Recent decades have seen a global emergence of candidaemia caused by non-Candida albicans Candida species, particularly the Candida parapsilosis complex.
AimsTo evaluate the clinical features and antifungal susceptibility profiles of isolates belonging to the C. parapsilosis species complex in patients with candidaemia in a midwestern Brazilian tertiary-care teaching hospital.
MethodsYeast identification was performed using an automated Vitek 2 Compact system. PCR-RFLP was employed for species differentiation.
ResultsFive cases of infection by C. parapsilosis sensu stricto and two by Candida orthopsilosis were found. Of the seven cases, five were adult patients undergoing haemodialysis. The only isolate of C. parapsilosis sensu stricto resistant to fluconazole (MIC=8μg/ml) was obtained from a patient on a long-term regimen with this drug. This was the only patient who evolved to death.
ConclusionsResistance to antifungal agents poses a therapeutic challenge, especially for non-C. albicans Candida species, and requires continuous monitoring using susceptibility tests because resistance in vitro can be predictive of treatment failure. In the present study, in vitro antifungal susceptibility proved consistent with clinical outcome.
En las últimas décadas se ha visto un surgimiento mundial de la candidemia causada por especies de Candida no-C. albicans, en particular del complejo Candida parapsilosis.
ObjetivosEvaluar las características clínicas y los perfiles de sensibilidad antifúngica en aquellos aislamientos del complejo de especies C. parapsilosis responsables de candidemia en un hospital universitario de tercer nivel en la región centro-oeste de Brasil.
MétodosLa identificación se realizó en un sistema automatizado Vitek 2 compact. Se utilizó PCR-RFLP para la diferenciación de las especies.
ResultadosSe encontraron cinco casos de candidemia por C. parapsilosis sensu stricto y dos por Candida orthopsilosis. Cinco eran pacientes adultos sometidos a hemodiálisis. El único aislamiento de Candida parapsilosis sensu stricto resistente a fluconazol (CIM, 8μg/ml) se obtuvo de un paciente en régimen largo de tratamiento con este antifúngico. Este fue el único paciente que murió.
ConclusionesLa resistencia a los antifúngicos constituye un desafío terapéutico, en especial contra las especies de Candida no-C. albicans, que requieren la monitorización continua por medio de pruebas de sensibilidad en vista de que la resistencia in vitro puede ser predictiva de fracaso del tratamiento. En el presente estudio la sensibilidad antifúngica in vitro resultó consistente con el curso clínico.
Recent decades have seen a global emergence of candidaemia caused by non-Candida albicans Candida species, particularly of the Candida parapsilosis complex.2,6,15 Determination of antifungal susceptibility is important to ensure effective monitoring and detection of resistance, contributing to appropriate treatment selection.13
The purposes of this study were to evaluate the clinical features of patients with candidaemia caused by species of the C. parapsilosis complex and to determine the antifungal susceptibility profiles of these agents.
The yeasts were isolated from a tertiary-care teaching hospital located in Campo Grande, Mato Grosso do Sul State, Midwest Brazil, from March 2010 to March 2012. The isolates were detected in blood culture using an automated Bact/Alert system and identified employing an automated Vitek 2 Compact system (both from bioMérieux, France).
Species differentiation was performed by polymerase chain reaction – restriction fragment length polymorphism (PCR-RFLP). DNA extraction, conducted as described by Sambrook et al.,16 was carried out at the Mycology Laboratory of the Instituto Adolfo Lutz, in São Paulo City. PCR-RFLP was performed according to Tavanti et al.17 The results were interpreted by comparison against standard strains (C. parapsilosis ATCC 22019, Candida orthopsilosis ATCC 96139, and Candida metapsilosis ATCC 96144).
The minimum inhibitory concentrations of the antifungal agents amphotericin B (Sigma Aldrich, USA), fluconazole (Sigma Aldrich), itraconazole (Sigma Aldrich), and voriconazole (Pfizer, USA) were determined using a broth microdilution method according to the M27-A3 guidelines of the Clinical and Laboratory Standards Institute.3 Interpretation of the minimum inhibitory concentrations for amphotericin B and itraconazole was based on the M27-S3 document3; for fluconazole and voriconazole, the M27-S4 guidelines4 were followed.
Retrospective demographic and clinical data were obtained from medical records. The study was approved by the Universidade Federal de Mato Grosso do Sul Research Ethics Committee (permit 1591/2010).
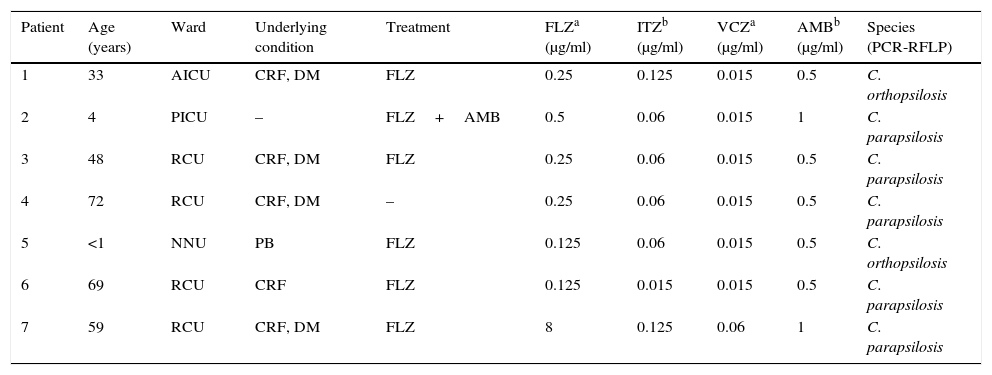
In the two-year period investigated, 32 patients were laboratory-diagnosed with candidaemia, predominantly caused by non-C. albicans Candida species (65.6% of cases). The C. parapsilosis complex predominated (7/32; 21.9%), comprising five isolates of C. parapsilosis sensu stricto and two of C. orthopsilosis. Except for one C. parapsilosis isolate resistant to fluconazole, all yeasts were susceptible to the antifungals tested. Table 1 shows the principal clinical and laboratory features of the seven patients.
