Hypertension has high global prevalence with suboptimal control rates. In Lebanon, its prevalence is estimated to around 37%. Antihypertensive monotherapy proved to have limited effectiveness in reducing blood pressure. A commonly used combination of irbesartan, an angiotensin ii receptor blocker, and amlodipine, a calcium channel blocker, has highly potent antihypertensive effects.
ObjectiveThe aim of this study was to assess the safety profile of irbesartan/amlodipine fixed-dose combination (FDC) in real-life practice, and to evaluate its use and its effectiveness in reducing blood pressure (BP) in the Lebanese population.
Patients and methodsA total of 603 patients, who provided written consent, were enrolled in this national, multicenter, non-interventional, longitudinal product registry, and data was collected at baseline and then at 3 follow-up visits separated by a period of 3 months.
ResultsMore than 80% of the patients were overweight or obese and 65% of them presented comorbidities, mainly dyslipidemia. Adverse events (AEs) were reported in around 12% of the cases, most of which were non-serious. Treatment-emergent (TE) AEs were experienced by around 6% of the patients, and none of the serious AEs were TE. BP level was significantly reduced at 3 months and 9 months after baseline. Around 80% of patients achieved their target BP at 3 months post-baseline. Patients’ weight and heart rate also sequentially decreased throughout the visits.
ConclusionThis study provides a better understanding of patients with hypertension treated with irbesartan-amlodipine FDC in Lebanon, and confirms the good safety and tolerability profile of this anti-hypertensive FDC.
La hipertensión tiene una alta prevalencia global con subóptimas tasas de control. En el Líbano, su prevalencia se estima alrededor del 37%. La monoterapia antihipertensiva resultó tener una eficacia limitada para reducir la presión arterial (PA). Una combinación corrientemente utilizada de irbesartán, un bloqueador del receptor de angiotensina ii, con amlodipino, un bloqueador de los canales de calcio, tiene efectos antihipertensivos muy potentes.
ObjetivoEl objetivo de este estudio fue evaluar el perfil de seguridad de la combinación de dosis fija (CDF) de irbesartán/amlodipino en la práctica de la vida real y describir su uso y su eficacia para reducir la PA en la población libanesa.
Pacientes y métodosUn total de 603 pacientes, que dieron su consentimiento por escrito, se inscribieron en este registro nacional, multicéntrico, no intervencionista y longitudinal. Los datos se recopilaron al inicio del estudio y luego en 3 visitas de seguimiento cada 3 meses.
ResultadosMás del 80% de los pacientes presentaban sobrepeso u obesidad y el 65% de ellos presentaban comorbilidades, principalmente dislipidemia. Se notificaron eventos adversos (EA) en casi el 12% de los casos, la mayoría de los cuales no fueron graves. Solo el 6% de los pacientes experimentaron EA aparecidos durante el tratamiento (EAAT) y ninguno de los EA graves fue atribuido al tratamiento. La PA se redujo significativamente a los 3 meses y 9 meses después del inicio del tratamiento con irbesartán/amlodipino. Alrededor del 80% de los pacientes alcanzaron su PA objetivo en la evaluación de los 3 meses. El peso y la frecuencia cardíaca de los pacientes también disminuyeron durante las visitas.
ConclusiónEste estudio proporciona una mejor comprensión de los pacientes con hipertensión tratados con irbesartán/amlodipino en CDF en el Líbano y confirma un perfil satisfactorio de seguridad y tolerabilidad de este tratamiento antihipertensivo.
Hypertension is the leading risk factor for cardiovascular disease and mortality worldwide, with a prevalence of 30–45%.1 It is estimated to cause 7.5 million deaths, which accounts for 12.5% of global deaths.2 In Lebanon, a high prevalence of hypertension, estimated to around 37%, was shown in the adult population.3 The same study highlights significant challenges to the management of hypertension, including the lack of blood pressure (BP) control in over half of the patients, and the undiagnosed cases of hypertension that also represent more than half of the total cases.3
Several epidemiological registries and studies were conducted in order to assess the management of hypertension patients, to better characterize patient profile, treatment patterns and safety. These studies showed that despite an improvement in hypertension control rates since 2000, hypertension management was still suboptimal and remains unacceptable in some countries. In fact, a 2013 study of 142,042 participants from 13 countries showed that only 32.5% of treated patients had controlled BP and only around 13%, are controlled.4
Guidelines from the European Society for Hypertension/European Society for Cardiology (ESC/ESH) encourage the use of combination treatment for most patients to better lower BP, preferably as single pill combination therapy to improve adherence to treatment, and the use of a simple treatment algorithm that is pragmatic and that applies to all patients.5 One of the most commonly used single-pill combinations is irbesartan and amlodipine. Irbesartan is an angiotensin II receptor blocker and amlodipine is a calcium channel blocker. Administration of both drugs in tandem provides an additive antihypertensive effect compared to their separate administration, as calcium influx blockade and reduction of angiotensin II-dependent vasoconstriction are complementary mechanisms in lowering BP.6 The irbesartan/amlodipine fixed-dose combination (IA-FDC) is indicated in the treatment of hypertension in adult patients in whom BP is not adequately controlled on irbesartan or amlodipine monotherapy.7 The safety and tolerability profile of IA-FDC was comparable to that of the approved antihypertensive mono-components throughout different studies.8,9
This observational study is aimed at assessing the safety and tolerability profile of IA-FDC in real-life practice by estimating the frequency, severity and seriousness of all adverse events in Lebanese patients with hypertension. Other objectives are to assess the profile of patients treated with different doses of IA-FDC and to describe BP change three months after IA-FDC treatment initiation.
