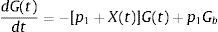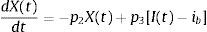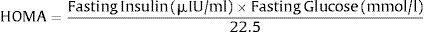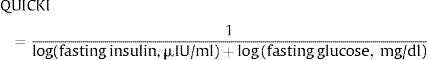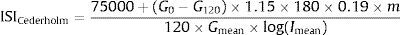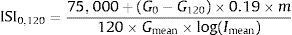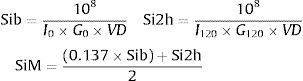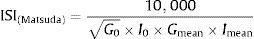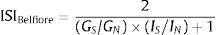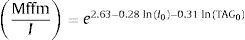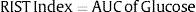Insulin resistance contributes to the pathophysiology of diabetes and is a hallmark of obesity, metabolic syndrome, and many cardiovascular diseases. Therefore, quantifying insulin sensitivity/resistance in humans and animal models is of great importance.
Various methods are used to assess insulin sensitivity both in individuals and in study populations. Validity, reproducibility, cost, and degree of subject burden are important factors for both clinicians and researchers to consider when weighing the merits of a particular method. Some methods rely on steady-state analysis of glucose and insulin, whereas others rely on dynamic testing. Each of these methods has distinct advantages and limitations. Thus, optimal choice and employment of a specific method depend on the nature of the studies being performed. Established direct methods for measuring insulin sensitivity in vivo are relatively complex. Finally, simple surrogate indexes for insulin sensitivity/resistance are available that are derived from blood insulin and glucose concentrations under fasting conditions (steady state) or in the postprandial state (dynamic). This article highlight merits, limitations, and appropriate use of current in vivo measures of insulin sensitivity/resistance and presents the advantages and disadvantages of each.
A resistência à insulina contribui para a fisiopatologia da diabetes e é uma característica marcante da obesidade, da síndrome metabólica, e de doenças cardiovasculares. Assim, quantificar a sensibilidade à insulina vs resistência à insulina em humanos e em modelos animais é de grande importância.
Existem vários métodos para avaliar a sensibilidade à insulina, tanto em indivíduos, como em populações de estudo. A validade, reprodutibilidade, custo e envolvimento dos indivíduos são fatores importantes a considerar para os clínicos e investigadores aquando da escolha de um determinado método de avaliação da sensibilidade e/ou resistência à insulina. Alguns métodos dependem da quantificação dos níveis de glucose e de insulina no estado estacionário, embora outros métodos possam ser utilizados no estado dinâmico. Cada um destes métodos tem vantagens e limitações distintas. Assim, a escolha e a aplicabilidade correta de um método específico depende da natureza dos estudos a serem realizados. O desenho de métodos diretos para medir a sensibilidade à insulina in vivo é relativamente complexo. Existem alguns índices simples para avaliar a sensibilidade e/ou resistência à insulina, que resultam da avaliação das concentrações de insulina e glucose em jejum (estado estacionário) ou no estado pós-prandial (estado dinâmico). Este artigo destaca as limitações e a utilização adequada dos atuais métodos de avaliação de sensibilidade e/ou resistência à insulina e apresenta as vantagens e desvantagens de cada um dos métodos.
Measurements of insulin sensitivity provide clinicians and researchers with excellent instruments to objectively evaluate the efficiency of both current and potentially useful interventional tools.
It is of great importance to develop tools for quantifying insulin sensitivity/resistance in humans, which may be used to appropriately investigate the epidemiology, pathophysiologic mechanisms, outcomes of therapeutic interventions, and clinical course of patients with insulin resistance.
Methods of insulin sensitivity/resistance assessmentHyperinsulinemic Euglycemic Glucose ClampThe Hyperinsulinemic Euglycemic Clamp (HIEC), originally developed by DeFronzo, is widely accepted as the “gold standard” for directly determining metabolic insulin sensitivity in humans.1 After an overnight fast, insulin is infused intravenously at a constant rate that may range from 5 to 120mU/m2/min (dose per body surface area per minute, during 180min). This constant insulin infusion results in a new steady-state insulin level that is above the fasting level (hyperinsulinemic). Consequently, glucose disposal in skeletal muscle and adipose tissue is increased while hepatic glucose production (HGP) is suppressed. Under these conditions, a glucose analyzer is used to frequently monitor blood glucose levels at 5–10min intervals, while 20% dextrose is given intravenously at a variable rate in order to “clamp” blood glucose concentrations in the normal range (euglycemic). After several hours of constant insulin infusion, steady-state conditions are typically achieved for plasma insulin, blood glucose, and the glucose infusion rate (GIR). Assuming that the hyperinsulinemic state is sufficient to completely suppress HGP, and since there is no net change in blood glucose concentrations under steady-state clamp conditions, the GIR must be equal to the glucose disposal rate (M). Thus, whole body glucose disposal at a given level of hyperinsulinemia can be directly determined. M is typically normalized to body weight or fat-free mass to generate an estimate of insulin sensitivity. Alternatively, an insulin sensitivity index (SI) derived from clamp data can be defined as SIClamp=MG×ΔI, where M is normalized for G (steady-state blood glucose concentration) and ΔI (difference between fasting and steady-state plasma insulin concentrations).2
The validity of glucose clamp measurements of insulin sensitivity depends on achieving steady-state conditions. “Steady-state” is often defined as a period greater than 30min (at least 1h after initiation of insulin infusion) during which the coefficients of variation for blood glucose, plasma insulin, and GIR are less than 5%.2 It is possible to use a radiolabeled glucose tracer under clamp conditions to estimate hepatic glucose production, so that appropriate corrections can be made to M in the event HGP is not completely suppressed.3–5 An alternative approach is to use an insulin infusion rate sufficiently high to completely suppress HGP according to the insulin sensitivity/resistance of the population to be studied.
M is routinely obtained at only a single insulin infusion rate, and therefore comparisons between M or SIClamp among different subjects is valid only if the same insulin infusion rate is used for all subjects.
The principal advantage of the glucose clamp in humans is that it directly measures whole body glucose disposal at a given level of insulinemia under steady-state conditions. Conceptually, the approach is straightforward but there is a limited number of assumptions that are clearly defined. In research settings where assessing insulin sensitivity/resistance is of primary interest and feasibility is not an issue, it is appropriate to use the glucose clamp technique.
