Human fungal infections have increased at an alarming rate in recent years, particularly in immunocompromised individuals. Cryptococcosis is the second most prevalent systemic fungal infection worldwide, and the most prevalent systemic infection in immunocompromised individuals, representing more than 70% of cases. The incidence of cryptococcosis is high in people with HIV/acquired immunodeficiency syndrome (AIDS), with recent estimates indicating that there are one million cases of cryptococcal meningitis globally per year in AIDS patients.
AimsThe aim of this research was to develop a rapid flow cytometric antifungal susceptibility test and to compare the results with the standard methods.
MethodsA reference strain and clinical isolates of Cryptococcus neoformans and Cryptococcus gattii were tested for susceptibility to amphotericin B by flow cytometry using propidium iodide as indicator of viability. Flow cytometry (FC) results were compared with the minimum inhibitory concentration (MIC) values determined by microdilution.
ResultsThe antifungal activity of amphotericin B ranged from MICs of 0.06 to 2μg/ml for the 11 isolates studied. The same results were found by FC.
ConclusionsThe FC method allows same-day results, assisting in the selection of appropriate antifungal therapies. These results demonstrate an excellent correlation between FC and the classic methods of testing for susceptibility to antifungal agents. This rapid diagnosis method makes it possible to quickly administer effective therapeutic interventions, often saving lives.
En los últimos años, las infecciones por hongos en el ser humano, particularmente en individuos inmunodeprimidos, han aumentado a un ritmo alarmante. La criptococosis es la segunda infección fúngica sistémica más frecuente en todo el mundo, y también la infección sistémica más frecuente en personas inmunodeprimidas, lo que representa más del 70% de los casos. La incidencia de la criptococosis es alta en las personas con VIH/síndrome de inmunodeficiencia adquirida. Estudios recientes indican que hay un millón de casos de meningitis criptocócica en pacientes con síndrome de inmunodeficiencia adquirida por año en todo el mundo.
ObjetivosEl objetivo de esta investigación fue desarrollar una prueba rápida de sensibilidad antifúngica por citometría de flujo (CF) y comparar los resultados con los métodos estándar.
MétodosSe estudió la sensibilidad a la anfotericina B por CF, con yoduro de propidio como indicador de la viabilidad, de una cepa de referencia y varios aislamientos clínicos de Cryptococcus neoformans y Cryptococcus gattii.
ResultadosLa actividad antifúngica de la anfotericina B varió de una concentración mínima inhibitoria de 0,06 a 2μg/ml para los 11 aislamientos estudiados. Los mismos resultados fueron encontrados por CF.
ConclusionesEl método de la CF permite resultados el mismo día, lo que hace posible una rápida selección de los tratamientos antifúngicos adecuados. Estos resultados demuestran una excelente correlación entre el método de la CF y los procedimientos clásicos de pruebas de sensibilidad a los agentes antifúngicos. Este método de rastreo ágil hace que sea posible realizar rápidamente intervenciones terapéuticas eficaces y, a menudo, salvar vidas.
In recent years, morbidity and mortality in patients with invasive fungal infections have increased,12,22 making this condition a major public health problem that affects a large part of the world's population, and reducing the quality and life expectancy.24 This increase is largely due to late diagnosis and initiation of appropriate antifungal therapy.19
Reference methods for antifungal susceptibility testing (AFST) recommended since 1985 by CLSI (Clinical Laboratory Standards Institute) were an initiative to standardise laboratory procedures and increase the interlaboratory reproducibility of broth microdilution in vitro methodology. The current CLSI M27-A3 procedure for yeasts allows better comparison of results among different laboratories.4,7,8,17 However, these reference methods are labour intensive in the laboratory for the evaluation of the susceptibility profile because they depend on the presence of biochemical substrates and the replicative capacity of cells, that are time-consuming (24 and 48h for the incubation of Candida spp. and Cryptococcus spp., respectively).17,26 New methodologies could decrease the incubation time and automate the collection of results.
Flow cytometry (FC) has been described as an excellent tool to study the susceptibility of microorganisms, including fungi, to drugs.5,11,26–29 Recent studies have shown that this technique does not require long incubation periods. The availability of a rapid, reproducible and sensitive detection method would allow earlier diagnosis and efficient administration of appropriate treatments.3,19,20,24,30 There is a clear positive correlation between the results of the FC and microdilution methods, but FC is preferable because it is faster.26
Given the increasing number of individuals with fungal infections,10,25,26 fast and efficient alternative methods are needed to measure the sensitivity of these fungi to drug treatments and prophylaxis of infections. In this study, we aimed to standardise the FC method used to determine the susceptibility of Cryptococcus neoformans and Cryptococcus gattii to amphotericin B in a shorter period of time.