Patients and methodsStudy designThis was a national, multicenter, non-interventional, longitudinal product registry on the safety of IA-FDC in the real-life management of hypertension in Lebanon. Twenty-five participating sites were randomly selected from an exhaustive list of physicians who treat patients with hypertension, with a geographical distribution that covers all Lebanese governorates. Eighteen of the participating investigators were cardiologists, three nephrologists, two family physicians, and two endocrinologists. Most of them (18 out of 25) practiced in private clinics, and the rest in hospital settings. Patients were recruited over a 12-month period, from March 30, 2017 until March 31, 2018. Patients attended the baseline visit, and were followed up at the 3-month, the 6-month, and the 9-month visits. This study was approved by all applicable ethics committees/institutional review boards of participating centers.
Study patientsMale or female adult patients who provided informed consent and who have been treated with IA-FDC for at least three months and at most six months prior to baseline visit were consecutively recruited in this study. The decision to initiate IA-FDC was at the physician's discretion independently of study participation. Patients participating in another clinical trial or with history of allergies or contraindication to any of the IA-FDC product, as well as pregnant or breastfeeding female patients were excluded from the study.
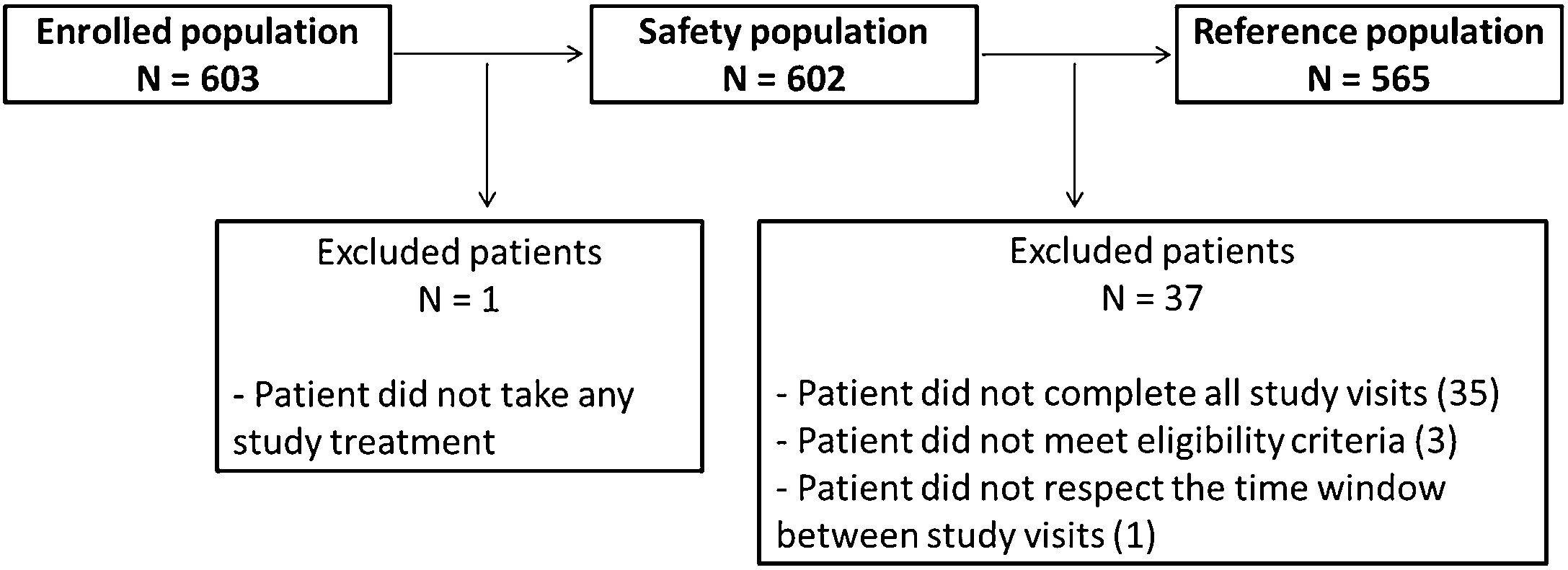
Study populationsThe Enrolled population is constituted by all patients included in the study. The Safety population is constituted by all patients who have taken at least one dose of the study treatment. The Reference population is constituted by all hypertensive patients, fulfilling the eligibility criteria, and who came to the selected sites during the specified study period, and attended the 3 follow up visits after the baseline visit.
