This study compares the effectiveness of a new early intervention service for firstepisode psychosis (FEP) in patients under conventional treatment. Six primary and 10 secondary outcome measures are used to better characterize the comparative effectiveness between two FEP groups.
MethodsThis study plans to enroll 250 patients aged 15–55 years with FEP from all inpatient and outpatient mental health services and primary health care from January 2020 until December 2022. The control group will be composed of 130 FEP patients treated in mental health centers in the 2 years prior to the start of PEPsNa (Programa de Primeros Episodios de Psicosis de Navarra). The primary outcome measures are symptomatic remission, functional recovery, personal recovery, cognitive performance, functional capacity in real-world settings, and costs. The secondary outcome measures are duration of untreated psychosis, substance abuse rate, antipsychotic monotherapy, minimal effective dose of antipsychotic drugs, therapeutic alliance, drop-out rate, number of relapses, global mortality and suicidality, resource use, and general satisfaction in the program.
DiscussionThis study arises from the growing need to broaden the scope of outcome measures in FEP patients and to account for unmet needs of recovery for FEPs. It aims to contribute in the dissemination of the NAVIGATE model in Europe and to provide new evidence of the effectiveness of early intervention services for stakeholders of the National Health Service.
The lifetime prevalence of psychotic disorders in the general population is 3.5%,1 and they usually have their onset in late adolescence or early adulthood. Around 1% of the general population seems to meet the criteria for an at-risk mental state for psychosis at age 24.2 First-episode psychosis (FEP) involves significant personal, social and health burden, and its outcomes are varied, ranging from full to incomplete remission with poor outcome.3
Early intervention services (EISs) for FEP patients provide multicomponent and intensive interventions during the first 2 or 3 years, which is acknowledged as the ‘critical period’ for the prevention of poor outcomes.4,5 EISs have been implemented all over the world in the last two decades, but few studies have specifically addressed their effectiveness.6
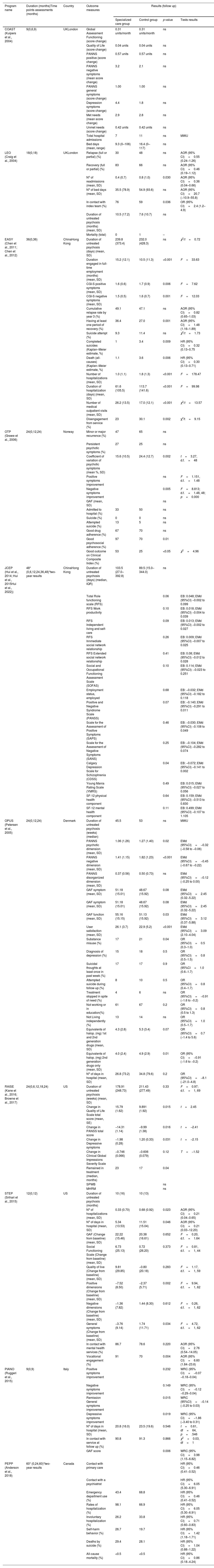
There is consistent evidence demonstrating that between 1–2 years and longer duration or early intervention for FEP patients is cost-effective.7–18 A recent comprehensive meta-analysis of 10 RCTs concluded that EIS programs were associated with superior outcomes compared with TAU across a wide range of clinically relevant outcomes, such as hospitalization risk, bed-days, symptoms, and global functioning.19 However, it has been reported that existing studies are not exempt of methodological limitations20 and the superiority of EISs over treatment-as-usual (TAU) may require longer follow-up periods to be definitively verified.21Table 1 shows a summary of the 10 FEP studies that provide consistent results of effectiveness with their outcome measures and significant results.
Summary of the outcome variables of the intervention programs in first-episode psychosis.
| Program name | Duration (months)Time points assessments (months) | Country | Outcome measures | Results (follow up) | |||
|---|---|---|---|---|---|---|---|
| Specialized care group | Control group | p value | Tests results | ||||
| COAST (Kuipers et al., 2004) | 9(0,6,9) | UKLondon | Global Assessment Functioning (score change) | 0.31 units/month | 0.31 units/month | ns | |
| Quality of Life (score change) | 0.04 units | 0.04 units | ns | ||||
| PANNS positive (score change) | 0.57 units | 0.57 units | ns | ||||
| PANNS negative symptoms (mean score change) | 3.2 | 2.1 | ns | ||||
| PANNS general symptoms (score change) | 1.00 | 1.00 | ns | ||||
| Depression symptoms (score change) | 4.4 | 1.8 | ns | ||||
| Met needs (mean score change) | 2.9 | 2.8 | ns | ||||
| Unmet needs (score change) | 0.42 units | 0.42 units | ns | ||||
| Total hospital admissions | 7 | 11 | ns | MWU | |||
| Bed days (mean, range) | 9.3 (0–106) | 16.4 (0–117) | ns | ||||
| LEO (Craig et al., 2004) | 18(0,18) | UKLondon | Relapse (full or partial) (%) | 30 | 48 | ns | AOR (95% CI)=0.55 (0.24–1.26) |
| Recovery (full or partial) (%) | 83 | 66 | ns | AOR (95% CI)=0.46 (0.19–1.12) | |||
| No of readmissions (mean, SD) | 0.4 (0.7) | 0.8 (1.0) | 0.030 | AOR (95% CI)=0.36 (0.04–0.66) | |||
| No of bed days (mean, SD) | 35.5 (78.9) | 54.9 (93.6) | ns | AOR (95% CI)=20.7 (−10.9–55.8) | |||
| In contact with index team (%) | 76 | 59 | 0.036 | OR (95% CI)=2.4 (1.2–4.9) | |||
| Duration of untreated psychosis (months) (mean, SD) | 10.5 (17.2) | 7.6 (10.7) | ns | ||||