Characteristics of seven patients with haematogenous candidaemia caused by species of the Candida parapsilosis complex and respective antifungal susceptibility profiles.
| Patient | Age (years) | Ward | Underlying condition | Treatment | FLZa (μg/ml) | ITZb (μg/ml) | VCZa (μg/ml) | AMBb (μg/ml) | Species (PCR-RFLP) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | AICU | CRF, DM | FLZ | 0.25 | 0.125 | 0.015 | 0.5 | C. orthopsilosis |
| 2 | 4 | PICU | – | FLZ+AMB | 0.5 | 0.06 | 0.015 | 1 | C. parapsilosis |
| 3 | 48 | RCU | CRF, DM | FLZ | 0.25 | 0.06 | 0.015 | 0.5 | C. parapsilosis |
| 4 | 72 | RCU | CRF, DM | – | 0.25 | 0.06 | 0.015 | 0.5 | C. parapsilosis |
| 5 | <1 | NNU | PB | FLZ | 0.125 | 0.06 | 0.015 | 0.5 | C. orthopsilosis |
| 6 | 69 | RCU | CRF | FLZ | 0.125 | 0.015 | 0.015 | 0.5 | C. parapsilosis |
| 7 | 59 | RCU | CRF, DM | FLZ | 8 | 0.125 | 0.06 | 1 | C. parapsilosis |
AICU: adult intensive care unit; PICU: pediatric intensive care unit; RCU: renal care unit; NNU: newborn nursery unit; CRF: chronic renal failure, DM: diabetes mellitus; PB: preterm birth; FLZ: fluconazole; ITZ: itraconazole; VCZ: voriconazole; AMB: amphotericin B.
C. parapsilosis sensu stricto and C. orthopsilosis jointly accounted for the majority of non-C. albicans Candida species isolated from the blood cultures – a finding that corroborates data collected from other Brazilian hospitals.5,10
In a tertiary-care hospital in Spain,8C. parapsilosis sensu stricto was the most frequent microorganism (90.3%) out of the 62 isolates pertaining to the C. parapsilosis complex isolated from blood, followed by C. orthopsilosis (9.7%).
Fever, tachycardia, and dyspnea were the most frequent clinical symptoms (3/7; 42.9%) at the time of candidaemia diagnosis. All patients had previously undergone procedures known for their associated high risk of nosocomial infection by Candida species – namely, haemodialysis or previous use of vancomycin (5 cases each); surgery within 30 days before the onset of candidaemia (3 cases); and use of nasogastric, orogastric, nasoenteral, or indwelling urinary catheter (2 cases each). These findings are similar to those of previous studies conducted in Brazil.1,2,12
All patients with candidaemia had used central venous catheters. In the two pediatric patients included in the present study, the yeasts were also isolated from the catheter tips, corroborating studies that underscore the risks of invasive fungal infection associated with these devices, given the ability of these microorganisms to form biofilms.8,14 All five adult subjects were chronic renal failure patients undergoing haemodialysis with double-lumen catheters. The research literature reports cases of infection by species of the C. parapsilosis complex in patients with this profile.5,9 In the present study, four of the five adults with chronic renal failure had diabetes mellitus, which also constitutes a risk factor for invasive fungal infection.
A 33-year-old patient with diabetes mellitus and chronic renal failure on haemodialysis developed endocarditis. C. orthopsilosis was isolated not only from blood cultures but also from a post-surgical fragment of cardiac valve – a documented case of infective endocarditis caused by this microorganism, albeit rarely reported in the literature.11 Endocarditis as a complication in patients with candidaemia not previously submitted to heart surgery is an uncommon event.5
Of the seven patients with candidaemia, the majority (6/7; 85.7%) received fluconazole as a first-choice antifungal agent. The single untreated patient developed transient candidaemia, recognizable by an episode of shivering and chills following a haemodialysis session, although no subsequent manifestations of invasive fungal disease were reported.
Blood cultures from a patient with chronic renal failure on haemodialysis were positive for C. parapsilosis sensu stricto for nine consecutive months. This patient's clinical condition required haemodialysis via central venous catheter and proved too critical for catheter replacement. Antifungal therapy was therefore maintained, as was the catheter. Previous long-term use of fluconazole by this patient is thought to have facilitated the development of in vitro (MIC=8μg/ml) and in vivo resistance to this antifungal. This was the only patient who evolved to death. Extensive use of fluconazole has been associated with the emergence of resistant clinical isolates.7
Antifungal resistance is an increasing clinical issue, especially in non-C. albicans Candida species, and requires continuous monitoring using susceptibility tests because resistance in vitro can be predictive of treatment failure.13 In the present study, in vitro antifungal susceptibility proved consistent with clinical outcome.
Financial support was provided by the Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul, Brazil.