Data collectionAt each study visit, patient data were collected on individual paper case report forms, then entered into a database and validated. Collected data included all adverse events (AEs), demographic and socioeconomic profile, vital signs, medical history and specifically hypertension profile, and concomitant medication.
Data analysisData analysis was performed under SAS 9.4. Numeric values were summarized as observed valued, mean and standard deviation (SD), minimum and maximum, and two-sided 95% Wald confidence interval (CI) of the mean for all endpoints. Categorical variables were summarized as number of non-missing data, counts and percentages, two-sided 95% CI using Agrest-Coull method for all endpoints. Variables were analyzed according to gender, age categories, and body mass index (BMI) categories. Analysis presented the Odds ratio (OR) between subgroups.
Sample sizeThe primary objective of the study was to assess in real-life practice the safety profile of IA-FDC when used to treat hypertension in Lebanon by estimating the frequency, severity and seriousness of AEs. As the primary and secondary criteria were described as rates, a good precision was needed in estimating these percentages. With 600 patients from all over Lebanon, the study would allow the estimation of any prevalence among patients such as prevalence of AEs or certain comorbidities to a margin of error of maximum ±4% using 95% CI.
Statistical considerationsFor statistical tests, the type I error risk was set at 5% (bilateral situation). No adjustment for multiplicity was performed. For inter-group comparison, a Student t-test or a Wilcoxon/Mann–Whitney test was performed, depending on the normality of the data, to test the hypothesis involving quantitative variables. In case of normality in all groups and Student t-test is chosen, a test of equality of variance before a Satterthwaite's correction was applied. The Chi square test or the Fisher's exact test, depending on the expected counts, was used to test the hypothesis involving qualitative variables. To test the differences between baseline and post-baseline values, a paired t-test or a Wilcoxon signed rank test was used depending on the normality of the data. Missing data or unknown responses were not included in the percentages and probabilities associated with tests.
ResultsStudy participantsOver a 12-month period, 603 patients were recruited in 25 centers across Lebanon, with 602 included in the safety population and 565 in the reference population (Fig. 1). The reference population included more males (315 [55.75%]). The age of the patients ranged from 22 to 93 years, with an average of 58.3±11.0 years and a median of 58 years. The majority of patients were married (73.81%) and most patients went to school (63.54%) or held a university degree (26.73%). Only 5.49% of the patients reached higher education and 1.77% could not read or write. Most of the patients were covered by health insurance (78.58%) and a fifth was unemployed.
Weight at baseline ranged from 50.00 to 130.00kg, with a mean of 83.36±12.56kg. Height was available for 502 patients and ranged from 150.00 to 188.00cm with a mean of 170.9±7.5. BMI was therefore computed for 501 patients who had both their weight and height collected at the study visit, and ranged from 19.0 to 44.4kg/m2, with a mean of 28.28±4.09kg/m2. According to the World Health Organization classification, only 96 patients (19.16%) had a BMI within the normal range, and the remaining 405 patients were overweight (53.09%) or obese (27.74%).