| Mortality (total) | 0 | 1 | – | ||||
| EASY (Chen et al., 2011; Chen et al., 2012) | 36(0,36) | ChinaHong Kong | Duration of untreated psychosis (days) (mean, SD) | 239.8 (373.4) | 232.0 (428.3) | ns | χ2/t=0.72 |
| Duration engaged in full-time employment (months) (mean, SD) | 15.2 (12.1) | 10.5 (11.3) | <0.001 | F=33.63 | |||
| CGI-S positive symptoms (mean, SD) | 1.6 (0.6) | 1.7 (0.9) | 0.006 | F=7.62 | |||
| CGI-S negative symptoms (mean, SD) | 1.5 (0.5) | 1.6 (0.7) | 0.001 | F=12.03 | |||
| Cumulative relapse rate by year 3 (%) | 49.1 | 47.1 | ns | AOR (95% CI)=0.82 (0.65–1.03) | |||
| Having at least one period of recovery (%) | 36.4 | 27.0 | 0.001 | AOR (95% CI)=1.48 (1.16–1.89) | |||
| Suicide attempt (%) | 9.3 | 11.4 | ns | χ2/t=1.73 | |||
| Completed suicides (Kaplan–Meier estimate, %) | 1 | 3.4 | 0.009 | HR (95% CI)=0.32 (0.13–0.75 | |||
| Death (all-causes) (Kaplan–Meier estimate, %) | 1.1 | 3.6 | 0.006 | HR (95% CI)=0.30 (0.13–0.71) | |||
| Number of hospitalizations (mean, SD) | 1.0 (1.1) | 1.8 (1.3) | <0.001 | F=178.47 | |||
| Duration of hospitalization (days) (mean, SD) | 61.6 (105.5) | 113.7 (141.6) | <0.001 | F=99.98 | |||
| Number of medical outpatient visits (mean, SD) | 26.2 (13.5) | 17.0 (12.1) | <0.001 | χ2/t=13.57 | |||
| Disengagement from service (%) | 23 | 30.1 | 0.002 | χ2/t=9.15 | |||
| OTP (Grawe et al., 2006) | 24(0,12,24) | Norway | Minor or major recurrence (%) | 47 | 65 | ns | |
| Persistent psychotic symptoms (%) | 27 | 25 | ns | ||||
| Coefficient of variation of psychotic symptoms (mean %, SD) | 15.6 (10.5) | 24.4 (12.7) | 0.002 | t=3.27, d.f.=48 | |||
| Positive symptoms improvement | ns | F=1.151, d.f.=1.48 | |||||
| Negative symptoms improvement | 0.005 | F=8.813; d.f.=1.48, 48; p=0.000 | |||||
| GAF (mean, SD) | ns | ||||||
| Admitted to hospital (%) | 33 | 50 | ns | ||||
| Suicide (%) | 0 | 0 | ns | ||||
| Attempted suicide (%) | 13 | 5 | ns | ||||
| Good drug adherence (%) | 67 | 70 | ns | ||||
| Good psychosocial adherence (%) | 97 | 70 | 0.01 | ||||
| Good outcome on Clinical Composite Index (%) | 53 | 25 | <0.05 | χ2=4.96 | |||
| JCEP (Hui et al., 2014; Hui et al., 2015Hui et al., 2022)) | 48* (0,6,12,24,36,48)*two-year results | ChinaHong Kong | Duration of untreated psychosis (days) (median, IQR) | 103.5 (27.0–392.8) | 89.5 (15.0–344.0) | ns | |
| Total Role functioning scale (RFS) | 0.06 | EB: 0.048; EMd (95%CI) −0.002 to 0.099 | |||||
| RFS Work productivity | 0.10 | EB: 0.018; EMd (95%CI) −0.004 to 0.039 | |||||
| RFS Independent living and self-care | 0.09 | EB: 0.013; EMd (95%CI) −0.002 to 0.027 | |||||
| RFS Innmediate social network relationship | 0.26 | EB: 0.009; EMd (95%CI) −0.007 to 0.025 | |||||
| RFS Extended social network relationship | 0.41 | EB: 0.08; EMd (95%CI) −0.012 to 0.028 | |||||
| Social and Occupational Functioning Assessment Scale (SOFAS) | 0.10 | EB: 0.114; EMd (95%CI): −0.023 to 0.251 | |||||
| Employment status, employed | 0.68 | EB: −0.032; EMd (95%CI) −0.182 to 0.118 | |||||
| Positive and Negative Syndrome Scale (PANSS) | 0.07 | EB: −0.140; EMd (95%CI) −0.291 to 0.011 | |||||
| Scale for the Assessment of Positive Symptoms (SAPS) | 0.46 | EB: −0.030; EMd (95%CI) −0.108 to 0.049 | |||||
| Scale for the Assessment of Negative Symptoms (SANS) | 0.25 | EB: −0.104; EMd (95%CI) −0.282 to 0.074 | |||||
| Calgary Depression Scale for Schizophrenia (CDSS) | 0.04 | EB: −0.072; EMd (95%CI) −0.141 to 0.002 | |||||
| Young Mania Rating Scale (YMRS) | 0.49 | EB: 0.015; EMd (95%CI) −0.027 to 0.056 | |||||
| SF-12 physical health component | 0.64 | EB: 0.159; EMd (95%CI) −0.513 to 0.830 | |||||
| SF-12 mental health component | 0.11 | EB: 0.499; EMd (95%CI) −0.107 to 1.105 | |||||
| OPUS (Petersen et al., 2005) | 24(0,12,24) | Denmark | Duration of untreated psychosis (weeks) (median) | 45.5 | 53 | ns | MWU |
| PANNS psychotic dimension (mean, SD) | 1.06 (1.26) | 1.27 (1.40) | 0.02 | EMd (95%CI)=−0.32 (−0.58 to −0.06) | |||
| PANNS negative dimension (mean, SD) | 1.41 (1.15) | 1.82 (1.23) | <0.001 | EMd (95%CI)=−0.45 (−0.67 to −0.22) | |||
| PANNS disorganized dimension (mean, SD) | 0.37 (0.56) | 0.50 (0.73) | ns | EMd (95%CI)=−0.12 (−0.25 to 0.00) | |||
| GAF symptom (mean, SD) | 51.18 (15.01) | 48.67 (15.92) | 0.08 | EMd (95%CI)=2.45 (0.32−5.22) | |||
| GAF symptom (mean, SD) | 51.18 (15.01) | 48.67 (15.92) | 0.08 | EMd (95%CI)=2.45 (0.32−5.22) | |||
| GAF function (mean, SD) | 55.16 (15.15) | 51.13 (15.92) | 0.03 | EMd (95%CI)=3.12 (0.37−5.88) | |||
| User satisfaction (mean, SD) | 26.1 (3.7) | 22.9 (5.2) | <0.001 | EMd (95%CI)=3.09 (2.10−4.04) | |||
| Substance misuse (%) | 17 | 21 | 0.04 | OR (95%CI)=0.5 (0.3−1.0) | |||