Materials and methodsMicroorganisms and growth conditionsThe C. neoformans ATCC 90012 strain and 10 clinical isolates were obtained from the mycology collection at the Clinical Mycology Laboratory, Department of Clinical Analysis, Faculty of Pharmaceutical Sciences, UNESP, Araraquara, Brazil. Clinical isolates with different susceptibility profiles are described in Table 1. Yeasts were grown and maintained on Sabouraud dextrose agar for 48h at room temperature.
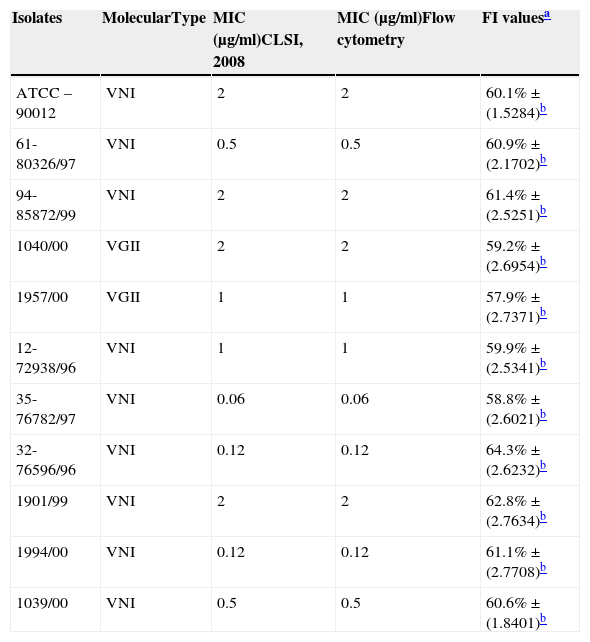
Molecular identification, minimum inhibitory concentration (MIC) and percentage value of fluorescence intensity (FI) of isolates of Cryptococcus spp.
| Isolates | MolecularType | MIC (μg/ml)CLSI, 2008 | MIC (μg/ml)Flow cytometry | FI valuesa |
|---|---|---|---|---|
| ATCC – 90012 | VNI | 2 | 2 | 60.1%±(1.5284)b |
| 61-80326/97 | VNI | 0.5 | 0.5 | 60.9%±(2.1702)b |
| 94-85872/99 | VNI | 2 | 2 | 61.4%±(2.5251)b |
| 1040/00 | VGII | 2 | 2 | 59.2%±(2.6954)b |
| 1957/00 | VGII | 1 | 1 | 57.9%±(2.7371)b |
| 12-72938/96 | VNI | 1 | 1 | 59.9%±(2.5341)b |
| 35-76782/97 | VNI | 0.06 | 0.06 | 58.8%±(2.6021)b |
| 32-76596/96 | VNI | 0.12 | 0.12 | 64.3%±(2.6232)b |
| 1901/99 | VNI | 2 | 2 | 62.8%±(2.7634)b |
| 1994/00 | VNI | 0.12 | 0.12 | 61.1%±(2.7708)b |
| 1039/00 | VNI | 0.5 | 0.5 | 60.6%±(1.8401)b |
C. neoformans and C. gattii isolates were grown on Sabouraud dextrose agar at 30°C for 48h, and cells from the culture were suspended in YPD medium with 2.9% NaCl and then incubated at 30°C overnight. Genomic DNA was extracted using glass beads.14 Quantification of DNA was performed in a spectrophotometer at 260nm,22 and its purity was determined at wavelengths of 260nm and 280nm. The URA5 fragment was amplified by PCR in a final volume of 50μl, digested with HhaI and Cfr13I13 and separated by electrophoresis on 3.0% agarose at 100V.
Antifungal agentAmphotericin B (AMB) was provided as a stock solution (Sigma Chemical Co., St. Louis, MO, EUA). The powder was dissolved in sterile DMSO and stored at 20°C.