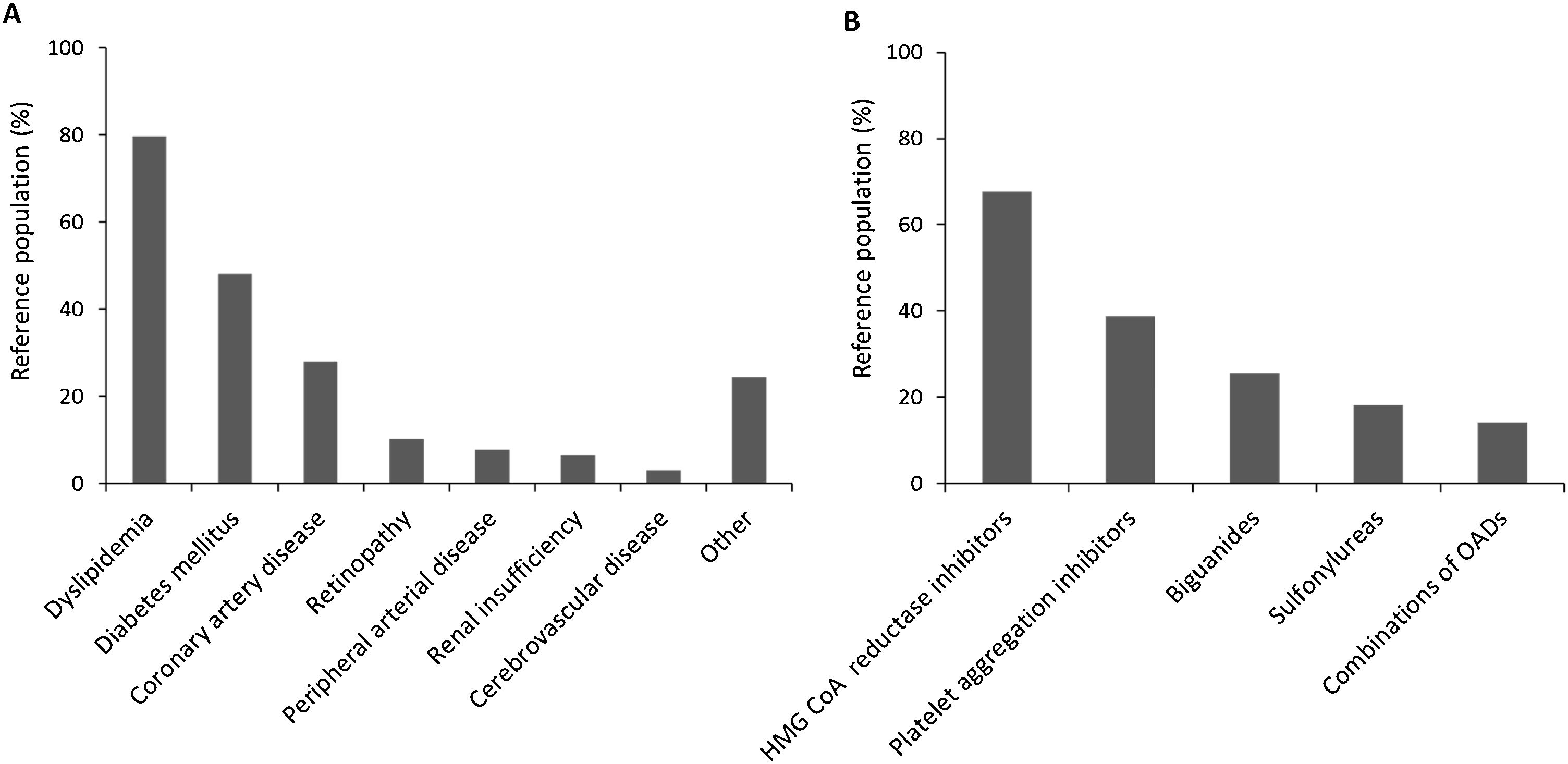
Medical history was also described for all patients in the reference population, and 64.78% presented comorbidities, while 49.91% took at least one concomitant medication. Fig. 2 displays comorbidity reported and concomitant medications. Dyslipidemia topped the list with 79.51% of patients with comorbidity suffering from it, while HMG CoA reductase inhibitors were the most prescribed in 67.73% of patients treated with concomitant medications.
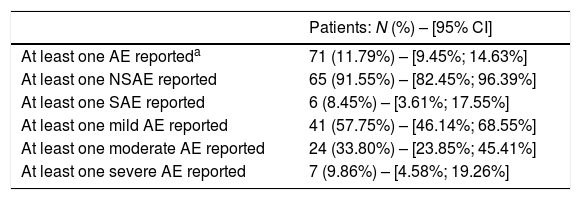
Analysis of primary endpointsSafety events, their frequency, severity and seriousness were analyzed to describe the safety profile of IA-FDC. Within the safety population, 71 (11.79%) patients reported at least one AE, of which, 65 (91.55%) reported at least one non-serious AE (NSAE), and 6 (8.45%) reported at least one serious AE (SAE). No AE of special interest (AESI) was reported in any of the study patients. One AE led to permanent treatment discontinuation and was classified as non-serious. Moreover, 41 (57.75%) patients reported at least one mild AE, 24 (33.80%) reported at least one moderate AE, and 7 (9.86%) reported at least one severe AE. Percentages of patients reporting AEs are summarized in Table 1.