| Diagnosis of depression (%) | 15 | 18 | 0.5 | OR (95%CI)=0.8 (0.5−1.5) | |||
| Suicidal thoughts at least once in past week (%) | 17 | 17 | 0.9 | OR (95%CI=1.0 (0.6−1.7) | |||
| Attempted suicide during follow-up (%) | 8 | 10 | 0.5 | OR (95%CI)=0.8 (0.4−1.7) | |||
| Treatment stopped in spite of need (%) | 4 | 6 | ns | OR (95%CI)=−0.91 (−1.6 to −0.2) | |||
| Not working or in education(%) | 61 | 67 | 0.2 | OR (95%CI)=0.8 (0.5 to 1.3) | |||
| Not Living independently (%) | 13 | 14 | ns | OR (95%CI)=1.0 (0.5−1.7) | |||
| Equivalents of halop. (mg) 1st and 2nd generation drugs (mean, SD) | 4.3 (2.8) | 5.3 (3.4) | 0.07 | OR (95%CI)=0.7 (−1.4 to 5.6) | |||
| Equivalents of halop. (mg) 2nd generation drugs only (mean, SD) | 4.0 (2.4) | 4.9 (2.9) | 0.01 | OR (95% CI)=−0.91 (−1.6 to −0.2) | |||
| No of days in hospital (mean, SD) | 26.8 (73.2) | 34.8 (79.6) | 0.2 | OR (95%CI)=−8.1 (−21.0−4.8) | |||
| RAISE (Kane et al., 2016; Browne et al., 2017) | 24(0,6,12,18,24) | US | Duration of untreated psychosis (weeks) (mean, SD) | 178.91 (248.73) | 211.43 (277.49) | 0.33 | F=0.97; d.f.=1, 69 |
| Change in Quality of Life Scale total score (mean, SE) | 15.79 (1.62) | 9.891 (1.92) | 0.015 | t=2.45 | |||
| Change in PANSS total score | −14.31 (1.14) | −9.99 (1.38) | 0.016 | t=−2.41 | |||
| Change in Depressive symptoms | −1.98 (0.28) | 1.20 (0.33) | 0.031 | t=−2.15 | |||
| Change in Clinical Global Impressions Severity Scale | −0.746 (0.066) | −0.606 (0.079) | 0.12 | T=−1.52 | |||
| Remained in treatment (median, months) | 23 | 17 | 0.04 | ||||
| SPWB | ns | ||||||
| MHRM | ns | ||||||
| STEP (Srihari et al., 2015) | 12(0,12) | US | Duration of untreated psychosis (months) | 10 (16) | 10 (13) | ||
| No of hospitalizations (mean, SD) | 0.33 (0.70) | 0.68 (0.92) | 0.023 | AOR (95% CI)=0.21 (0.04−0.65) | |||
| No of days in hospital (mean, SD) | 5.34 (13.53) | 11.51 (15.04) | 0.046 | AOR (95% CI)=0.21 (0.03−12.20) | |||
| GAF (Change from baseline) (mean, SD) | 22.22 (15.46) | 20.38 (16.61) | 0.652 | F=0.20, d.f.=1.64 | |||
| Social Functioning Scale (Change from baseline) (mean, SD) | 6.73 (25.13) | 0.72 (26.20) | 0.373 | F=0.81, d.f.=1, 44 | |||
| Quality of live (Change from baseline) (mean, SD) | 9.81 (29.85) | −0.80 (20.18) | 0.283 | F=1.17, d.f.=1, 59 | |||
| Positive dimensions (Change from baseline) (mean, SD) | −7.52 (8.50) | −2.37 (5.71) | 0.002 | F=9.94, d.f.=1, 62 | |||
| Negative dimensions (Change from baseline) (mean, SD) | −1.36 (7.82) | 1.44 (8.30) | 0.612 | F=0.26, d.f.=1, 62 | |||
| General symptoms (Change from baseline) (mean, SD) | −3.76 (9.14) | 1.74 (11.71) | 0.034 | F=4.72, d.f.=1, 62 | |||
| In contact with mental health services (%) | 86.7 | 78.6 | 0.220 | AOR (95% CI)=2.76 (0.54−14.05) | |||
| Vocational engagement (%) | 91 | 70 | 0.004 | AOR (95% CI)=6.60 (1.84−23.6) | |||
| PIANO (Ruggeri et al., 2015) | 9(0,9) | Italy | Positive symptoms improvement | 0.232 | WRC (95% CI)=−0.07 −0.18−0.04) | ||
| Negative symptoms improvement | 0.149 | WRC (95% CI)=−0.12 −0.29−0.04) | |||||
| Remission General symptoms improvement | 0.015 | WRC (95%CI)=−0.14 (−0.25 to 0.03) | |||||
| Depressive symptoms improvement | 0.019 | WRC (95% CI)=−1.86 (−3.40 to 0.31) | |||||
| No of days in hospital (mean, SD) | 20.8 (16.0) | 23.5 (19.6) | 0.546 | t=0.61, df=64, p=.546 | |||
| In contact with service at follow up (%) | 90.8 | 91.3 | 0.866 | χ2=0.03, df=1 | |||
| GAF score | 0.006 | WRC (95% CI)=3.98 (1.15−6.82) | |||||
| PEPP (Anderson et al., 2018) | 60* (0,24,60)*two-year results | Canada | Contact with primary care | HR (95% CI)=0.46 (0.41−0.52) | |||
| Contact with a psychiatrist | HR (95% CI)=6.05 (5.30−6.91) | ||||||
| Emergency department use (%) | 43.4 | 68.8 | HR (95% CI)=0.46 (0.41−0.52) | ||||
| Rates of hospitalization (%) | 98.1 | 66.9 | HR (95% CI)=6.05 (5.30−6.91) | ||||
| Involuntary hospitalization (%) | 26.2 | 33.8 | HR (95% CI)=0.71 (0.60−0.83) | ||||
| Self-harm behavior (%) | 26.7 | 19.7 | HR (95% CI)=1.42 (1.18−1.71) | ||||
| Deaths by suicide (%) | 29.4 | 28.1 | HR (95% CI)=1.04 (0.88−1.22) | ||||
| All-cause mortality (%) | <0.5 | <0.5 | HR (95% CI)=0.86 (0.18−4.24) | ||||
PANNS: Positive and Negative Syndrome Scale (Kay et al., 1987); CGI-S: Clinical Global Impressions-Severity Scale; IQR: interquartile range; RFS: Role Functioning Scale (Goodman, Sewell, Cooley, & Leavitt, 1993); SOFAS: Social and Occupational Functioning Assessment Scale; SF-12: Short Form-12 Health Survey; GAF: Global assessment of functioning; AOR: Adjusted Odds ratio; OR: Odds ratio; EMd: Estimated mean difference; HR: Hazard ratio; EB: Interaction estimated between groups; SPWB: Scales of Psychological Well-Being; MHRM: Mental Health Recovery Scale; WRC: Weighted Regression Coefficient. MWU: Mann–Whitney U-test.
