated with basiliximab. A heart transplant recipient was diagnosed with invasive pulmonary aspergillosis 22 days after therapy was basiliximab was started.13 The patient was also concomitantly in use of tacrolimus, mycophenolate mofetil and prednisone. In a second paper29 two cases of Aspergillus infection were reported in renal transplant recipients receiving a graft from the same donor. Both patients received an induction therapy consisting of cyclosporine, corticosteroids and basiliximab. Diagnosis was confirmed by culture in both cases, both manifesting an iliac pseudoaneurysm. Two cases of aspergillosis were also reported in a Chilean study in which renal transplant recipients received induction therapy with basiliximab.20 It is important to note, however, that in all the cases cited the patients were in concomitant use of other immunosuppressive drugs.
DaclizumabDaclizumab is a humanized anti-IL-2r antibody. The drug is 90% human, retaining 10% murine compartments in critical hypervariable segments for binding specificity.70 Daclizumab has been used in association with TNF-α blockers in corticosteroid-refractory acute GVHD,88,102 and cases of aspergillosis have been described in patients receiving such drug combinations. Three cases of aspergillosis were reported in patients taking daclizumab in association with infliximab,88 with five additional cases described when daclizumab was combined with etanercept.102 In another study,87 66% of HSCT patients with corticosteroid-refractory GVHD treated with anti-thymocyte globulin and mycophenolate mofetil developed IA, with no IA case being reported in patients treated with daclizumab (alone or in combination with infliximab or anti-thymocyte globulin; p<0.01). Willenbacher et al.99 reported only one case of IA amongst 16 patients who received daclizumab for the treatment of GVHD. In a cohort study of heart-transplanted patients the use of daclizumab was not related with increased incidence of early or late intensive care unit (ICU) admission secondary to aspergillosis.64 One case of A. versicolor infection was reported in a pancreas transplant recipient with pure cell aplasia following immunosuppressive therapy with daclizumab, mycophenolate mofetil and alemtuzumab.18 In a retrospective analysis of lung transplant patients,40 induction of immunosuppression with daclizumab in comparison with polyclonal preparations (anti-thymocyte globulin or anti-lymphocyte globulin) was associated with a higher recovery rate of Aspergillus species from clinical samples (including colonisation and invasive disease; OR 2.05, 95% CI 1.14–3.75). However, more patients on the daclizumab group were also receiving therapy with corticosteroids.
CD52 blockers: alemtuzumabThe CD52 antigen is expressed on T and B lymphocytes, monocytes, macrophages and eosinophil cells, as well as on the lining epithelia of the male reproductive tract. This is a short glycoprotein consisting of a sequence of only 12 amino acids—it is attached to the outer layer of the cell membrane by a glycosyl phosphatidylinositol lipid anchor. The CD52 antigen is one of the most abundant antigens on the surface of lymphocytes, accounting for approximately 5% of the surface antigens.
Alemtuzumab is a CD52 blocker now widely used in patients with aplastic anaemia,89 transplant recipients54 and patients with B cell malignant lymphoma.25 Alemtuzumab binds to 95% of normal human blood lymphocytes as well as malignant B- and T-cell lymphocytes. Malignant T cells express the CD52 antigen in high density and the intensity of the expression appears to be correlated with clinical effects. There is a profound and long-lasting depletion of mature B- and T lymphocytes, natural killer cells and monocytes.91
Several cases of IA have been associated with alemtuzumab.19,23,34,45,53,57 The risk of infection in patients treated with alemtuzumab is exacerbated by prior exposure to intensive chemotherapy when used in salvage of chronic lymphocytic leukaemia, non-Hodgkin lymphoma and other malignancies, and with other immunosuppressive agents for solid organ transplants and GVHD. Lymphocyte depletion lasting for as long as 12 months may be seen in these patients,62 who may as well be already immunocompromised due to their underlying disease. Clinical studies on the efficacy of alemtuzumab in patients with heavily pre-treated B-cell chronic lymphocyte leukaemia have reported an IFD incidence of approximately 20%.59 In patients receiving alemtuzumab as first-line agent, no increased risk of IFD has been related. One case of Aspergillus infection has been reported in a kidney transplant recipient treated with alemtuzumab.21 This patient has secondary to hereditary C4 deficiency and was also co-infected with Neisseria meningitidis. A review article from 2005 founded 7 cases of aspergillosis in patients receiving alemtuzumab for chronic lymphocytic leukaemia and 7 cases in patients with cutaneous T cell lymphoma.91 A Dutch study evaluating adverse events with alemtuzumab use in chronic lymphocytic leukaemia found 4 Aspergillus infections amongst 27 patients.49 There were 2 cases of proven and 2 cases of possible IA, and 3 of these patients died. Additional cases of pulmonary and lumbar spine aspergillosis were reported in pancreas transplant recipients using alemtuzumab.68 Two cases of IA were reported in a series of 17 patients with T-lymphoid neoplasms treated with a combination of alemtuzumab and pentostatin.76 One fatal case of Aspergillus terreus infection was associated with the use of alemtuzumab in a patient with chronic lymphocytic leukaemia before autologous HSCT.61 In another study, a patient with acute lymphoblastic leukaemia died due to IA after treatment with alemtuzumab plus cyclophosphamide.30