Summary of patients recording adverse events in the safety population.
| Patients: N (%) – [95% CI] | |
|---|---|
| At least one AE reporteda | 71 (11.79%) – [9.45%; 14.63%] |
| At least one NSAE reported | 65 (91.55%) – [82.45%; 96.39%] |
| At least one SAE reported | 6 (8.45%) – [3.61%; 17.55%] |
| At least one mild AE reported | 41 (57.75%) – [46.14%; 68.55%] |
| At least one moderate AE reported | 24 (33.80%) – [23.85%; 45.41%] |
| At least one severe AE reported | 7 (9.86%) – [4.58%; 19.26%] |
AE: adverse event; NSAE: non-serious adverse event; SAE: serious adverse events.
Treatment-emergent AEs (TEAEs) were experienced by 36 (5.98%, 95% CI [4.33%; 8.19%]) patients of the safety population. A total of 7 SAEs were also reported in 6 (1.00%) patients and none of them was a TEAE. They included a hip fracture, an acute myocardial infarction, one sudden cardiac death, one case of cholelithiasis, one case of Meniere's disease and two cases of rectal hemorrhage. Also, 68 NSAEs were reported in 65 (10.80%) patients, among which 36 were TEAEs and were divided into 33 peripheral edema events, two hypotension events and one tachycardia event.
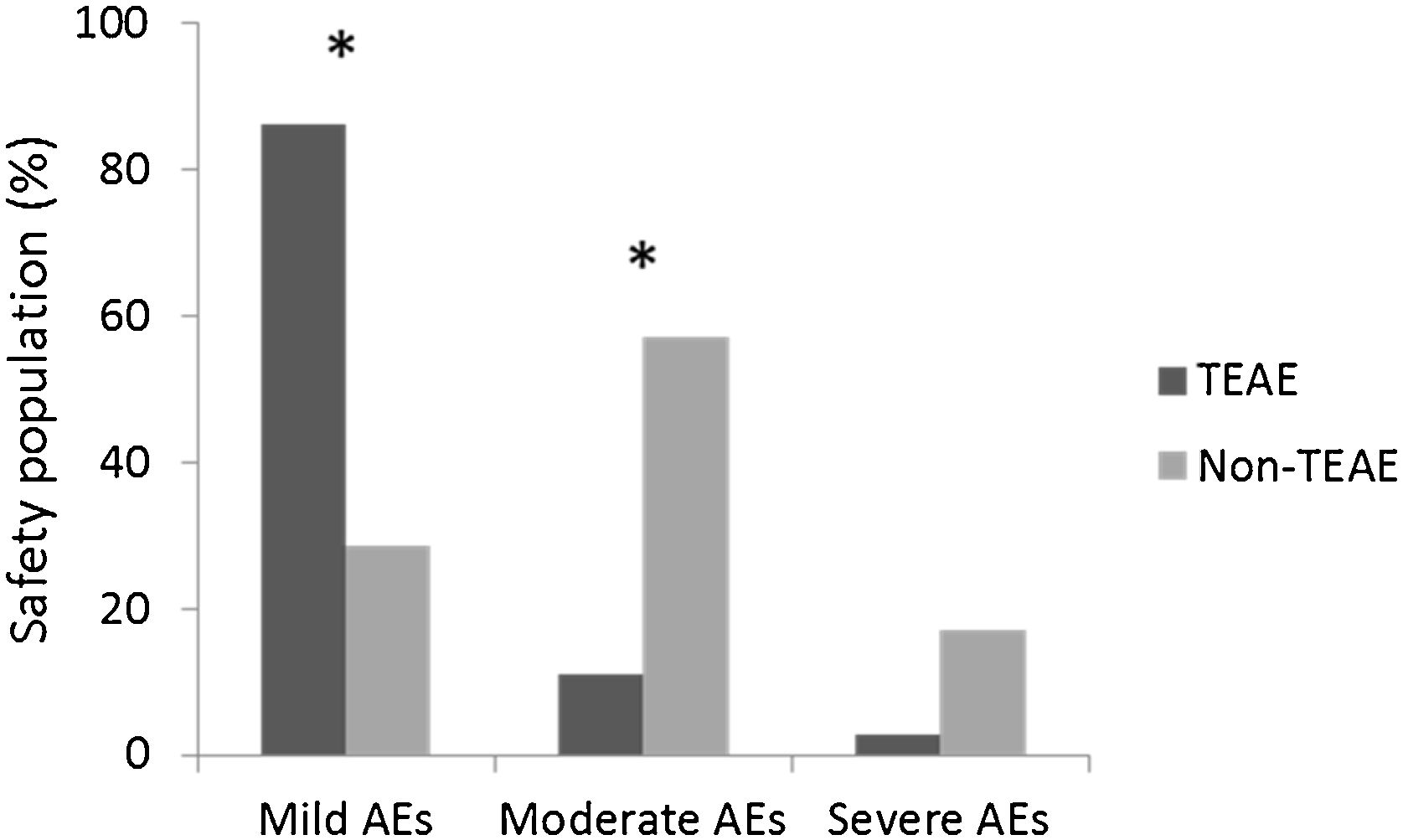
Subgroup analysis showed that there was no statistically significant effect of patients’ gender, age or BMI on the occurrence of most AEs. Only moderate AEs seemed to be reported more frequently by women (p=0.001). Fig. 3 shows that among patients with mild AEs, significantly more reported AEs were TEAEs, whereas among patients with moderate AEs, significantly more reported AEs were non-TEAEs. Treatment-emergence was not statistically significant when severe AEs were considered.