The main limitations of the HIEC approach are that it is time-consuming, labor intensive, expensive, and requires an experienced operator to manage technical difficulties. Another limitation is that the clamp utilizes steady-state insulin levels that may be supraphysiological. This results in a reversal of the normal portal to peripheral insulin gradient. Thus, the glucose clamp may not accurately reflect insulin action and glucose dynamics under physiological conditions that a dynamic test, such as, an oral meal or oral glucose load may determine. Further, in the HIEC insulin sensitivity is measured only under a steady-state condition, and therefore, the test does not realistically portray dynamic conditions such as those occurring after normal meals. Because HIEC is dependent on steady-state conditions, insulin infusion is continuous for ≈3h, and the subjects are in the fasted state. The results of the HIEC may be limited by these restraints, because insulin release is pulsatile,6–8 and insulin action is sensitized in the postprandial state.9 Nevertheless, it should be remembered that the HIEC measures insulin-stimulated glucose disposal only at insulin levels in the upper physiological range; information on the effects of insulin on glucose uptake and production in the basal condition, which is physiologically very important, is not provided (unless tracers are used).10
Insulin Tolerance TestThe Insulin Tolerance Test (ITT) was one of the first methods developed to assess insulin sensitivity in vivo.11 In this method, a fixed bolus of regular insulin (0.1IU/kg bw) is given iv after an 8–10h fast. Blood samples are collected at 15 and 5min before and 3, 6, 9, 12, 15, 20 and 30min after insulin injection, and the plasma glucose decrement is then measured. Glucose is injected at 30min to stop the fall in plasma glucose.12,13 The faster the decline in glucose concentration, the more insulin sensitive the subject is. The slope of the linear decline in plasma glucose (KITT) can be calculated by dividing 0.693 by the plasma glucose half-time (50% from baseline):
where t1/2 represents the half-life of plasma glucose decrease, and is calculated from the slope of least square analysis of the plasma glucose concentrations from 3 to 15min after iv insulin injection, when the plasma glucose concentration declined linearly. Normal KITT is >2.0%/min and values <1.5%/min are considered abnormal. This method gives an indirect estimate of overall insulin sensitivity.The advantages of the ITT include its simplicity, rapidity and the use of a bolus injection of insulin. The bolus injection of insulin mimics the physiological pulsatile release of insulin.6 Furthermore, because glucose tolerance after a meal is dependent on insulin sensitivity, measuring insulin sensitivity in the prandial state is physiologically relevant.
Some of the drawbacks of this method include the supraphysiological insulin dose used, and also the fact that the test does not differentiate peripheral vs hepatic insulin resistance.14 Another major limitation of this test is the risk of hypoglycemia. Hypoglycemia triggers hormonal responses, which may interfere with insulin sensitivity and in turn slows the disappearance rate of glucose from plasma.15 In this view, the fall in plasma glucose concentration would be a function of the interplay between insulin, on the one hand, and glucagon, catecholamines, growth hormone and cortisol, on the other. Given that, the counterregulatory response occurs only 15–20min after insulin injection. The glucose fall occurring in the first 15min after iv insulin administration is probably a function of insulin-stimulated glucose uptake by tissues as well as insulin ability to suppress glucose output by the liver.16
A lower insulin dose method of 0.05YIU/kg bw, or shortening the test to 15min was suggested as an attempt to decrease the risk of hypoglycemia.14,17 The shorter version12,16 derived from the notion that the counterregulatory hormone response occurs only after 20min of the insulin infusion.18
The ITT has been shown to correlate with the HIEC in several studies.12,16 However, arterialization of blood is essential in the ITT, as data from standard venous blood measurements showed no significant relationship with HIEC-derived glucose disposal.16
In conclusion, the ITT should be used with great caution in insulin sensitive individuals because of the increased risk of hypoglycemia, even when the smaller dose version of the test is used. The shorter ITT is a valid test in large-scale studies, especially when the site of resistance is not of importance.
Insulin Suppression TestThe insulin-suppression test (IST), another method that directly measures metabolic insulin sensitivity/resistance, was introduced by Shen et al. in 1970 and subsequently modified by Harano et al.19,20 After an overnight fast, somatostatin (250μg/h) or the somatostatin analog octreotide (25μg bolus, followed by 0.5μg/min)21 is intravenously infused, to suppress endogenous secretion of insulin and glucagon. Simultaneously, insulin (25mU/m2/min) and glucose (240mg/m2/min) are intravenously infused over 3h. Blood samples for glucose and insulin determinations are taken every 30min for 2.5h, and then at 10min intervals from 150 to 180min of the IST. The constant infusions of insulin and glucose determine steady-state plasma insulin (SSPI) and glucose (SSPG) concentrations. The steady-state period is assumed to be from 150 to 180min after initiation of the IST. SSPI concentrations are generally similar among subjects. Therefore, the SSPG concentration will be higher in insulin resistant subjects and lower in insulin sensitive subjects, i.e., SSPG values are inversely related to insulin sensitivity. The IST provides a direct measure (through SSPG) of the ability of exogenous insulin to mediate disposal of an iv glucose load, under steady-state conditions, where endogenous insulin secretion is suppressed.22
The SSPG is a highly reproducible direct measure of metabolic actions of insulin, that is, less labor intensive and less technically demanding than HIEC. Indeed, since there are no variable infusions with the IST, steady-state conditions are more easily achieved with the IST than with HIEC. In research settings, the IST can be used for larger populations that may pose difficulties for application of HIEC. ELIMINAR esta frase.
