Cerebellar infarcts are relatively infrequent, assuming between 1.5 and 3% of total ischaemic strokes. Haemorrhagic transformation is a possible complication, reaching in some series up to 23.4%, in multiple territories involvement and posteroinferior cerebellar artery (PICA).1 In immobilised post-stroke patients, deep venous thrombosis (DVT) incidences vary from 10 to 75%, depending on the diagnostic method and evaluation time.2 Pulmonary embolism (PE) generally arises from DVT in a paralysed lower extremity after a stroke3 and its incidence after a cerebrovascular accident; without heparin prophylaxis, varies across studies ranging from 0.8 to 1% in the two following weeks and 4% in those with an extended rehabilitation period. Advanced age, high NIHSS score, haemiparesis, immobility, female gender, atrial fibrillation, receipt of blood tissue plasminogen activator, and teaching hospital admission are risk factor.2 Incidences of PE from 10 to 13% have been documented in thromboembolic complications studies,4 as well as mortality between 13 and 25%.2
We present the case of a 75-year-old man who came to the emergency room with a two-day history of vomiting and vertiginous sensation. He had been discharged a week earlier, after presenting a PICA stroke 18 days earlier, and haemorrhagic transformation 7 days ago, causing moderate hydrocephalus. He presented as sequelae vestibular vertigo, ataxia, dysphagia, desaturation of 90% and a pO2 of 54mmHg (83.00–108) due to an acute heart failure in treatment with diuretics drugs, so home oxygen therapy was prescribed. His medical history presented high blood pressure, type II diabetes mellitus, and hypercholesterolemia. Since hospital discharge, the patient reports little mobilisation, but he used pneumatic compression stockings on lower limbs during hospital admission. The patient was haemodynamically stable. Physical examination showed a moderate general state impairment, with ambient air saturation by indirect pulse oximetry of 87%. Pulmonary auscultation and respiratory dynamics did not show alterations.
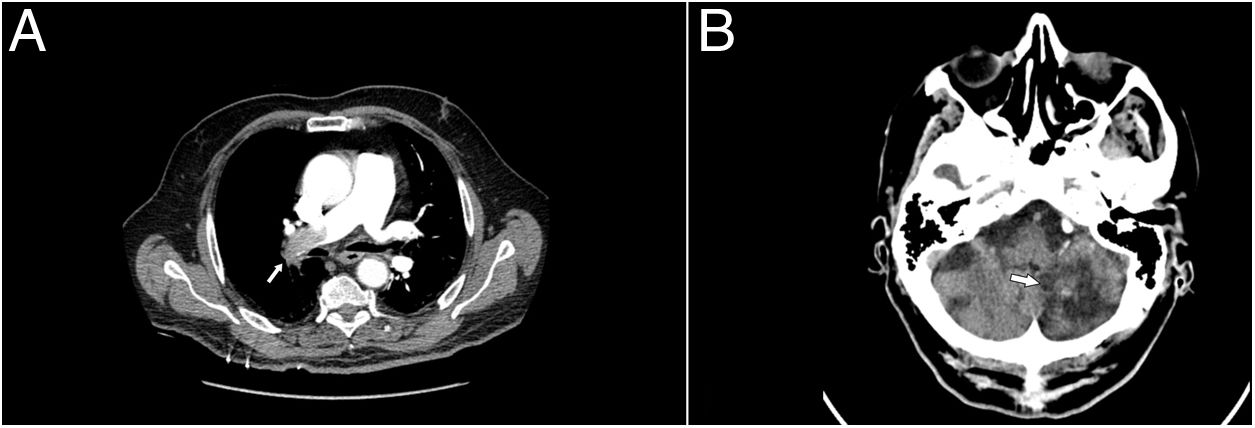
Complementary tests determined partial respiratory failure with a pO2 of 51mmHg, the glucose of 186mg/dl, a troponin T hs of 17.74pg/ml (0.00–14), and a d-dimer of 20,000ng/ml (0.10–500), during hospitalisation for PICA stroke it was 2200.00. Arterial blood PaCO2, creatinine levels, coagulation study, and EKC record were in normal range. A computed tomography chest angiography (CTA chest) revealed a filling defect in right main artery with extension to all lobar branches and most of its segmental, contralateral segmental arteries of the left lobe and superior lobar artery with extension to lingula, an increase in the calibre of the pulmonary artery and a mild rectification of the interventricular septum (Fig. 1, Image A). Transthoracic echocardiogram (TTE) performed one day after admission showed moderate pulmonary hypertension without signs of ventricular dysfunction, and Doppler ultrasonography in lower extremities demonstrated thrombus in right popliteal vein. The diagnosis of intermediate-high risk acute pulmonary embolism, PESI 105, class III, was made