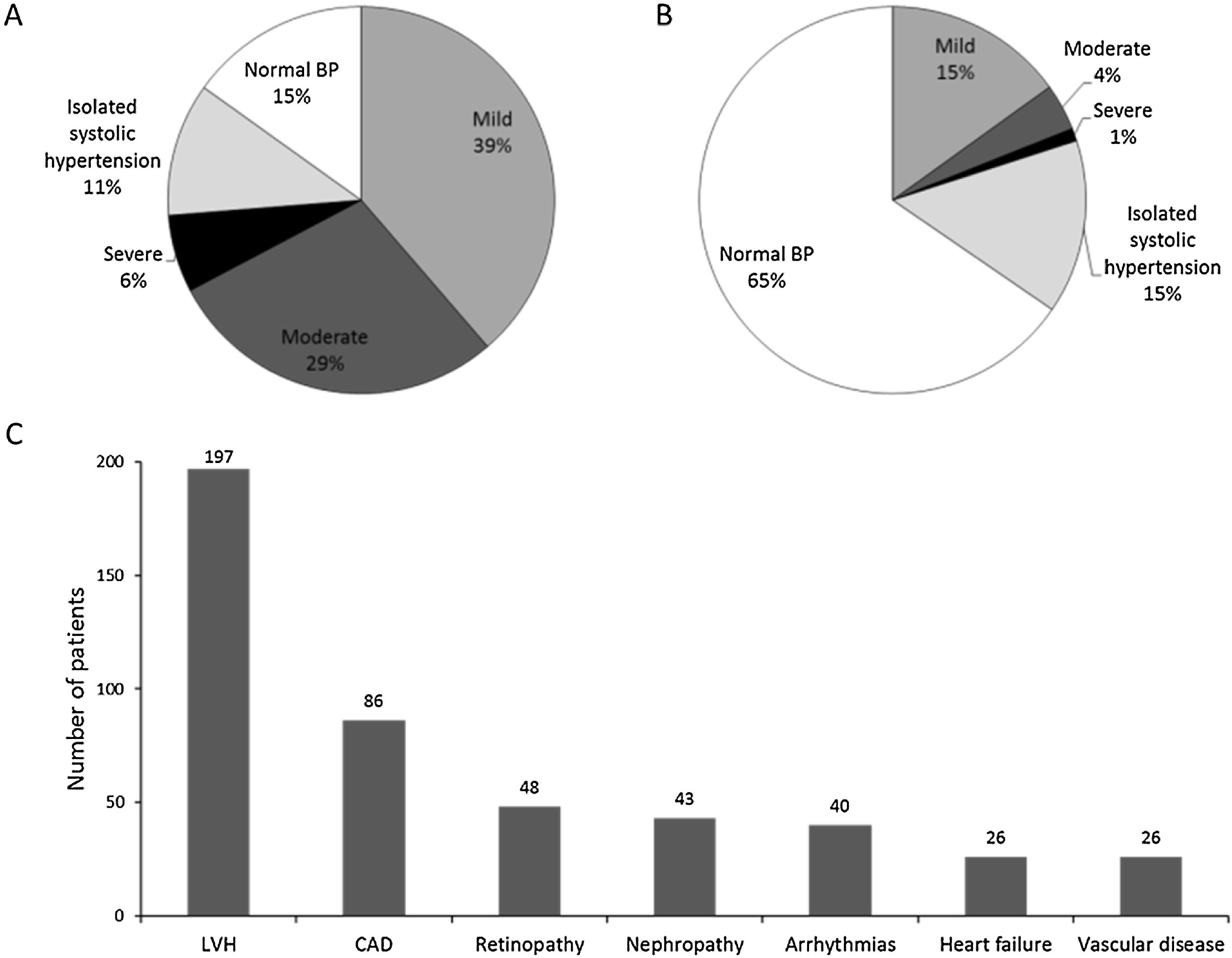
Patient hypertension profilePatients in the reference population had had hypertension for a maximum of 32.0 years prior to study initiation. In particular, 39.9% of patients were diagnosed with hypertension within the year leading up to study start, 41.9% between one and five years, and 28.2% over five years before study start. The highest systolic BP (SBP) recorded during the last three months before patient enrollment ranged from 110 to 230mmHg with an average of 160.9±21.5mmHg. The highest diastolic BP (DBP) recorded during the same period ranged from 60 to 120mmHg with an average of 93.0±10.4mmHg. Fig. 4A displays the distribution among hypertension grades of the 556 patients for whom BP measurements were available within three months prior to study entry. Only around 15% of them had controlled BP, more than 70% of patients reported a family history of hypertension, and around half of them applied lifestyle measures to reduce their BP, mainly through diet, physical activity, and smoking cessation.