A comprehensive approach to broaden the scope of outcome measures and analyze their clinical relevance in EISs for psychosis is a clear unmet need that has to be accomplished, and it is the innovation of our study protocol.
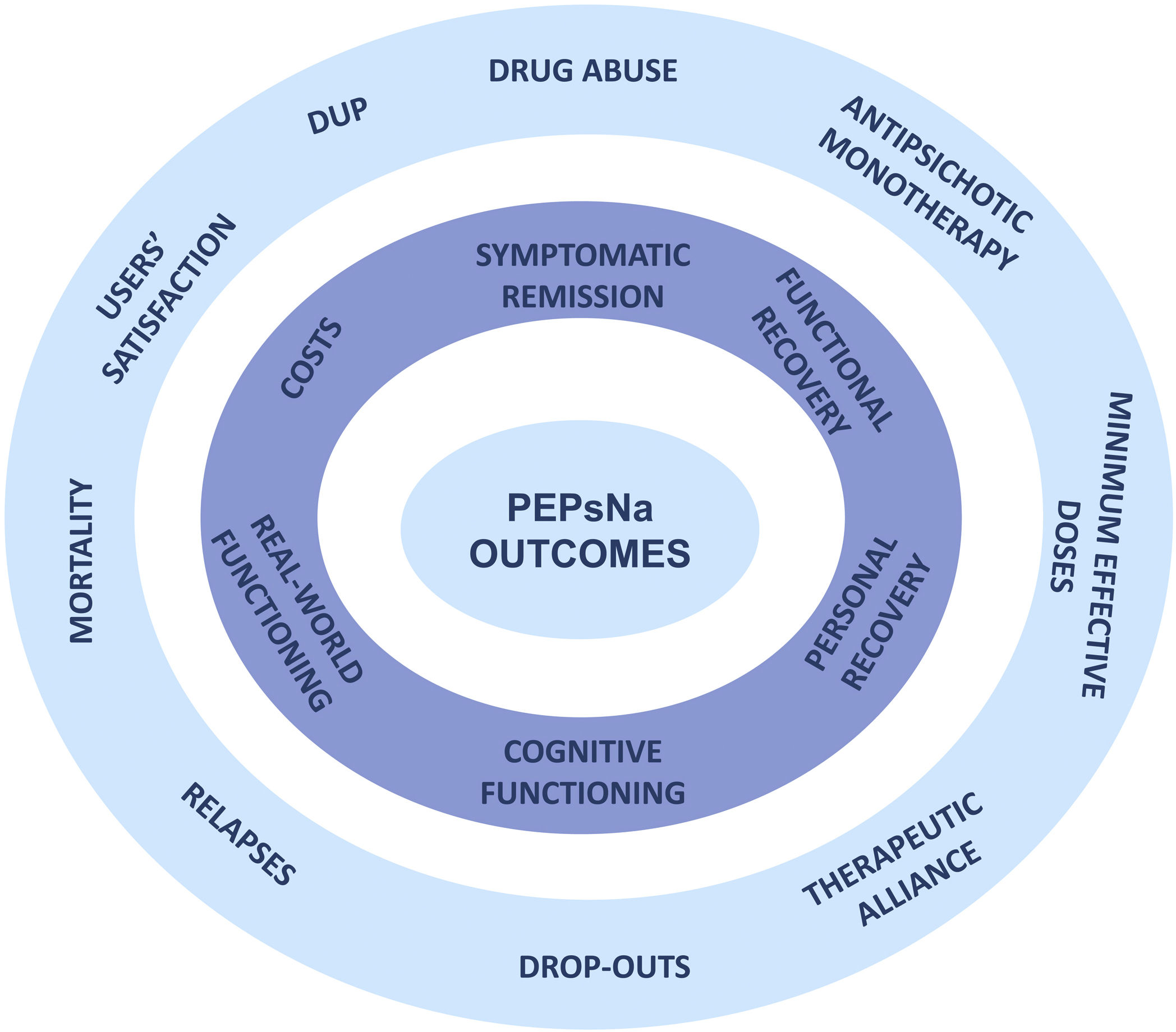
Aims and hypothesisThis study seeks to compare the effectiveness of the 2-year Programa de Primeros Episodios de Psicosis de Navarra (PEPsNa) in individuals experiencing a first episode of psychosis under conventional treatment (CT). To have a deeper insight into the effectiveness of the program, main outcome measures are broadening to cover relevant domains beyond clinical efficacy. The primary outcome measures are symptomatic remission, functional recovery, personal recovery, cognitive performance, functional capacity in real-world settings, and cost-effectiveness of the EIS.
Moreover, we chose 10 secondary outcome measures, including DUP shortening, substance abuse reduction, treatment with antipsychotic monotherapy, treatment with minimal effective doses of antipsychotic drugs, good to excellent therapeutic alliance, reducing the drop-out rate, lower number of relapses, reduction of global mortality and suicidality (attempts and completed suicide), reduction of resources use, and general satisfaction in the program.