Therapy with alemtuzumab is associated with cytomegalovirus reactivation in up to two third of cases,39,51,57 which may secondarily increase the risk for IA. CMV reactivation is now a recognised risk factor for IA in general solid organ transplantation,29 haematopoietic stem cell,11 heart,65 liver27 and lung transplantation.37 It has been hypothesized that the association between CMV and Aspergillus is well explained by the immunosuppressive effect of the virus, dysregulating the cytokines production,27 involving lymphocyte suppression with reversal of the helper–suppressor cell ratio and suppressed function of natural killer cells.37
Since patients receiving alemtuzumab are at increased risk for IA, antifungal prophylaxis has been recommended for these patients. Alemtuzumab therapy is now considered a major indication for antifungal prophylaxis, like marked neutropenia after HSCT.85 Although the majority of fungal infections occur within 3 months of alemtuzumab therapy, prophylaxis may be maintained for 6 months after initiation of therapy.91
CD20 blockers: rituximabRituximab is a chimeric monoclonal antibody against CD20 on pre-B lymphocytes and mature B lymphocytes. It is used in the treatment of auto-immune diseases, non-Hodgkin lymphoma and as a promising tool for the treatment of post-transplant lymphoproliferative disease. Binding induces B-lymphocyte lysis, depleting B-cells lineages.80 Rituximab use may increase the duration of severe neutropenia if associated with chemotherapy.31 Therapy with rituximab may result in hypogammaglobulinaemia that is usually mild but may result in bacterial, viral, and fungal infections. Antibodies in general are considered to contribute little to the immune response to these infections.94 A study performed in B-lymphocyte-deficient mice showed restoration of defective antifungal immunity when opsonising antibodies were administered, implicating a role for antibodies in the development of long-lasting immunity.94 B lymphocytes may act against several intracellular pathogens, by activating Th1 lymphocytes.31 However, there are insufficient data to make any conclusion on the role of B cells in the pathogenesis of IA.94
A few case reports have associated therapy with rituximab with the occurrence IA, mostly in HSCT recipients4,31,33,84,94,95 but also in patients with idiopathic thrombocytopenic purpura24 and acute lymphoblastic leukaemia.30 Disseminated aspergillosis (with pulmonary and cutaneous involvement) was diagnosed in a patient with non-Hodgkin lymphoma after therapy with rituximab.94 Although autologous HSCT recipients are usually considered to be at low risk for IA, a recent prospective study showed that previous treatment with fludarabine (p=0.008) or rituximab (p=0.039) both increased the risk for IA at univariate analysis.31 Therapy with fludarabine was the only variable predicting IA at multivariate analysis (p=0.008). No evidence for disease progression was observed after 15 months of follow up in a patient with rheumatoid arthritis treated with rituximab who was previously diagnosed with pulmonary aspergillosis and TB.43 The patient received antifungal prophylaxis with voriconazole.
IL-1 receptor antagonists: AnakinraAnakinra is a recombinant human variant of the naturally occurring IL-1 receptor antagonist (IL-1Ra), which blocks the IL-1 signal and inhibits IL-1.2 This drug has been used in the treatment of autoimmune diseases, especially rheumatoid arthritis. Infections occur at a similar rate as that of the placebo groups. In vitro studies have shown that A. fumigatus may signal via TLRs—a family of receptors that mediate cellular responses to structurally conserved pathogen-associated microbial products. TLRs participate in the IL-1 inflammatory cascade, involved in the activation of polymorphonuclear leukocytes.7 Expressions of TLR2 and TLR4 in alveolar macrophages and polymorphonuclear cells play an important role in innate immunity against Aspergillus species. TLR4 knockout has been associated with increased mortality in mice challenged intranasally with Aspergillus conidia, whereas TLR2 knockout was associated with increased mortality rates in one of two studies.79 Mice deficient in either TLR2 or TLR4 have higher pulmonary fungal burdens than wild-type mice. TLR signalling is mediated by the adapter protein MyD88 and results in activation of nuclear factor kappa B (NF-κB) and release of TNF-α and other pro-inflammatory cytokines. An in vivo study showed that MyD88-dependent pathway, a gene responsible for activate innate immunity by transcription of IL-1R-associated kinases, associated with IL-1 and TLR cascade, is essentially required for the innate and the Th1-mediated resistance to Aspergillus infections.7
Based on the above, patients on treatment with IL-1 inhibitors are theoretically at an increased risk for IFD—however, no epidemiological or clinical data yet exist to confirm that.