Given the background of stroke with haemorrhagic transformation and the bleeding risk (RIETE bleeding score 3, intermediate risk), we decided to use prophylactic enoxaparin doses (40mg/24h), and placed a temporary vena cava filter on the second day of admission. Ten days after admission, we repeated the brain computed tomography scan, without signs of rebleeding three weeks after the haemorrhagic transformation (Fig. 1, Image B), so we decided to start apixaban 2.5mg/12h. Admission was maintained four more days for evolutionary surveillance, and since no new neurological symptoms were present, he was discharged and followed up in consultation two weeks later. We removed the vena cava filter on scheduled intervention one week after the start of the anticoagulation therapy.
In this revision, the patient reported that vomiting and vertigo had subsided and had a baseline oxygen saturation of 94%, so we stopped the home oxygen therapy. In control TTE, we did not observed alterations. Subsequently, the patient went to the emergency room due to an episode of syncope, where a CTA chest to rule out a new episode of PE was performed. This test revealed no filling defects.
Our patient had DVT risk factors; arterial hypertension, diabetes mellitus, high d-Dimer levels, and prolonged immobilisation. A higher incidence of DVT was described in the 14 days after the start of the stroke.5 Our patient may develop an asymptomatic DVT that later leads to PE. There are no prospective studies on the risk/benefit of anticoagulation after cerebellar bleeding. Some evidence indicates that anticoagulation can be resumed after 4–8 weeks of a cerebral haemorrhage in atrial fibrillation patients if a control image test does not show rebleeding and we use drugs with a low risk of hemorrhage.6 A meta-analysis suggests that anticoagulation resumption with warfarin in patients with recent intracranial haemorrhage is associated with a low risk of arterial embolism without increased cerebral bleeding.7 The noninferiority of the direct-acting anticoagulants over antivitamin K has been demonstrated in PE.7 Among them, apixaban has shown lower haemorrhagic risk,8 so we decided to treat our patient with 2.5mg/12h given the recent cerebellar haemorrhage (three weeks). Other studies described dabigatran as the safest drug,9 but we had more experience with apixaban management.
The temporary vena cava filter placement may be useful in patients with deep vein thrombosis and high risk of PE associated with a decrease incidence.10 In the absence of haemodynamic instability, no reperfusion treatment was considered.11 Given the prolonged immobilisation situation without thromboembolic prophylaxis due to intracranial bleeding as a probable aetiology of pulmonary embolism, we did not consider thrombophilias study. The control CTA chest is reserved for selected cases in PE follow-up. The patient went to the emergency room due to a syncope episode, where they decided to perform CTA chest to rule out a new episode of PE. As no filling defects were detected, we decided to use it as a follow-up test.11 There are no studies on the use of low doses of enoxaparin as bridge to anticoagulation, but given the affectation caused by PE, the risk of new episode and the recent haemorrhagic transformation, it could be a therapeutic option. The heterogeneity of PE with recent intracranial bleeding history and the absence of specific guidelines requires individualisation of each case.
Conflict of interestNone of the authors has any conflict of interest of any type.