Many of the limitations of the IST are similar to those described for HIEC (with the exception that the IST is less technically demanding). Thus, it is impractical to apply the IST in large epidemiological studies or in the clinical care setting. SSPG under ideal conditions determines primarily skeletal muscle insulin sensitivity, and is not designed to reflect hepatic insulin sensitivity.22
Continuous infusion of glucose with model assessmentThe continuous infusion of glucose with model assessment (CIGMA) is a procedure that assesses insulin sensitivity through the evaluation of the near steady-state glucose and insulin concentrations after a continuous infusion of glucose, with model assessment.23 This method mimics postprandial glucose and insulin concentrations. CIGMA not only provides information about glucose tolerance and insulin sensitivity, but also about β-cell function. Using a mathematic model of glucose homeostasis, glucose and insulin values are compared with known physiologic data of glucose, and insulin kinetics in response to glucose infusion, which are derived from healthy lean subjects with no family history of diabetes.23
The glucose and insulin values used for CIGMA are obtained during the last 15min of the 60min continuous glucose infusion (5mg glucose/kg bw/min). Samples are collected at 5min intervals and the average is then compared with predicted values from the computer model. The median value for normal subjects is 1.35, and for diabetic patients with mild hyperglycemia is 4.0.23
There are two main advantages of CIGMA over Homeostasis Model Assessment (HOMA). First, the insulin values that are measured in CIGMA are much higher than those in HOMA owing to the glucose stimulus and second, higher insulin concentration in CIGMA stimulates peripheral glucose uptake producing a steady-state glucose concentration, which is a better reflection of the peripheral insulin sensitivity.18
Although CIGMA is more practical, cheaper and less invasive than the frequently sampled intravenous glucose tolerance test (FSIVGTT) and HIEC procedure, the model incorrectly assumes that levels of insulin resistance at the liver and peripheral tissues are equal. Furthermore, in insulin-deficient subjects, where the insulin response is insufficient to stimulate glucose uptake, the interpretation of CIGMA is difficult.
Minimal model analysis of frequently sampled intravenous glucose tolerance testThe minimal model, developed by Bergman, Cobelli and colleagues in 1979, provides an indirect measure of metabolic insulin sensitivity/resistance based on glucose and insulin data obtained during a frequently sampled intravenous glucose tolerance test (FSIVGTT).24 After an overnight fast, an intravenous bolus of glucose (0.3g/kg bw) is infused over 2min starting at time 0.
Currently, a modified FSIVGTT is used, where exogenous insulin (4mU/kg/min) is also infused over 5min beginning 20min after the iv glucose bolus.25,26 Some studies use tolbutamide (a potassium channel blocker) instead of insulin in the modified FSIVGTT, to stimulate endogenous insulin secretion.27,28
Blood samples are taken for plasma glucose and insulin measurements at different time points, before and 180min after glucose infusion. The data obtained are then subjected to minimal model analysis using the computer program MINMOD (minimal model approach – MINMOD), to generate an index of insulin sensitivity (SI).
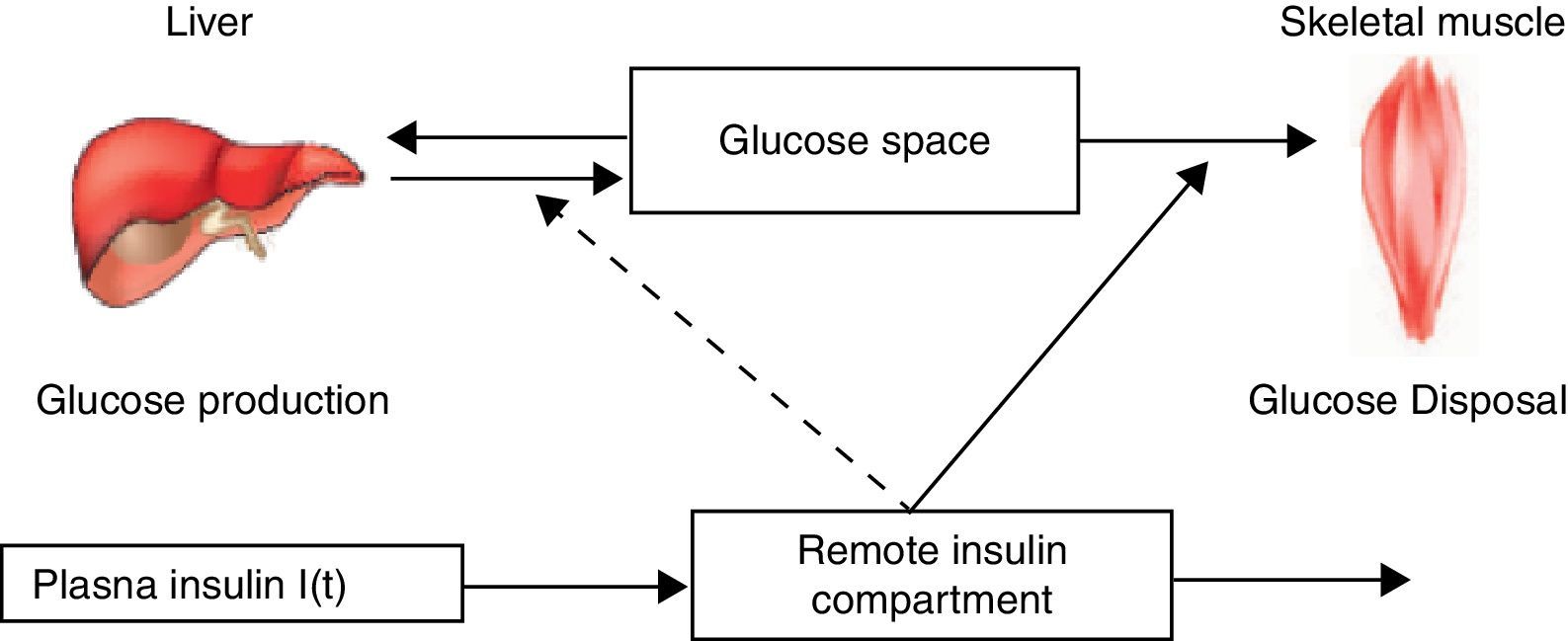
The MINMOD is defined by two coupled differential equations with four model parameters (Fig. 1). The first equation describes plasma glucose dynamics in a single compartment. The second equation describes insulin dynamics in a “remote compartment”. The structure of the MINMOD allows it to uniquely identify model parameters, which determine a best fit to glucose disappearance during the modified FSIVGTT. SI is calculated from two of these model parameters, and is defined as fractional glucose disappearance per insulin concentration unit.29
Schematic equations and parameters for the minimal model of glucose metabolism. Differential equations describing glucose dynamics [G(t)] in a monocompartmental “glucose space” and insulin dynamics in a “remote compartment” [X(t)] are shown at the top. Glucose leaves or enters its space at a rate proportional to the difference between plasma glucose level, G(t) and the basal fasting level, Gb. In addition, glucose also disappears from its compartment at a rate proportional to insulin levels in the “remote” compartment [X(t)]. In this model, t – time; G(t) – plasma glucose at time t; I(t) – plasma insulin concentration at time t; X(t) – insulin concentration in “remote” compartment at time t; Gb – basal plasma glucose concentration; Ib – basal plasma insulin concentration; G(0) – G0 (assuming instantaneous mixing of the iv glucose load); p1, p2, p3 and G0 – unknown parameters in the model that are uniquely identifiable from FSIVGTT; glucose effectiveness (SG) – p1 and insulin sensitivity – p3/p2.