Minimum inhibitory concentration (MIC)The microdilution method was performed as described in the M27A3 document.6 RPMI 1640 medium buffered to pH 7.0 with 0.165M morpholinepropanesulfonic acid (MOPS) and supplemented with 2% glucose and l-glutamine was used. C. neoformans and C. gattii suspensions were prepared in RPMI to a final concentration of 5.0×102–2.5×103 colony forming units (CFU)/ml. In 96-well plates, serial dilutions of amphotericin B were added at concentrations ranging from 0.03 to 16μg/ml. The plates were incubated with shaking at 35°C for 48h. The results were assessed visually and spectrophotometrically at 490nm.
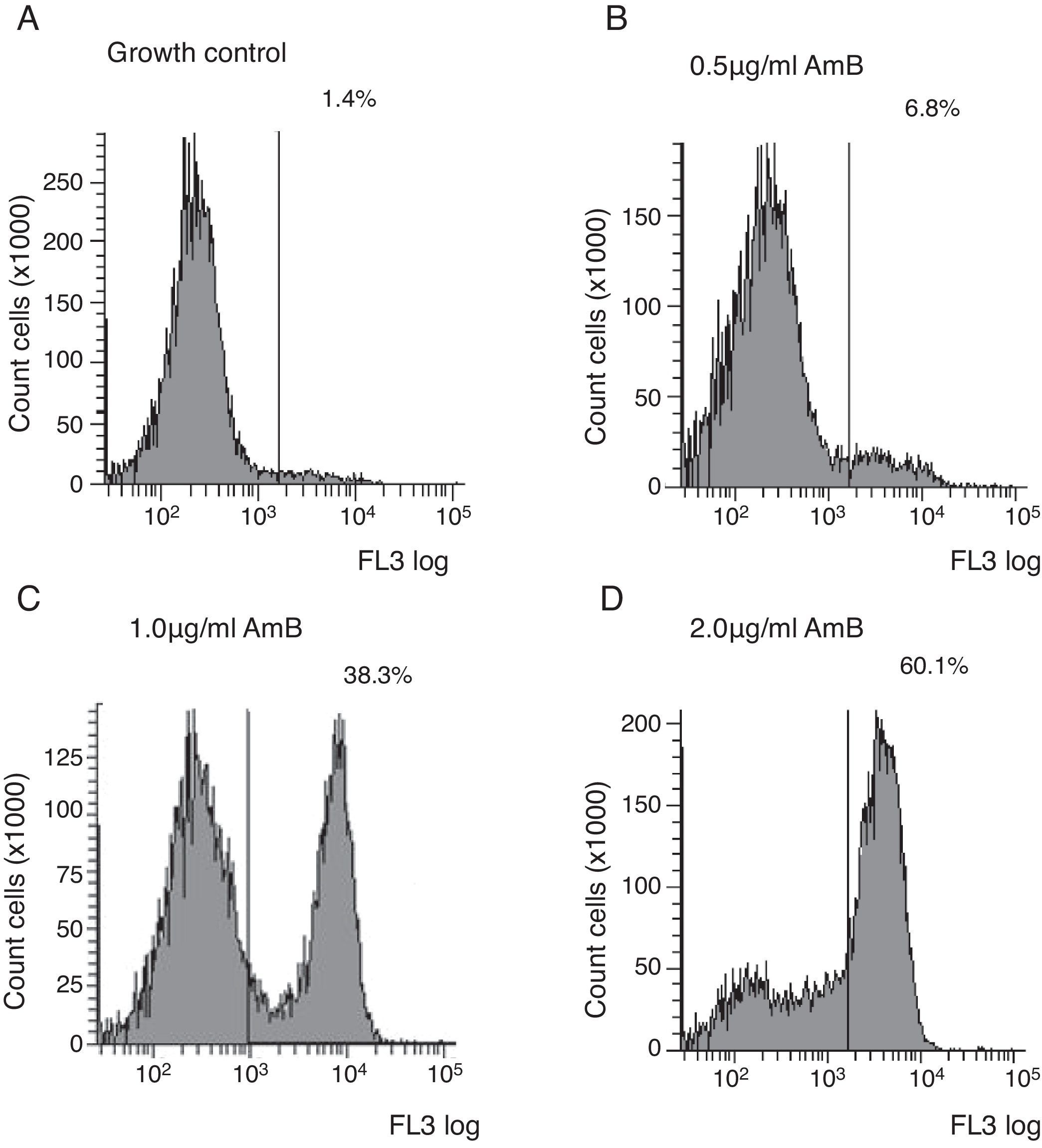
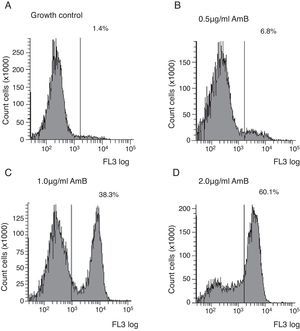
Flow cytometry assayYeast suspensions were prepared in sterile phosphate-buffered saline supplemented with 2% glucose (pH 7.0). Suspensions containing 106cells/ml in RPMI 1640 buffered to pH 7.0 with 0.165M MOPS and supplemented with 2% glucose and l-glutamine were incubated under shaking at 35°C with each antifungal for 4h. The AMB concentrations ranged from 0.03 to 16μg/ml and were diluted as described in the M27-A3 document.6 After the antifungal treatment, cells were centrifuged and washed three times in HEPES buffer supplemented with 2% glucose (pH 7.2). The cells were suspended in 0.125% sodium deoxycholate, containing 1μg/ml propidium iodide (PI; Sigma, Chemical Co., St. Louis, MO, EUA), and incubated in the dark for 10min. Controls were incubated in RPMI and washed three times in the same buffer. A PI-positive control was included using a strain treated with 70% ethanol for 1h at room temperature. Untreated cells were used as the growth control. Ten thousand cells per tube were used on a FACSCanto™ flow cytometer (Becton & Dickinson, San Diego, CA, USA), and studies were conducted with a flow cytometric protocol in which size forward scatter (FSC) and granularity side scatter (SSC) at 620nm fluorescence emission (FL3) resulted in graphs representing the fluorescence intensity (FI) of PI-labelled yeasts that were analysed using FACSDiva™ software. The graphs of PI fluorescence intensity were divided into two quadrants, i.e., left comprehending PI negative cells (viable cells), and right comprehending PI positive cells (non-viable cells). The MIC of AMB was defined as the lowest concentration that showed ∼50% increase in FI compared to growth control,5 corresponding to the right quadrant of the graphs.
Statistical analysisAll data were statistically analysed using ANOVA. p values<0.01 were considered significant.
ResultsIdentification of Cryptococcus spp.Cryptococcus spp. strains used were ATCC 90012 (C. neoformans serotype A) and 10 clinical isolates. Molecular analyses (URA5-RFLP) identified eight (80%) of the isolates as C. neoformans serotype A, and two isolates (20%) as C. gattii.