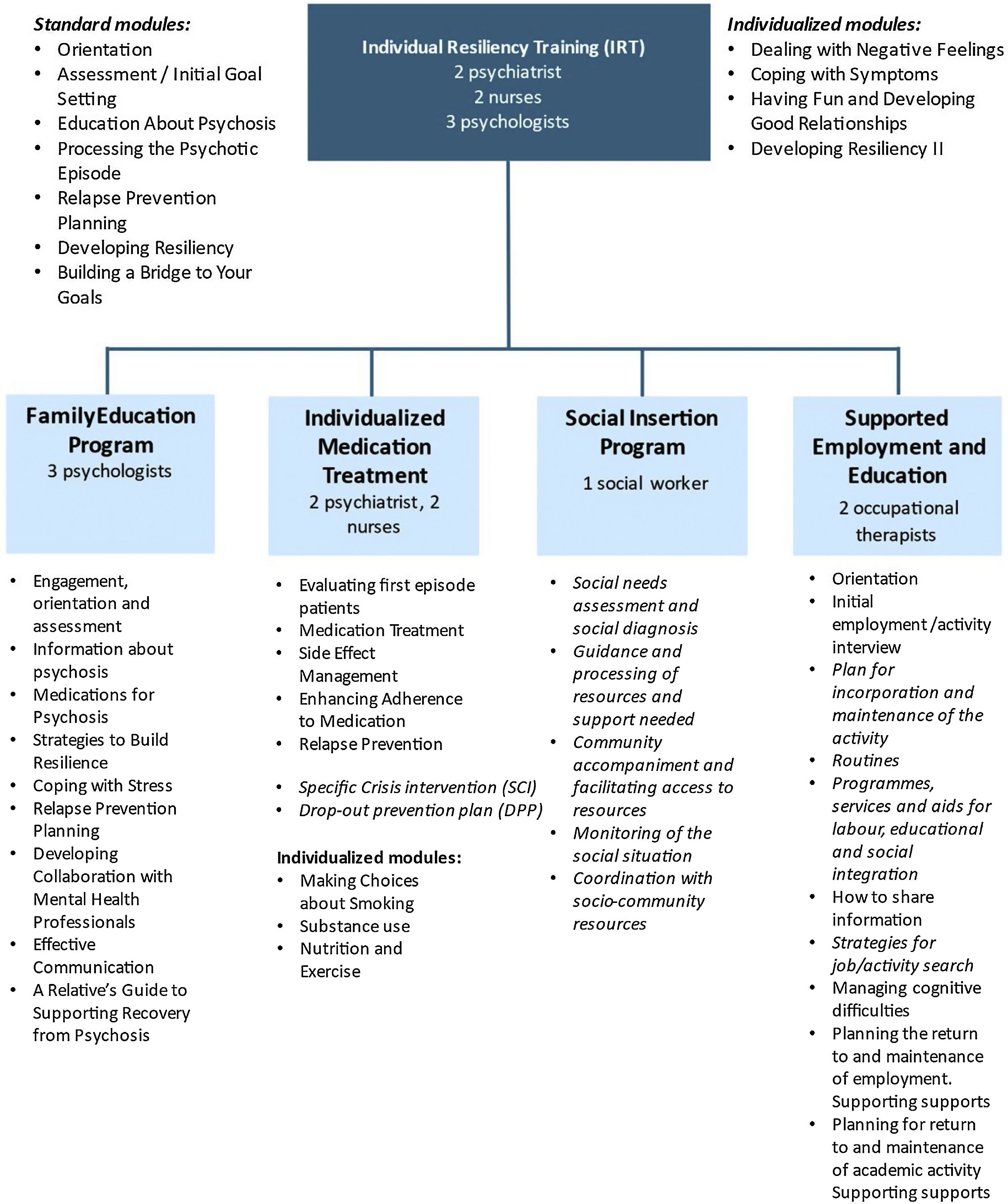
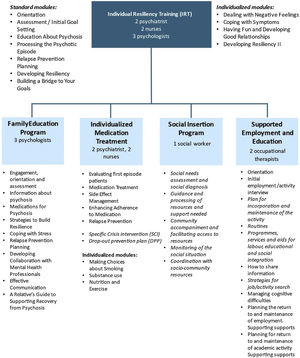
MethodsDesign rationale and populationThis is a naturalistic observational longitudinal study of FEP patients admitted to the PEPsNa, which is a new EIS for an epidemiological catchment area comprising 600,000 inhabitants (Navarra, Spain). The PEPsNa is an adaptation of the NAVIGATE program (https://navigateconsultants.org/index.html) (Fig. 1). The PEPsNa covers the first 2 years after the FEP, and it is carried out by a multidisciplinary team, with a focus on intensive assertive-community intervention.
The computerized clinical decision support system (COMPASS) of the Navigate22 are not included though the recommendations of drug prescriptions from their manual are followed for the index group. In the control group all antipsychotic drugs are transformed to a global chlorpromazine equivalent dose score.
Eligible subjectsThe PEPsNa receives FEP patients from psychiatric wards, psychiatric emergencies services, mental health centers and primary care. The inclusion criteria are as follows: (a) residence in the catchment area; (b) age between 15 and 55 years; (c) FEP diagnosis according to DSM-5 criteria (F20–F29)23; (d) no previous treatment for FEP; and (e) providing informed written consent for the participation in the program. This study plans to enroll 215 patients from January 2020 to December 2022.
The same inclusion criteria are applied to the CT group of FEP patients that were treated before the development of the PEPsNa. Exclusion criteria in both treatment groups are as follows: (a) age<15 or >55 years; (b) psychosis exclusively related to illicit drugs, medications or medical conditions; or (c) IQ<70. FEP that followed CT between January 2018 and December 2019 will be traced to invite them to participate in the study. It is planned enrolling 115 participants.
Sample recruitment and tracing proceduresEvery patient who accepts treatment in the PEPsNa is fully informed about the study, and their cooperation and consent for using all available information in the study is requested at the end of the 2-year intervention. The control group includes FEP patients receiving conventional inpatient or outpatient treatment in the mental health centers of our community. Potential candidates will be traced through the records of the Navarra Health Register Service (NaHRS) and contacted by telephone to participate in the study. Alternatively, a contact with their psychiatrist or primary care doctor will be established to request their collaboration in the study.
The research team will not interfere with the care in the new FEP program nor in the conventional follow-up of control subjects.
RatersPatients in the PEPsNa will be systematically assessed at baseline and at the end of the 2-year intervention. A similar 2-year period after the FEP will be gathered from the control subjects during their re-contact and thorough assessment. All assessments will be accomplished by means of face-to-face interviews with patients and a close relative, and all clinical information available from the NaHRS will be accessed. Furthermore, regular team meetings to minimize criteria variance and to reach a consensus in psychopathological and diagnostic assessments will be scheduled.
At 24 months, in the PEPsNa cohort and at the time of re-contact in control subjects, two neuropsychologists (AS and GG) unrelated to the PEPsNa and blind to psychopathological status will administer the neuropsychological evaluation.