In addition to SI, other minimal model parameters may be used to estimate a “glucose effectiveness” index (SG). SG is defined as the ability of glucose per se to promote its own disposal and inhibit hepatic glucose production (HGP) in the absence of an incremental insulin effect (i.e., when insulin is at basal levels).
Minimal model analysis of the modified FSIVGTT is easier than HIEC method because it is slightly less labor intensive, steady-state conditions are not required, and there are no iv infusions that require constant adjustment. Unlike HIEC or IST, information about insulin sensitivity, glucose effectiveness, and β-cell function can be derived from a single dynamic test. The minimal model generates excellent predictions of glucose disappearance during the FSIVGTT.
In research settings, where assessing insulin sensitivity along with glucose effectiveness and β-cell function is of interest, minimal model analysis of the insulin-modified FSIVGTT may be appropriate. The minimal model approach is simpler than direct methods for determining insulin sensitivity. Nevertheless, it still involves iv infusions with multiple blood sampling over a 3h period, that is, nearly as labor intensive as the HIEC or IST. In addition, many limitations of minimal model analysis stem from the fact that the model oversimplifies the physiology of glucose homeostasis.29
Another oversimplification of the minimal model involves lumping together effects of insulin to promote peripheral glucose utilization and suppress HGP. As insulin sensitivity/resistance varies, the relative contribution of HGP to SI may vary significantly. Since the minimal model relies on a dynamic test to evaluate insulin sensitivity, estimates of SI are much less reliable in individuals with impaired insulin secretion and/or significant insulin resistance (when compared with healthy subjects). Under these conditions, the minimal model may overestimate SG to accurately predict the disappearance of glucose during the FSIVGTT. Indeed, estimates of SG are spuriously affected by differences in insulin secretory capacity.26,30 Moreover, for similar reasons, minimal model analysis often generates senseless negative values for SI in a substantial proportion of subjects with diabetes, who have minimal insulin secretory capacity and significant insulin resistance.2,30 These nonsystematic errors inherent in the minimal model approach are highlighted by calibration model analysis, demonstrating that some simple surrogate indexes of insulin sensitivity have better absolute accuracy for predicting SIClamp than the minimal model-derived SI.31
Oral Glucose Tolerance TestThe Oral Glucose Tolerance Test (OGTT) is a simple test, widely used in clinical practice to diagnose glucose intolerance and type 2 diabetes.18,32,33 After an overnight fast, blood samples for determinations of glucose and insulin concentrations are taken at 0, 30, 60, and 120min following a standard oral glucose load (75g).33 A diagnosis of diabetes is conferred if an individual has a plasma glucose level ≥200mg/dl (11Ymmol/l) as measured 2h after the ingestion of a 75g glucose load. If an individual has a value in the range of 140–199mg/dl (7.7–11Ymmol/l) 2h post-glucose load, it is designated as having impaired glucose tolerance.33 Oral glucose tolerance reflects the efficiency with which the body handles glucose after an oral glucose load.
The OGTT mimics the glucose and insulin dynamics of physiological conditions more closely than conditions of the HIEC, IST or frequently sample intravenous glucose tolerance test (FSIVGTT).34 However, it is important to recognize that glucose tolerance and insulin sensitivity are not equivalent concepts. In addition to metabolic actions of insulin, insulin secretion, incretin effects, and other factors contribute importantly to glucose tolerance. Thus, the OGTT, by itself, provides useful information about glucose tolerance but not insulin sensitivity/resistance per se.35,36 During the OGTT, the use of a glucose tracer and both insulin and C-peptide plasma measurements at specific time points, allows the calculation of glucose clearance.
The OGTT is technically quite simple to perform and certainly lower in cost than HIEC or FSIVGTT. These considerations have made the OGTT the glucose challenge test of choice in clinical situations.37 However, there are some problems with the OGTT that make it less desirable for use in research situations. First, there is variability in the rate of gastric emptying and glucose absorption from the gastrointestinal tract, causing some imprecision from the start. This variability can partially account for poorly reproducible results even within the same individual.38 Second, glucose measurements in the standard OGTT do not give adequate information regarding the dynamics of glucose and insulin action.33
The OGTT is a relatively crude measure of glucose tolerance. It does not measure the components of insulin sensitivity and insulin secretion. In light of this limitation, attempts have been made to obtain indices from OGTT data that might better reflect β-cell function and insulin sensitivity.39,40
Meal Tolerance TestIn an attempt to study the ability to regulate blood glucose in a more physiological situation than the OGTT, some authors measure the glycemic profile in response to the ingestion of a mixed meal containing carbohydrates, fat and proteins; the Meal Tolerance Test (MTT).
The experimental procedure for the MTT is similar to the OGTT, that is, after an overnight fast (10–12h), a mixed meal (liquid or solid) is given and the glycemic profile is measured throughout 2h; usually the insulin profile is also determined during the same period of time.34,36
The MTT is a “physiologic” variant of OGTT41 offering several advantages: (a) lack of artifactual postload hypoglycemia, thus making this test suitable for the study of postprandial hypoglycemia, a situation which is frequently due to high values of insulin sensitivity, but also to hyperinsulinism in a context of insulin resistance42; (b) use of a physiologic stimulus triggering a cephalic phase proportional to palatability scores43; (c) possibility to measure insulin sensitivity with a modified algorithm based on the minimal model36 as well as glucose effectiveness and insulin secretion; (d) potential for evaluating the physiologic effects of incretins.44
The MTT can represent a simple procedure, less unpleasant for the patient than the standard OGTT, and providing both a physiologic picture of glucoregulation, and a sophisticated and precise analysis of this glucoregulation, in terms of insulin sensitivity, glucose effectiveness, and insulin secretion.41
The β-cell response is stronger after a mixed meal than after an OGTT with equal carbohydrate quantity, both for classical and model-based parameters. The higher response was mostly explained by higher β-cell sensitivity during the meal, which may lead to lower glucose excursions.45
Several factors may contribute to differences in insulin secretion following an MTT compared with the OGTT. The MTT has a lower glycemic index than the OGTT, which may lead to lower glucose excursions.46 Moreover, slower gastric emptying following the MTT due to larger volume,47 solid character,48 and fat content49 will lead to a slower entry of nutrients into the circulation.