Minimum inhibitory concentration (MIC)The antifungal activity of amphotericin B (AMB) ranged from MICs between 0.06 and 2μg/ml for C. neoformans, and 1–2μg/ml for C. gattii (Table 1).
Flow cytometric assayFC results were expressed as the fluorescence intensity (FI) of PI-labelled yeasts. Several experiments were conducted to optimise the dead-cell control, yeast inoculum size, length of AMB exposure, presence of sodium deoxycholate and dye incubation times. Higher FI percentages were observed in ethanol-treated cells compared to drug-free cells after 10min of incubation with PI.
Untreated cells stained with PI had a very low FI; thus, these cells were used as the growth control. The FC assay was standardised using the C. neoformans ATCC 90012 strain and AMB in RPMI at concentrations ranging from 0.03 to 16μg/ml for 4h. The specified test conditions produced results comparable to the CLSI microdilution technique. The gradual increase in the FI percentage observed in yeasts treated with high AMB concentrations agreed with the positive control values as demonstrated in Fig. 1. The MIC value considered for AMB was 2μg/ml because the FI increased 50% above the FI value of growth control (1.4%). The experiments were repeated five times, and the results described above represent the average of all experiments.
Each isolate and its MIC value, as determined by the CLSI microdilution method and subsequent evaluation by FC, are shown in Table 1. The AMB MICs for the 10 isolates ranged from 0.06 to 2μg/ml based on the microdilution method and FC. The assays were repeated in three independent experiments and the results described above were obtained by averaging the trials. The two tested methods showed the same MICs.
DiscussionC. neoformans has become increasingly important with the rise in HIV infections, solid-organ transplantations and the use of immunosuppressive therapies.12 In vitro antifungal susceptibility testing of emerging pathogens has become a significant issue for fungal therapeutics and is required to increase our understanding of the fungicidal characteristics of the administered drugs, particularly AMB because it is used during the induction phase of cryptococcosis therapy.2,15,16
The ability of the broth microdilution method to differentiate between CLSI-susceptible and resistant strains is still under debate for AMB. Data regarding the susceptibility of C. neoformans, dermatophytes and Malassezia spp. to any antifungal agent, or the susceptibility of yeasts to posaconazole and amphotericin B, are not available.4 Thus, we have studied alternatives to minimise such problems. The availability of rapid and reliable tools to determine the susceptibility to antifungal agents is essential. We have standardised the FC method to determine the susceptibility of C. neoformans and C. gattii to AMB.