Other agentsAlefaceptAlefacept is a monoclonal antibody used in the treatment of psoriasis and psoriatic arthritis. It binds to CD2, a receptor on the surface of lymphocytes, inhibiting their interaction with leukocyte functional antigen 3 (LFA-3). Interaction between CD2 and LFA-3 is important for the activation of T lymphocytes. Activated T lymphocytes secrete a number of inflammatory mediators, including IFN-γ. Since CD2 is primarily expressed on T lymphocytes, treatment results in a reduction in CD4+ and CD8+ T lymphocytes, with lesser effects on other cell populations (e.g., natural killer and B lymphocytes). No IFD, especially aspergillosis, has been related to alefacept use.
NatalizumabNatalizumab is a chimerical monoclonal antibody that blocks α-4-integrin, a membrane protein present in all leukocytes, except neutrophils, which is involved in leukocyte migration beyond vascular space and into parenchyma. Thus, the α-4-integrin binding prevents transmigration of inflammatory cells across endothelial cells and into tissues.80 Natalizumab is used for multiple sclerosis and Crohn's disease.
Although no case of aspergillosis has been associated with natalizumab use, pre-marketing trials identified cases of Aspergillus infections,80 a finding that was not observed in post-marketing trials.67
EfalizumabEfalizumab is a recombinant monoclonal antibody that binds to CD11a, a subunit of leukocyte function antigen-1 (LFA-1) found on leukocytes. By binding to CD11a, efalizumab blocks multiple T-cell-mediated responses. This drug may be used in the treatment of psoriatic plaques. Although reactivation of latent infections may occur, no efalizumab-related case of aspergillosis has been described.
AbataceptAbatacept is a fusion protein that mimics CTLA-4, inhibiting CD80 or CD86, receptors presenting on presenting antigen cells. This results in inability of these ligands to engage the CD28 receptor on T cells, down-regulating and decreasing T-cell activation and proliferation, inhibiting TNF-α, IL-6, IL-2 and, possibly, IFN-γ. Abatacept may be used for rheumatoid arthritis treatment. To the best of our knowledge, only one case of abatacept-related aspergillosis has been reported, in a patient treating rheumatoid arthritis, in a randomised controlled trial.47
BevacizumabThis is a vascular endothelial growth factor (VEGF) inhibitor used mainly in the treatment of metastatic colorectal cancer. Aspergillus hyphae are known to have marked tropism for blood vessels and angioinvasion is a central feature of invasive pulmonary aspergillosis, especially in patients with quantitative or functional deficits in polymorphonuclear cells. Angioinvasion leads to vascular thrombosis, tissue infarction and dissemination of Aspergillus to distant organs. In addition, tissue necrosis limits the penetration of immune cells as well as antifungal agents to the site of infection. VEGF may be involved in infection because VEGF expression may be implicated in response to fungal infections—Aspergillus infections have been shown to secrete angiogenesis inhibitors, inducing ischemia and preventing neutrophil clearance of the fungal infection. In addition, VEGF levels are elevated in the presence of fungal balls.38
Very limited data exist on aspergillosis in association to bevacizumab use. In one report a patient with pronounced neutropenia had invasive pulmonary aspergillosis following therapy with bevacizumab.100 In a trial analysing bevacizumab use in non-small cell lung cancer, 2 cases of IA occurred (out of 99 patients treated with bevacizumab), with no case being reported in the control group (a non-significant difference).42 It remains speculative therefore if the use VEGF inhibitors such as bevacizumab will modify the frequency of IA or its disease presentation.
Diagnostic considerationsIn the recently reviewed EORTC/MSG consensus for the diagnosis of IFD, the ‘host factor’ was expanded to include patients receiving therapy with anti-TNF-α agents or other monoclonal antibodies such as like alemtuzumab. However, it is not clear how diagnostic tests such as galactomannan and computed tomography will perform in these patients.
It has been demonstrated that galactomannan test performance varies largely depending on the patients being tested.3,73 For instance, test sensitivity is higher for neutropenic patients and lower for solid organ transplant recipients or other non-neutropenic patients treated with corticosteroids. Unfortunately, not enough data are available to allow for a conclusion regarding the performance of galactomannan testing in patients using monoclonal antibodies. In this review, we were able to find four reports only in which galactomannan was used as a diagnostic tool, all in patients treated with rituximab.17,31,33,94 Similarly, the prevalence of typical tomographic signs such as the halos or cavities could not be assessed due to the limited data published so far in patients taking monoclonal antibodies.17,24,33 Moreover, the distribution of Aspergillus species causing infection in these patients is also unknown.