The MTT might be considered as an additional tool for the assessment of metabolic abnormalities, in glucose-intolerant and insulin-resistant states.34
Thus, the MTT is a more physiological test than the OGTT, in regard to human diet, and is potentially able to give useful information concerning islet β-cell function in the different categories of glucose intolerance,50 but not insulin sensitivity/resistance per se.36
As any other method that measures glucose tolerance, the MTT does not assess insulin sensitivity directly and may not be repeated in the same subject or animal on the same day.
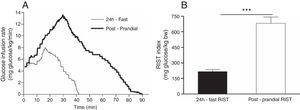
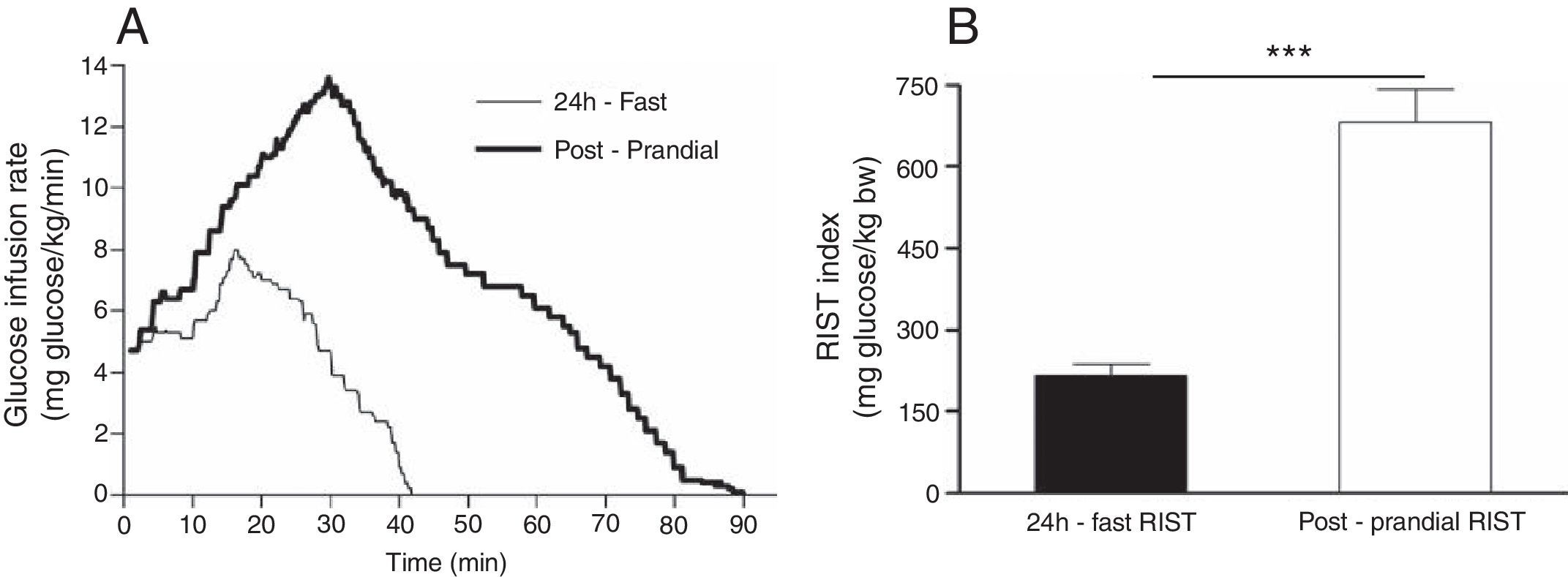
Rapid Insulin Sensitivity TestA new method for insulin sensitivity quantification, called the Rapid Insulin Sensitivity Test (RIST), was described and evaluated for use in rats,51,52 cats53,54 mice55 and humans.56 The standard dynamic profile for the RIST in fed and fasted humans as well as the RIST insulin sensitivity index is shown in Fig. 2.
(A) Mean profile using the dynamic analysis of the pattern of glucose infusion during the Rapid Insulin Sensitivity Test (RIST). The mean RIST curves were obtained by averaging glucose infusion rates at 0.1-min intervals throughout the test (Postprandial RIST (bold line) and the RIST obtained after 24h-fast (simple line)). (B) Calculation of the area under the curve during the 24 h-fast and postprandial RIST, that corresponds to the total amount of glucose infused to maintain euglycemia over the test period, which is terminated when no further glucose infusion is required (RIST index).56
The RIST is an euglycemic test and is carried out after establishing the glycemic baseline, which is done by taking arterialized venous blood samples at 5min intervals until three consecutive measurements are stable. An insulin infusion is commenced (50YmIU/kg administered over 5min) and, after 1min, glucose samples are taken at 2min intervals, and glucose is infused intravenously at a variable rate to maintain euglycemia. The test is completed when no more glucose is required. At the standard test dose of insulin of 50YmIU/kg, the RIST in the fasted state is complete within approximately 40min. The RIST index, the insulin sensitivity parameter, is simply the amount of glucose that had to be administered in order to maintain euglycemia after the bolus administration of insulin.52
The majority of the insulin sensitivity tests are done in the fasted state, when insulin sensitivity would be logically anticipated to be at its lowest level. Studies performed by Patarrão et al. and Lautt et al. indicated that the fasted state results in a very low insulin responsiveness. It is reasonable that insulin sensitivity should be under a regulatory mechanism such that in the fasted state insulin effect would be minimized, and inappropriate release of insulin would not, therefore, lead to life-threatening hypoglycemia. The RIST can be carried out in the fed state.9,56 Furthermore, the RIST allows insulin sensitivity assessment before and after a meal, making it possible to test both meal and drug effects on insulin sensitivity.56,57
The RIST is extremely sensitive and can be shown to generate dose-response relationships to insulin, which makes the RIST the most advantageous method in the determination of small differences in insulin sensitivity. This method is able to be carried out more than one time in the same subject with high reproducibility, and is sufficiently versatile to permit paired experimental designs, in the same subjects and on the same day. Both the accuracy and precision of the test can be assessed from determination of the deviation from the ideal euglycemic target.52
Insulin release normally occurs in a pulsatile manner, and hormones released in a pulsatile manner are best studied by pulsatile administration.58 Based on this assumption, the intravenous insulin bolus administered at the beginning of the RIST mimics the physiological insulin action. It also avoids the vagal withdrawal and sympathetic activation induced by sustained hyperinsulinemia, during the HIEC55,59 and the hypoglycemia caused by the acute ITT.59 It does not alter levels of counter-regulatory hormones, such as catecholamines, somatostatin or glucagon.54 Moreover, both insulinemia and glycemia return to basal levels after each RIST.