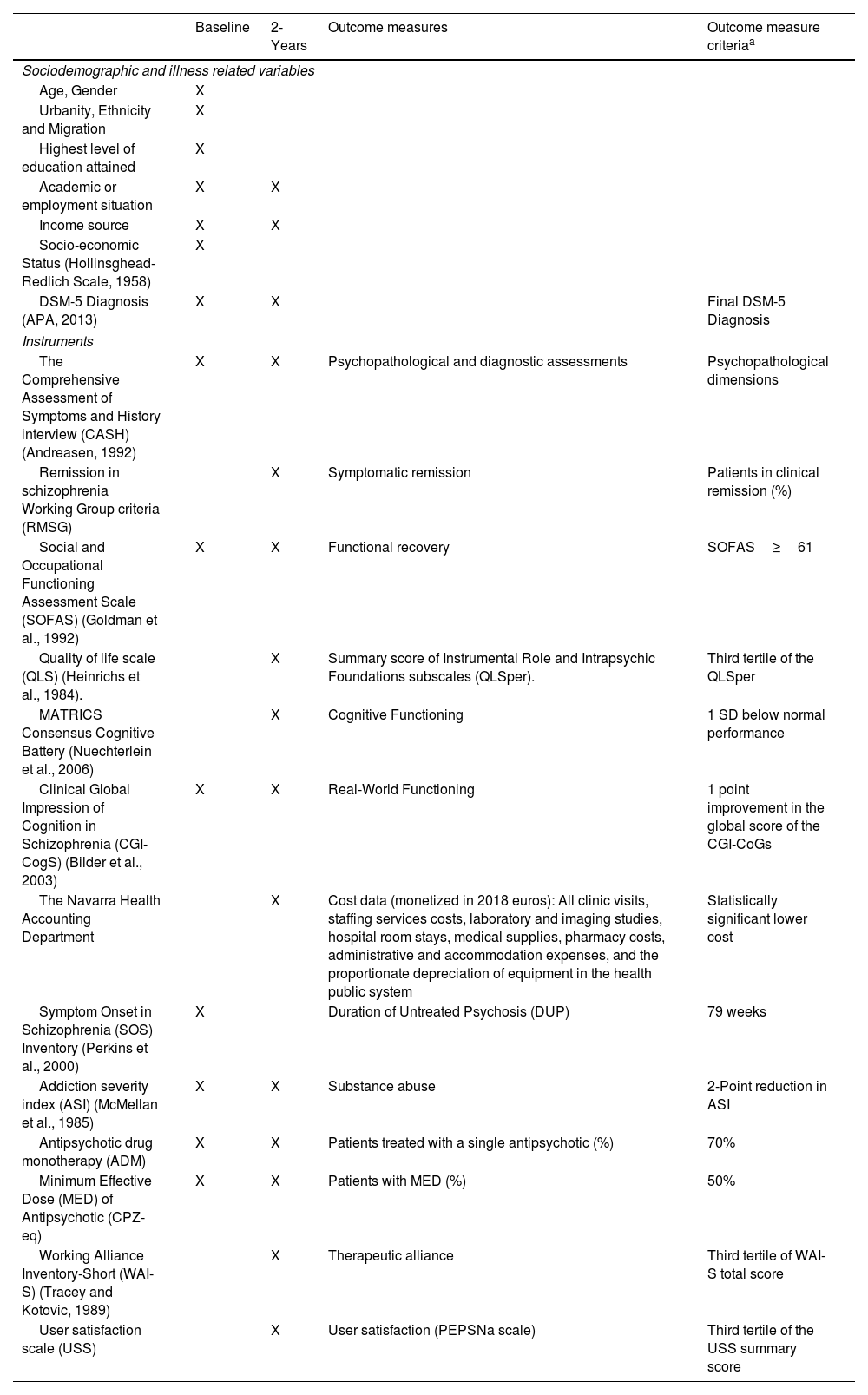
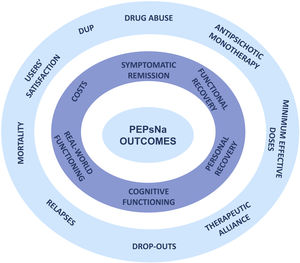
Primary outcome measures (Fig. 2)Patients will be evaluated by means of the Comprehensive Assessment of Symptoms and History (CASH) interview.24 The CASH is a semi-structured interview able to document sociodemographic, psychopathological and illness-related variables that allows for establishing DSM-5 diagnoses in FEP patients.25 Other assessment instruments are displayed in Table 2.
Assessment scales, time-points of assessment and outcome measures criteria.
| Baseline | 2-Years | Outcome measures | Outcome measure criteriaa | |
|---|---|---|---|---|
| Sociodemographic and illness related variables | ||||
| Age, Gender | X | |||
| Urbanity, Ethnicity and Migration | X | |||
| Highest level of education attained | X | |||
| Academic or employment situation | X | X | ||
| Income source | X | X | ||
| Socio-economic Status (Hollinsghead-Redlich Scale, 1958) | X | |||
| DSM-5 Diagnosis (APA, 2013) | X | X | Final DSM-5 Diagnosis | |
| Instruments | ||||
| The Comprehensive Assessment of Symptoms and History interview (CASH) (Andreasen, 1992) | X | X | Psychopathological and diagnostic assessments | Psychopathological dimensions |
| Remission in schizophrenia Working Group criteria (RMSG) | X | Symptomatic remission | Patients in clinical remission (%) | |
| Social and Occupational Functioning Assessment Scale (SOFAS) (Goldman et al., 1992) | X | X | Functional recovery | SOFAS≥61 |
| Quality of life scale (QLS) (Heinrichs et al., 1984). | X | Summary score of Instrumental Role and Intrapsychic Foundations subscales (QLSper). | Third tertile of the QLSper | |
| MATRICS Consensus Cognitive Battery (Nuechterlein et al., 2006) | X | Cognitive Functioning | 1 SD below normal performance | |
| Clinical Global Impression of Cognition in Schizophrenia (CGI-CogS) (Bilder et al., 2003) | X | X | Real-World Functioning | 1 point improvement in the global score of the CGI-CoGs |
| The Navarra Health Accounting Department | X | Cost data (monetized in 2018 euros): All clinic visits, staffing services costs, laboratory and imaging studies, hospital room stays, medical supplies, pharmacy costs, administrative and accommodation expenses, and the proportionate depreciation of equipment in the health public system | Statistically significant lower cost | |
| Symptom Onset in Schizophrenia (SOS) Inventory (Perkins et al., 2000) | X | Duration of Untreated Psychosis (DUP) | 79 weeks | |
| Addiction severity index (ASI) (McMellan et al., 1985) | X | X | Substance abuse | 2-Point reduction in ASI |
| Antipsychotic drug monotherapy (ADM) | X | X | Patients treated with a single antipsychotic (%) | 70% |
| Minimum Effective Dose (MED) of Antipsychotic (CPZ-eq) | X | X | Patients with MED (%) | 50% |
| Working Alliance Inventory-Short (WAI-S) (Tracey and Kotovic, 1989) | X | Therapeutic alliance | Third tertile of WAI-S total score | |
| User satisfaction scale (USS) | X | User satisfaction (PEPSNa scale) | Third tertile of the USS summary score | |
DSM-5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; CPZ-eq: chlorpromazine equivalents.
To determine the rate of patients who achieve symptomatic remission during the last 6 months of the 2-year follow-up, we will rely on the Remission in Schizophrenia Working Group (RSWG) criteria by Andreasen, Carpenter, Kane, Lasser, Marder, Weinberger.26 The remission rate in previous studies was 54–58%.19,27–29
Functional recoveryThe individual's level of social and occupational functioning not directly influenced by the severity of the psychiatric symptoms will be assessed with the Social and Occupational Functioning Assessment Scale (SOFAS).30 Functional recovery is established in this study by a SOFAS score≥61 sustained over the last 6 months.