Patient hypertension status. Data were analyzed for patients in the reference population. Percentage of patients with controlled BP and distributed among hypertension grades prior to study entry (A) and at the baseline visit (B); number of patients reporting the main hypertension-related complications (C). CAD: coronary artery disease; LVH: left ventricular hypertrophy.
At study entry (baseline visit), patients had been treated with IA-FDC for at least 3 months, with 369 (65.43%) having their BP controlled (Fig. 4B) and 168 (29.73%) patients were taking at least one additional antihypertensive medication. Beta blocking agents were by far the most prescribed medication (around 60%), followed by diuretics (around 30%). At least one complication due to hypertension was reported by 242 (42.83%) patients. These complications were mostly cardiovascular (90.50%) and microvascular (35.54%). Fig. 4C displays ongoing hypertension complications reported by more than 10% of patients at study entry, left ventricular hypertrophy topping the list, followed by coronary artery disease.
Use of IA-FDCThe absolute majority of the reference population (99.47% of patients) was taking four doses of IA-FDC (150/5mg; 150/10mg; 300/5mg; 300/10mg) and the treatment was initiated for an average of 3.86±0.85 months prior to baseline visit. Around 11.68% of patients had an IA-FDC escalation at the baseline visit, but more than 95% of the patients had no change in their IA-FDC doses during the 3-month, 6-month, and 9-month follow up visits.
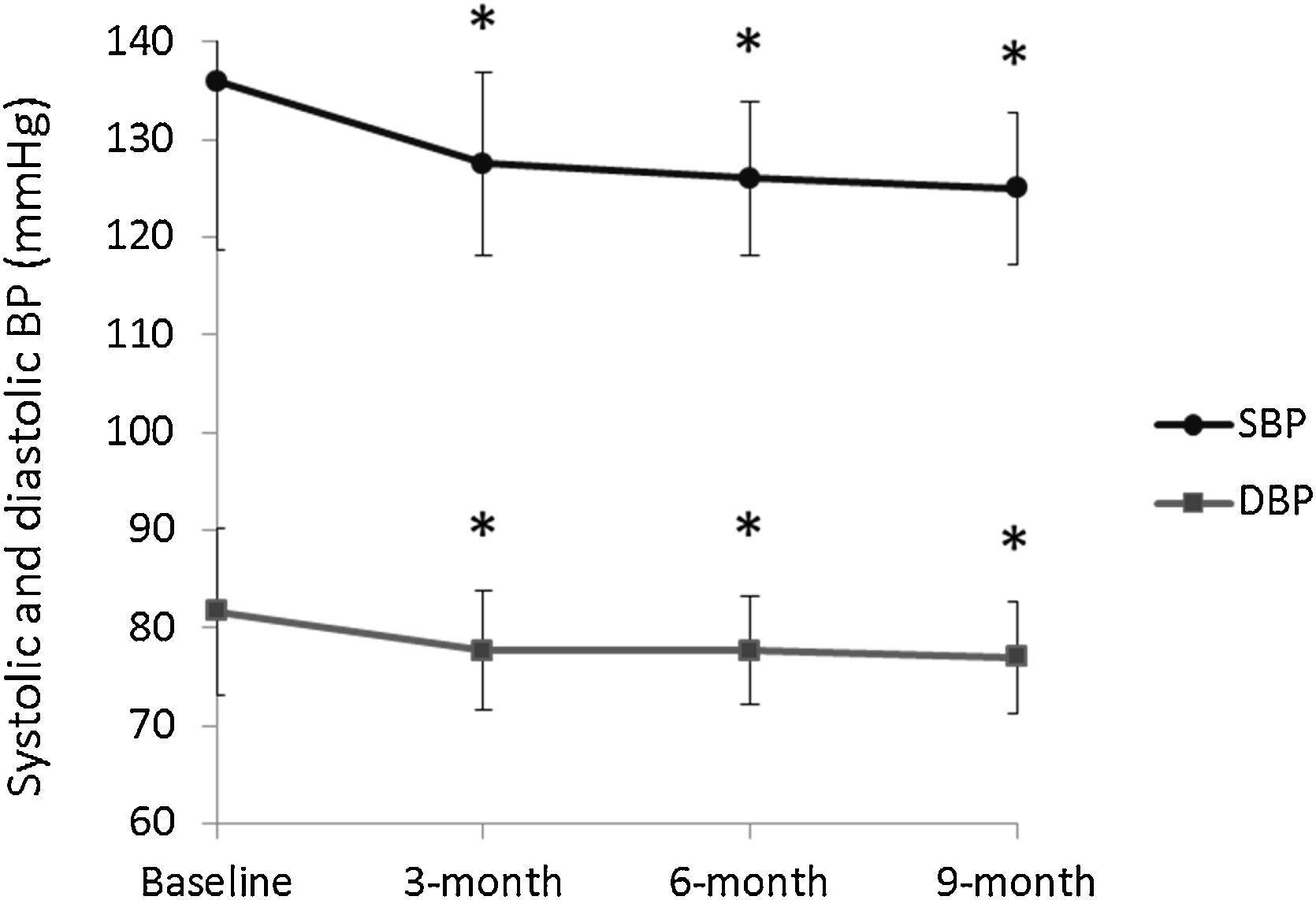
Efficacy of IA-FDC in reducing BPDBP and SBP values were recorded for patients on every study visit. Fig. 5 displays the change in these values from baseline through all follow up visits. Average SBP was 136.0±17.3mmHg at baseline and 125.0±7.8mmHg at the 9-month follow up visit, showing a significant decrease of 11.0±18.8mmHg (p<0.001). Similarly, average DBP was 81.7±8.5mmHg at baseline and 77.0±5.7mmHg, showing a significant decrease of 4.60±9.67mmHg (p<0.001). Of note, the decrease in SBP and DBP was significant when comparing the values at baseline to those at any of the study visits. Around 80% of patients for whom BP values were available at all study visits, achieved their target BP within 3 months post-baseline, 13.27% within 6 months, and 7.10% within 9 months. At the end of the study, 86.19% of the patients had their BP controlled.