One methodological issue relates to the basal glucose concentration determined before and after the RIST. Previous studies demonstrate clearly that there is no mean change in basal blood glucose levels used as the euglycemic target when, for example, compared before and after denervation of the hepatic plexus in rats60 or atropine.61 In addition, we have also determined that there is no correlation between the magnitude of the RIST index and basal glucose levels when compared using a large number of data points.52 Of more concern is the importance that glucose uptake or output should not change during the RIST. Whatever stimulus is used, including either ablation or stimulation protocols, the stimulus is administered prior to conducting the RIST, and a new stable glycemic baseline must be demonstrated. In addition, at the conclusion of the RIST index, the re-established baseline must not be significantly altered. In the event that such alteration occurs, it suggests that glucose output either increased or decreased during the test. This is usually obvious by comparing the shape of normal RIST curves with that obtained in the presence of the altered baseline. In such situations, the data must be excluded, and the RIST repeated.
None of the available methods available to estimate insulin sensitivity/resistance proved to be a reliable way to assess insulin sensitivity/resistance since most of them have non-physiological continuous infusion of insulin and/or glucose, which interfere with peripheral insulin sensitivity/resistance; take a long time to be performed; could not avoid counter-regulatory responses to the hypoglycemia that follows an insulin bolus; could not allow the assessment of insulin sensitivity in different conditions in the same subject, and in the same day; and they only evaluate insulin sensitivity/resistance in the fasted state. Based on all of these drawbacks, it was necessary to develop another method for assessing insulin sensitivity/resistance.
To summarize, the RIST is a quick method to evaluate insulin sensitivity, reproducible in the same subject and on the same day, utilizes a bolus of insulin to mimic pulsatile insulin release, and can be performed in the fed or fasting state. In addition, since the RIST is an euglycemic test, avoids hypoglycemia and prevents the activation of counter-regulatory hormones. The RIST provides a new powerful tool to dissect insulin action in the fasted and fed state, and may provide a means to detect the pre-diabetic state, where early insulin resistance can be detected well before the impairment of the direct effect of insulin at a time when lifestyle interventions can be readily tested.
Simple surrogate indexes for insulin sensitivity/resistanceHomeostasis Model AssessmentThe Homeostasis Model Assessment (HOMA), developed in 1985, is a model of interactions between glucose and insulin dynamics, that is then used to predict fasting steady-state glucose and insulin concentrations, for a wide range of possible combinations of insulin resistance and β-cell function.62 The model assumes a feedback loop between the liver and β-cell62,63; and glucose concentrations are regulated by insulin-dependent hepatic glucose production, while insulin levels depend on the pancreatic β-cell response to glucose concentrations. Thus, a diminished response to glucose-stimulated insulin secretion reflects deficient β-cell function. Likewise, insulin resistance is reflected by diminished suppressive effect of insulin on hepatic glucose production.
HOMA model describes this glucose-insulin homeostasis by a set of empirically derived non-linear equations. The model predicts fasting steady-state levels of plasma glucose and insulin for any given combination of pancreatic β-cell function (HOMA%B) and insulin sensitivity (HOMA%S).
In practical terms, most studies using HOMA employ an approximation described by a simple equation to determine a surrogate index of insulin resistance. This is defined by the product of the fasting glucose and fasting insulin, divided by a constant. The formula for the HOMA model is:
The denominator of 22.5 is a normalizing factor, i.e., the product of normal fasting plasma insulin of 5μIU/ml and normal fasting plasma glucose of 4.5Ymmol/l obtained from an “ideal and normal” individual.62 Therefore, for an individual with normal insulin sensitivity, HOMA=1. It is important to note that, over wide ranges of insulin sensitivity/resistance, log (HOMA) transforms the skewed distribution of fasting insulin values to determine a much stronger linear correlation with HIEC estimates of insulin sensitivity.2
HOMA or log (HOMA) is extensively used in large epidemiological studies, prospective clinical trials, and research studies. In research settings where assessing insulin sensitivity/resistance is of secondary interest or feasibility issues preclude the use of direct measures by HIEC, it may be appropriate to use log (HOMA).63
Quantitative insulin sensitivity check indexQuantitative insulin sensitivity check index (QUICKI) is an empirically derived mathematical transformation that uses fasting blood glucose and plasma insulin concentrations. It provides a reliable, reproducible, and accurate index of insulin sensitivity with excellent positive predictive power.2,64 Since fasting insulin levels have a non-normal skewed distribution, log transformation improves its linear correlation with reference standard glucose clamp (SIClamp). However, as with 1/(fasting insulin) and the glucose/insulin ratio, this correlation is not maintained in diabetic subjects with fasting hyperglycemia and impaired β-cell function that is insufficient to maintain euglycemia. To accommodate these clinically important circumstances where fasting glucose is inappropriately high, insulin is inappropriately low, application of logarithm to both fasting glucose, and fasting insulin provides a reasonable correction such that the linear correlation with SIClamp is maintained, in both diabetic and non-diabetic subjects. The reciprocal of this sum results in further transformation of the data generating an insulin sensitivity index that has a positive correlation with SIClamp. Thus, QUICKI is defined by the following formula:
QUICKI is among the most thoroughly evaluated and validated surrogate index for insulin sensitivity. As a simple, useful, inexpensive, and minimally invasive surrogate for HIEC-derived measures of insulin sensitivity, QUICKI is appropriate and effective for use in large epidemiological or clinical research studies, to follow changes after therapeutic interventions, and for use in studies where evaluation of insulin sensitivity is not of primary interest.2,65
QUICKI and HOMA were derived in a completely different conceptual fashion. Nevertheless, these two surrogate indexes are mathematically related, i.e., QUICKI is proportional to 1/log (HOMA).