Personal recoveryWe use the Heinrichs et al's Quality-of-Life Scale (QLS).31 The QLS is a self-assessment inventory that includes a broad range of outcome items but only some of them may reflect the recovery concept.32 Therefore, two modifications were introduced in the QLS. First, patients were asked by the rater to answer the QLS items that are scored exclusively according to the patient's answer. Second, we only include a summary score of two out the four QLS subscales (Instrumental Role and Intrapsychic Foundations) as outcome measures of personal recovery (QLSper). These two measures seems to tap well with a set of personal qualities reflecting interest in and engagement with the environment, including “Sense of purpose, Curiosity and Motivation”, which are core recovery constructs.33 Personal recovery is defined as a cut-off on the basis of the third tertile of the QLSper.
Cognitive functioningThe neurocognitive assessment will be carried out by means of the MATRICS Consensus Cognitive Battery (MCCB) that was validated in Spain for FEP patients.34 This battery evaluates seven cognitive functions and provides a global score.35
The MCCB scoring program allows producing age-, gender- and education-corrected T-scores for the seven cognitive domains (normative mean=50; standard deviation=10).36 Thus, as the scores in the subjects with psychosis were approximately 0.5–1 standard deviation below the healthy subjects, we established a MCCB global score of 1 SD below normal performance (cut-off point=40).
Real-world functioningTo evaluate the real-world performance in our study, the Clinical Global Impression of Cognition in Schizophrenia (CGI-CogS)37 is used. CGI-CogS is a semi-structured interview designed to evaluate cognitive function by means of clinical questions and was adapted to Spanish by our team.38 The CGI-CogS is administered at baseline and after 2 years in PEPsNa participants and at after the same 2-year ‘time-window’ period in the control group. Real-world functioning recovery is defined in this study as an improvement of at least 1 point in the global score of the CGI-CoGs.
Cost analysisThe costs of each participant will be calculated over the full period of study (2 years). All clinic visits, staffing services costs, laboratory and imaging studies, hospital room stays, medical supplies, pharmacy costs, administrative and accommodation expenses, and the proportionate depreciation of equipment in the health public system were monetized in 2018 euros by the professionals in the Navarra Health Accounting Department by gathering direct data from the NaHRS. Moreover, it is planned to extend this period to 5 years after the FEP to account for the later costs of both FEP groups.
Since there are no previous gold standards in these measures, we estimate that PEPsNa participants will have significantly lower global costs than patients undergoing CT.
Secondary outcome measures (Fig. 2)Duration of untreated psychosisThe duration of untreated psychosis (DUP) will be determined by means of the SOS-Perkins Scale.39 The DUP was defined as the months elapsed between the first psychotic symptoms and the beginning of antipsychotic treatment. A cut-off point of 79 weeks for the DUP was set, according to the results of Correll et al.19
Substance abuseThe Addiction Severity Scale (ASI)40 defines the severity of the use of illicit drugs. We set an ASI global score 2 points lower 2 years after the FEP as a cut-off for recovery.
Antipsychotic drug monotherapy (ADM)Bioque et al.41 reported that 68.7% of FEP patients are treated with ADM at 2-year follow-up of a large sample of FEP patients. We set this criterion at 70% of patients on ADM.
Minimum effective dose (MED) of antipsychoticsThe mean dose of antipsychotics in each evaluation period will be converted to chlorpromazine equivalent doses,42,43 and the MED will be defined as 200mg/day in chlorpromazine equivalents.44 Following the rates reported by Liu et al.,45 it is established as criterion that 50% of PEPsNa patients will receive MED.
Therapeutic allianceThe Spanish validation of the Working Alliance Inventory (WAI-S)46 will be used as a measure of therapeutic alliance at the 6-month point of the follow-up. The cut-off point for good therapeutic alliance will be set as the third tertile of the summary score of the WAI-S.
Drop-out rateThe interruption of treatment is a good indicator of treatment failure due to lack of efficacy/tolerability, safety or acceptability. It has been reported that treatment disengagement from EISs in psychosis is high (21–30%).19,47,48 In our study, this outcome is set at lower than 20% of FEP patients.
Number of relapsesRelapse was defined when participants stop fulfilling the RSWG criteria during at least 1 week of the follow-up.49 The relapse rate in FEP patients is 20–50% in the first 5 years.19,49,50 A relapse rate of 20% is established as the outcome in this measure.
Mortality, suicide and suicide attemptsBased on the rates reported in the literature,15,51,52 we estimate that the overall mortality of the PEPsNa subjects will be at least 3% lower than that of the CT group. Regarding deaths by suicide, PEPsNa patients will be 0.5% lower than the CT group. The number of deaths will be gathered through accessing the NaHRS.
Resource useResource use data will be gathered from the 2-years period after the FEP in both groups. Additionally, it is planned to extended this period to 5 years after the FEP to account for the later resource use of FEP patients in both groups.
Data will be collected from the NaHRS, which is a central registry for all public healthcare services. We will consider the following indicators for comparison between groups: number and days of hospital admissions and partial hospitalizations, number of outpatient contacts, and number of emergency visits. A summary score of global resource use after 2 years in both groups will be obtained. Best resource use is defined as a cut-off on the basis of the third tertile of the global resource use score.
User satisfactionAt the end of the 24-month period, the User Satisfaction Scale (USS) will be answered by PEPsNa participants and at the time of re-contact in CT participants. The USS used in this study is an inventory comprising 12 items to measure global satisfaction with the program, accessibility, awareness and shared decisions. The cut-off point was set as the third tertile of the USS summary score due to the lack of a previous gold standard in this scale.
Table 2 displays the operationalized criteria for primary and secondary measures of the effectiveness study of the PEPsNa.
Statistical analysisFindings from the study will be reported in accordance with the Strengthening the Reporting Observational Studies in Epidemiology (STROBE) guidelines.53 We have determined that we require at least 330 patients. This was calculated by setting the significance level at p≤0.05 with a power of 80% and relative sample size (PEPSNA group/CT group) of 0.25. We estimate that 65% of PEPsNa group and 35% of in the CT group would achieve recovery (SOFAS≥61) at the 2-year period after the FEP (https://statpages.info/proppowr.html). The inclusion of unequal numbers of cases with controls such as 2:1 or 4:1 might allow for increasing the power of the study in observational (not randomized) case-control studies.54
Descriptive statistics will be used to describe baseline characteristics in both groups. For continuous scales showing evidence of some skew, a median and IQR will be also presented, and they will be transformed in the most appropriate way in case of non-normality. Univariate and multivariate logistic regression analyses will be used to explore potential differences between both groups in sociodemographic and outcome variables.