Since proven IA requires a positive histopathology, we were interested to know what histopathologic findings are present when IA occurs in patients treated with monoclonal antibodies. Also again, not much information is available in the literature to answer this question. In one report in a patient treated with etanercept,86 biopsy showed extensive necrosis, granulation tissue and acute inflammatory process. Since TNF-α inhibitors have a marked capacity in inhibiting granuloma formation as well as dissolving granulomatous structures—as seen in sarcoidosis, Crohn's disease and Wegener's vasculitis32—it could be anticipated that necrosis would predominate in the histopathologic picture, with little or no granuloma formation. That remains however to be demonstrated in vivo.
TreatmentThe antifungal treatment of patients with IA following the use of biologic agents has not been systematically investigated. Most studies published so far have reported the use of deoxycholate amphotericin B, which was in some cases followed by oral voriconazole. A few patients received voriconazole as primary therapy. Death was the outcome in ∼50% of cases and no post-mortem studies are available. Diagnostic delays may be major contributors for the high mortality rate observed in these patients. As stated before, a high index of suspicion for IA is ultimately required for patients treated with monoclonal antibodies. Due to the theoretical risk of worsening or disseminating infection, patients with an active fungal disease should not start treatment with a biologic agent.92
Moreover, the type of underlying host immunosuppression may have a profound impact on the efficacy of the antifungal agent used to treat IA. Pre-clinical studies in animals show that amphotericin B deoxycholate has limited efficacy on prevention or treatment of invasive pulmonary aspergillosis in the corticosteroid-immunosuppressed animals, while the same effect is not observed in neutropenic mice with invasive pulmonary aspergillosis.5 These are clearly two distinct populations at risk for IA. Amphotericin B deoxycholate has a pro-inflammatory activity, releasing cytokines like TNF-α, IL-6, IL-1 and IL-1β. Since IA in hosts treated with corticosteroids are characterised by the presence of neutrophilic influx and marked inflammation, therapy with amphotericin B deoxycholate may have a deleterious effect in this context.5 The same effect has not been shown using triazoles, like voriconazole,5 although human prospective data are still lacking. The overall response rate to antifungal drugs in patients taking monoclonal antibodies is uncertain.
Regarding antifungal prophylaxis, no evidence-based protocol exists since patients on monoclonal antibodies were not considered for inclusion in most studies in the area.85 It has been recommended that antifungal prophylaxis should be given to patients with severe GVHD or prolonged neutropenia receiving anti-TNF-α agents. Prophylaxis should also be considered for acute lymphocytic leukaemia patients receiving chemotherapy as well as for patients on salvage therapy for non-Hodgkin lymphoma, when in association with alemtuzumab. The incidence of IFD in these groups reaches ∼10%.85 It seems also reasonable to institute prophylaxis in patients using biologic agents in the presence of a non-responding lymphoproliferative disease or previous use of immunosuppressors.85 Antifungal prophylaxis with broad-spectrum anti-mould azoles such as itraconazole or posaconazole may be warranted.91 The antifungal prophylaxis time is uncertain, since IFD may occur up to 12 months after alemtuzumab cessation.85
ConclusionsMonoclonal antibodies are a new recognised risk factor for IA,85 with most cases being associated with the use of anti-TNF-α agents, rituximab and alemtuzumab. There is no doubt that the biologic agents have improved quality of life patients receiving these therapies; however, blockade of key cytokines comes with risks. Since the use of these therapeutic options is expected to increase in the clinical practice, a higher frequency of IFDs is also anticipated. A proper index of suspicion will therefore be required for these patients. It is also important to note that immunosuppression alone is not sufficient to cause an IFD, with environmental as well as local pulmonary factors still playing a role. For instance, patients who have undergone HSCT frequently experience damage to the respiratory epithelium as a result of irradiation, cytotoxic drug use or GVHD disease, facilitating the adherence of Aspergillus conidia to epithelial cells, a critical early step in airway colonisation and subsequent invasion. In these cases, the use of monoclonal antibodies may be as a ‘trigger’ for Aspergillus infection.
The performance of diagnostic methods such as galactomannan, β-glucan and computed tomography has not been properly evaluated in patients taking biologic agents. In addition, the typical histopathological picture has not yet been defined for these patients, and the best antifungal agent for these patients is unknown. As shown in this review, the future of aspergillosis will probably mean more patients at risk—and diagnostic tests and antifungal drugs will have to be evaluated for these emerging populations.
Corresponding author.