Recently, susceptibility to antifungal agents has been evaluated by several FC methods that can save time and provide additional information regarding the mechanisms of action and resistance for the tested drugs.1,7,8,17,21
FC methods have been advocated because they are more reliable for studies of susceptibility to AMB.9,20–23 The scattered light detected by FC gives intrinsic cellular information, such as size and complexity, but the use of fluorochromes allows for the evaluation of a wide range of physiological or morphological parameters, such as the membrane integrity, pH, membrane potential and viability.17
The results of the current study suggest that C. neoformans susceptibility to AMB can be tested by FC after treating the yeast with that drug for 4h. AMB induces primary lesions in the cell membrane, and PI can enter cells after a short incubation period. The strain is considered susceptible if a low concentration of the drug produces a 50% increase in the intensity of fluorescence when compared to drug-free, PI-stained control cells.
Our results are in agreement with numerous studies. In one study, the authors used PI to obtain MICs in 3.5h, which showed good agreement between CLSI and FC methods.9 In another study, the authors observed that the agreement between CLSI and FC methods ranged from 96 to 99% for Candida albicans.5 Another study determined the feasibility of direct susceptibility testing of Candida species to fluconazole by a rapid flow cytometric method; a total of 50 Candida strains were identified as positive by the blood culture.28 Minimal inhibitory concentration (MIC) determined by fluorescent flow cytometry (FACS) showed excellent agreement to that determined by microdilution. The researchers concluded that the FC method can greatly assist in the selection of appropriate antifungal therapy in few hours.
PI is one of the most popular fluorescent probes to check viability that is used in susceptibility testing by cytometric methods.8,17 We found it to be adequate for evaluating the susceptibility to the fungicidal agents tested in this study. This may be explained by the significant damage that occurs at the cell membrane, which allows PI to access the target. According to previous studies in Candida, the activity of echinocandin could be studied in cells exposed to fungicidal drugs that cause direct membrane damage with PI after treatment for 3h.9 AMB has a relatively large structure and acts on the cell membrane of yeast in a way that likely interferes with the uptake of most molecules. Thus, the uptake of PI by AMB-treated cells is significantly reduced compared to untreated cells because the cells become progressively more impermeable in the presence of AMB.18 In the present study, combining PI with sodium deoxycholate yielded faster results because deoxycholate enhanced PI penetration. The addition of deoxycholate is essential for the prompt and optimal penetration of DNA-saturating concentrations of PI into damaged yeast cells after treatment with an antifungal agent. Sodium deoxycholate facilitates the diffusion of PI into the cells.19
Following this study, we attempted to standardise the FC method to test the antifungal susceptibility of Cryptococcus spp. to AMB. Clinical isolates were selected for analysis by FC, and the results were compared to the MIC values determined by the standard methodology described in the M27-A3 document. We selected 10 isolates belonging to the VNI and VGII types, and the MIC values ranged from 0.06 to 2μg/l, with no apparent relationship between molecular type and MIC (Table 1). No statistically significant difference was observed when comparing M27-A3 and FC methods (ANOVA, p≤0.01). The FC technique yielded results comparable to the CLSI technique but with the advantage of a short 4h incubation period for AMB. These data suggest that FC could be applied in studies of C. neoformans from clinical sources.
The results of this study suggest that the use of flow cytometry to check the susceptibility to amphotericin B is a reliable, fast and safe method. An investigation of quick alternative methods is needed to increase the survival of patients with serious infections that require a treatment with antifungal agents.
ConclusionOur results demonstrate an excellent correlation between the results of the FC analysis and the classic methods of susceptibility testing for the tested antifungal agents. FC saves significant amounts of time in antifungal susceptibility testing when compared to classical protocols. This is of particular interest and importance when reliable and accurate answers are needed in the shortest possible time, as is the case in immunocompromised patients such as those with leukaemia and bone marrow recipients for whom diagnostic mistakes cannot be made.
Conflict of interestThe authors declare that they have no conflict of interests.