Odds ratio risks for group effects on the outcome variables will be calculated using a generalized linear model, logistic regression, Cox proportional hazard regression models, or analysis of covariance when feasible. These analyses will be carried out according to the intention-to-treat principle. A post hoc sensitivity analysis will be performed to examine the overall attrition rates and between-group attrition differences of the primary outcomes.
In order to compare total costs involved between the PEPsNa and CT groups, a cost-effectiveness analysis will be carried out by expressing all the costs in monetary terms and outcomes in defined recovery cut-off points.
All statistical analyses will be carried out with SPSS (v.24).
DiscussionThis study will aim to examine the effectiveness of the PEPsNa EIS for FEP in comparison with a control group of FEP patients under CT. The main innovations are highlighted below:
- 1.
New effectiveness study contributing to provide evidence, support, engagement and dissemination of the EIS to stakeholders of the National Health System (NHS).
EISs have consistently demonstrated superior outcomes in relation to CT on symptomatic remission and functional improvement (see Table 1), although some caveats remain unascertained due to the great heterogeneity among samples and designs of previous studies.20
The NHS has to deal with the balance between effectiveness and the level or growth rate of the medical budget. In this regard, it is highly recommended to develop an effectiveness study to account for the expenses generated by the implementation of new EISs.55
- 2.
Broadening the scope of outcomes in FEP patients.
This study aims to broad the scope of outcome measures to better characterize and personalize the recovery process in FEP patients entering in the PEPsNa. The selection of the six primary and 10 secondary measures will provide a comprehensive profile of outcomes beyond the symptomatic and functional measures of previous studies (Fig. 2).
- 3.
Proposal of specific cut-off points in outcome instruments assessing effectiveness.
This study not only aims to broaden the scope of outcome domains but also intends to establish cut-off points for outcome measures in standardized instruments and direct outcome measures in order to ease comparison with other EISs in different settings or countries (Table 2).
- 4.
First adaptation of the NAVIGATE program in Europe.
To our knowledge, this study will examine the effectiveness of the first-adaptation of the NAVIGATE program in Europe. Moreover, three innovations will be introduced in the adaptation of the NAVIGATE program to our setting. First, the IMT module will be expanded to include a specific crisis intervention (SCI) module to prevent involuntary admissions. Second, a social intervention program (SIP) aimed at enhancing social and work outcomes will be included. Third, a new module will be developed to prevent dropping out of the PEPsNa (drop-out prevention plan, DPP) (Fig. 1).
Following the recommendations of EIS implementation, we will use the First Episode Psychosis Services Fidelity Scale (FEPS-FS).56 This scale aims to assess the degree to which programs deliver evidence-based practices by providing a list of objective criteria by which a program is judged to adhere to a reference standard for the intervention.57
LimitationsA common limitation in follow-up studies is the loss of patients throughout the process. In the pilot study carried out since 2020, drop-out rates from the PEPsNa have been low (<10%). However, our estimation of the loss of patients in the control group has been greater (25%). The recruitment of FEP control subjects may suffer from additional attrition due to several causes, such as abandon of mental health visits, refusal to cooperate and change of residence). These factors will contribute to sample size inequality between groups. Another potential differential factor for difference between both groups is the relapse since there is consistent evidence that psychotic relapse continue after the first 2 years of follow-up.49
Our case–control design study has certain limitations that may reduce the strength of results. First, the lack of blindness and randomization may provide relevant information about the effectiveness of the intervention but results should not be as conclusive as those resulting from randomized clinical trials. Our observational design may be affected by several due potential bias, such as selection, conflation and recall biases. To minimize these biases, all available evidence of the outcome of intervention in FEP control was gathered from information of patients and close relatives and through the access to patient's electronic medical record data. Moreover, potential differences in sample characteristics were examined as potential confounders in statistical analyses.
The 2-year period of intensive intervention of the PEPsNa will restrict the comparison regarding the EIS with longer intervention periods.17,58,59
The PEPsNa is mainly a personalized intervention program that precludes blind assessments of participants, except for the self-applied inventories and neuropsychological evaluation. This limitation will be taken into consideration by using highly structured evaluations and scales and achieving good to excellent interobserver reliability among raters.
COVID-19 and current researchCOVID-19 has already changed our world, and within this framework, our research systematics. However, the available literature is currently limited on the impact of the pandemic on the research of psychosis. One report found that FEP patients without COVID-19 infection hospitalized in the first four months since lockdown were significantly older than patients with FEP in 2021, and presented with significantly less substances abuse.60 In this study, sociodemographic and psychopathological variables will be examined between PEPSNA and FEP control groups and in case of significant differences a dichotomic covariate related to the time of assessment (COVID vs non-COVID time) will be included in statistical analyses.
ConclusionsWith the current study, we aim to examine the effectiveness of the first adaptation of the NAVIGATE in Europe by comparing the PEPSNA cohort to a FEP sample receiving TAU in the same setting. Our main aims are two. First, to broad the scope of potential outcome measures of FEP patients to go beyond symptomatologic remission to better address their unmet needs of recovery. Second, the study's results not only will have direct translation to clinical practice but also, they will have the potential to inform policy and maximize the quality and accessibility of mental health services for FEP patients and their caregivers.
Ethics and disseminationThis study follows the standards of good clinical practice accepted worldwide, in accordance with the Declaration of Helsinki and approved by the Clinical Research Ethics Committee (CEIC) of Navarra (756/2019).
The results from this investigation will be actively disseminated through peer-reviewed journal publications and conference presentations.
Financial supportThis study was funded by a grant from the Carlos III Health Institute of the Ministry of Science and Innovation of the Government of Spain (PI19/01698).
Conflict of interestNone.
The authors would like to thank all participants and their parents for their participation.
To Víctor Peralta, Matilde Martínez, Elena García de Jalón, María Otero, Mari Cruz Ariz, Tadea Lizarbe and Myriam Ochoa, who adapted the NAVIGATE program to our setting. Special thanks to MM, a great colleague and person we miss.
Matilde Martínez, María Otero, Leire Azcárate, Nahia Pereda, Fernando Monclús, Laura Moreno, Alba Fernández, Alba Sabaté, Izaskun Aguirre, Tadea Lizarbe, Myriam Ochoa Martínez and Mari Jose Begué.