The major advantage of both the QUICKI and HOMA models is that they both require only one blood draw from a fasting patient. They thus do not require extensive technical expertise, and constitute a much lower cost per subject when compared with the HIEC or the FSIVGTT, making the QUICKI and HOMA models much more practical for use in large-scale epidemiologic studies, and for clinical situations.63
However, the major disadvantage is that both of these methods fail to provide information about the stimulated glucose and insulin systems. Essentially, they provide information only about what is occurring with homeostatic mechanisms in the fasting state, largely reflecting insulin's effect on hepatic glucose production and not on peripheral glucose uptake, which is the more relevant aspect concerning insulin action/resistance.
Insulin sensitivity indexesCederholm and Wibell indexThe insulin sensitivity index proposed by Cederholm and Wibell represents mainly peripheral insulin sensitivity and muscular glucose uptake, due to the dominant role of peripheral tissues in glucose disposal after an oral glucose load.40
The formula for the Cederholm index is:
where 75,000 – oral glucose load in an OGTT in mg, G0 – fasting plasma glucose concentration (mmol/l), G120 – plasma glucose concentration in the 120th min of OGTT (mmol/l), 1.15 – factor transforming whole venous blood glucose to plasma values (not necessary, if glucose concentration is estimated in plasma), 180 – conversion factor to transform plasma glucose concentration from mmol/l into mg/dl, 0.19 – glucose space in liter per kg of body weight, m – body weight (kg), 120 – duration of OGTT (min), Imean – mean plasma insulin concentration during OGTT (mIU/l) and Gmean – mean plasma glucose concentration during OGTT (mmol/l).Values found in normal non-obese individuals were reported to be about 79±14mgl2/mmol/mIU/min, lower in obese individuals, in subjects with impaired glucose tolerance and in patients with type 2 diabetes.40
Gutt et al. indexThe ISI0,120 was adapted from the Cederholm insulin sensitivity index, by omitting the constant terms, and using the plasma glucose and insulin concentration from fasting (0min) and 120min samples from the OGTT.66
The ISI0,120 index is defined as:
where 75,000 – oral glucose load in an OGTT in mg, G0 – fasting plasma glucose concentration (mg/dl), G120 – plasma glucose concentration in the 120th min of OGTT (mg/dl), 0.19 – glucose space in l/kg of body weight, m – body weight (kg), 120 – duration of OGTT (min), Imean – mean plasma insulin concentration during OGTT (mIU/l) and Gmean – mean plasma glucose concentration during OGTT (mmol/l).The reference range for lean controls was 89±39, for obese 58±23 and for diabetic patients 23±19mgl2/mmol/mIU/min.18
Avignon et al. indexAvignon67 proposed 3 insulin sensitivity indices: Sib (derived from fasting plasma insulin and glucose concentrations), Si2h (derived from plasma insulin and glucose concentrations in the 120th min of OGTT) and SiM (derived by averaging Sib and Si2h after balancing Sib by a coefficient of 0.137 to give the same weight to both indices):
where I and G represent the plasma concentrations of insulin (mIU/l) and glucose (mmol/l), respectively and, VD is the glucose distribution volume calculated using a monocompartmental model: VD=150ml/kg of body weight.28Matsuda et al. indexOriginally proposed by Matsuda and DeFronzo,39 insulin sensitivity index-Matsuda (ISI(Matsuda)) is an whole body insulin sensitivity index that reflects a composite estimate of hepatic and muscle insulin sensitivity. This index is calculated from plasma glucose (mg/dl) and insulin (mIU/l) concentrations in fasting state and during OGTT.
The formula for the Matsuda index is:
where 10,000 – simplifying constant to get numbers from 0 to 12, √ – correction of the nonlinear values distribution, G0 – fasting plasma glucose concentration (mg/dl), I0 – fasting plasma insulin concentration (mIU/l), Gmean – mean plasma glucose concentration during OGTT (mg/dl), from 0 to 120min and Imean – mean plasma insulin concentration during OGTT (mIU/l), from 0 to 120min.The insulin secretion/insulin resistance (disposition) index calculated as the product of insulin secretion measured with (ΔI0–30/ΔG0–30 or ΔI0–120/ΔG0–120) and ISI(Matsuda) (or modified ISI(Matsuda) using plasma glucose and insulin concentrations at 30min during the OGTT), had excellent power to predict onset of type 2 diabetes.68
Belfiore et al. indexThe condition for calculation of the Belfiore formula is the definition of the normal value for basal glucose and insulin concentrations, and for mean normal value for glucose and insulin areas during OGTT.18 The main point of the Belfiore formula is the comparison of insulin and glucose values measured (fasting, 0–1–2h areas or 0–2h areas) with the defined normal reference values.
The ISIBelfiore index is defined as:
where Gs, GN – plasma glucose concentrations expressed as fasting values or as areas obtained during a standard OGTT at 0 and 2h (0–2h areas are equal to GS,N=G0+G120) or at 0, 1 and 2h (0–1–2h areas equal to GS,N=½G0+G60+G120, Is, IN – plasma insulin concentrations expressed as fasting values or as areas obtained during a standard OGTT at 0 and 2h (0–2h areas are equal to IS,N=I0+I120) or at 0, 1 and 2h (0–1–2h areas equal to IS,N=½I0+I60+I120. The subscripts S and N refer to “subjects” and “normal reference values”, respectively.Insulin sensitivity calculated using these formulas can achieve only values between 0 and 2. In subjects with normal insulin sensitivity is it around 1; in overweight subjects, in subjects with impaired glucose tolerance, and with type 2 diabetes this value is below 1.69,70
Stumvoll et al. indexStumvoll proposed a series of indices calculated from plasma glucose (mmol/l) and insulin (pmol/l concentrations during OGTT).70 The equations were generated using the multiple linear regression analysis and adapted to the availabilities of sampling times during OGTT, and of demographic parameters (BMI, age).
An example equation could be the index of insulin sensitivity calculated from data obtained in 0, 60 and 120min of OGTT either with or without demographic data:
ISIStumvoll=0.222−0.00333×BMI−0.0000779×I120−0.000422×age
ISIStumvoll=0.156−0.0000459×I120−0.000321×I0−0.00541×G120
The authors proposed a formula for predicting insulin resistance in normoglycemic individuals.71 Regression analysis was used to estimate the cut-off points and the importance of various data for insulin resistance (fasting concentrations of insulin, triglycerides, aspartate aminotransferase, BMI, waist circumference). A bootstrap procedure was used to find an index most strongly correlating with insulin sensitivity index, corrected for fat-free mass obtained by HIEC MffmI.1
An insulin sensitivity index obtained from HIEC of ≤6.3 (expressed as glucose disposal rate in mg/kg/min divided by average plasma insulin concentration in mIU/l) was seen as a cut-off for individuals with insulin resistance. The combination of fasting insulin (mIU/l) and triglycerides (TAG, mmol/l) showed the best prediction of insulin resistance as follows:
where I0 – fasting plasma insulin concentration (mIU/l) and TAG0 – fasting plasma triglycerides concentration (mmol/l).Oral Glucose Insulin SensitivityThe Oral Glucose Insulin Sensitivity (OGIS) is a method for the assessment of insulin sensitivity from the OGTT. OGIS provides an index that correlates to the index of insulin sensitivity obtained from the HIEC.
This method calculates insulin sensitivity with a model-derived equation of the form:
where G and I are glucose and insulin concentrations (subscripts represent time instant) and DO is the oral glucose dose (g/m2 body surface area).The function f is complex, but can be easily programmed on a spreadsheet (see http://www.isib.cnr.it/bioing/ogis/home.html, where a web-based calculator is also available). The expression of f contains some parameters, chosen to maximize the agreement with the HIEC. Glucose and insulin can be given in either common or international units (with appropriate parameters, see table 2 in72).
OGIS is a predictor of the HIEC insulin sensitivity, expressed as glucose clearance M/G, normalized to body surface area. The units of OGIS are thus ml/min/m2 of body surface area. OGIS has been validated against an 120mU/min/m2 insulin infusion HIEC (by direct comparison of the glucose clearance values), instead of the more standard 40mU/min/m2 used in the previous methods. Formulas for a 3h and 2h OGTT are also available.72
OGIS exploits the known quantitative relationships between the observed data and the HIEC insulin sensitivity to attempt a genuine insulin sensitivity prediction. However, this advantage is limited by the necessity to use empirical assumptions, and to calculate parameters from regression.72
Rapid Insulin Sensitivity Test indexThe RIST index is the parameter used to evaluate insulin sensitivity that represents the total amount of glucose infused during the Rapid Insulin Sensitivity Test (RIST), in order to maintain euglycemia after the exogenous bolus administration of insulin. It corresponds to the area under the curve (AUC) of total glucose infused (mg glucose/kg bw) throughout the test52:
ConclusionsThis paper has examined a wide variety of methods currently available for estimating insulin sensitivity/resistance and also introduces a new method – the RIST. The methods range from complex, time-consuming, labor-intensive, invasive procedures to simple tests involving a single fasting blood sample. It is important to understand the concepts underlying each method so that relative merits and limitations are appropriately matched to proposed applications. Developing valid, reliable, cost-effective methods of assessing insulin sensitivity is a major scientific challenge. Dynamic tests are useful if information about both insulin secretion and insulin action are needed. As with all measurement techniques, correct interpretation of data from different methods for measuring insulin sensitivity requires a complete understanding of the technique.
FundingThis study was supported by Fundação para a Ciência e Tecnologia (FCT) grants FCT/POCI/SAU-OBS/56716/2004 and PIC/IC/82956/2007 and by the Portuguese Diabetes Society (SPD).
Conflicts of interestThe authors declare no conflicts of interest.
We thank Fernandes AB, Afonso RA, Ribeiro RT and Inês S. Lima for helpful scientific discussions.




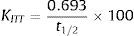
![Schematic equations and parameters for the minimal model of glucose metabolism. Differential equations describing glucose dynamics [G(t)] in a monocompartmental “glucose space” and insulin dynamics in a “remote compartment” [X(t)] are shown at the top. Glucose leaves or enters its space at a rate proportional to the difference between plasma glucose level, G(t) and the basal fasting level, Gb. In addition, glucose also disappears from its compartment at a rate proportional to insulin levels in the “remote” compartment [X(t)]. In this model, t – time; G(t) – plasma glucose at time t; I(t) – plasma insulin concentration at time t; X(t) – insulin concentration in “remote” compartment at time t; Gb – basal plasma glucose concentration; Ib – basal plasma insulin concentration; G(0) – G0 (assuming instantaneous mixing of the iv glucose load); p1, p2, p3 and G0 – unknown parameters in the model that are uniquely identifiable from FSIVGTT; glucose effectiveness (SG) – p1 and insulin sensitivity – p3/p2. Schematic equations and parameters for the minimal model of glucose metabolism. Differential equations describing glucose dynamics [G(t)] in a monocompartmental “glucose space” and insulin dynamics in a “remote compartment” [X(t)] are shown at the top. Glucose leaves or enters its space at a rate proportional to the difference between plasma glucose level, G(t) and the basal fasting level, Gb. In addition, glucose also disappears from its compartment at a rate proportional to insulin levels in the “remote” compartment [X(t)]. In this model, t – time; G(t) – plasma glucose at time t; I(t) – plasma insulin concentration at time t; X(t) – insulin concentration in “remote” compartment at time t; Gb – basal plasma glucose concentration; Ib – basal plasma insulin concentration; G(0) – G0 (assuming instantaneous mixing of the iv glucose load); p1, p2, p3 and G0 – unknown parameters in the model that are uniquely identifiable from FSIVGTT; glucose effectiveness (SG) – p1 and insulin sensitivity – p3/p2.](https://static.elsevier.es/multimedia/16463439/0000000900000001/v1_201407280026/S1646343913000734/v1_201407280026/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)