In an attempt to evaluate how SBP and DBP responded to the different doses across study visits, total BP measurements were recorded and reported for the four different IA-FDC doses. Values came as follow: for the 150/5mg dose (N=518), SBP was 128.9±12.6mmHg and DBP was 78.4±7.5mmHg; for the 150/10mg does (N=4), SBP was 135.0±23.8mmHg and DBP was 80.0±14.1mmHg; for the 300/5mg does (N=1240), SBP was 128.2±11.4mmHg and DBP was 78.4±6.5mmHg; and for the 300/10mg does (N=494), SBP was 129.3±13.2mmHg and DBP was 79.1±6.9mmHg. These differences were not statistically significant.
There was also a significant and sequential decrease in the average heart rate from the baseline (76.1±8.4bpm) to the 9-month visit (74.3±6.9bpm) (p<0.001) and in the average weight from the baseline (83.36±12.56kg) to the 9-month visit (81.18±11.48kg) (p<0.001). This is of utmost important since at baseline, more than half of the patients were physically inactive (61.24%), smokers (57.52%), following an unhealthy diet (55.93%) and under stress (52.57%).
Subgroup analysis showed that patients presenting IA-FDC TEAEs (N=34) had a significantly higher weight (88.14±14.91kg) than those presenting AEs not related to IA-FDC (N=33; 6.14±14.29kg) (p=0.001). They were also diagnosed with hypertension for a significantly longer period (5.85±5.80 years versus 2.00±2.66 years, p<0.001). Moreover, patients having taken higher doses of IA-FDC prior to the study (p<0.001), at the baseline visit (p=0.04) and at the 3-month visit (p=0.03) had significantly more TEAEs. In particular, peripheral edema seemed to be more significantly associated with higher doses of amlodipine, namely IA-FDC 150/10mg and 300/10mg compared to IA-FDC 150/5mg and 300/5mg (p=0.001). None of the other AEs had a significantly different occurrence in the doses subgroups.
DiscussionThis study examined the safety of IA-FDC prescribed in the treatment of hypertension in Lebanese patients. The enrolled population included 603 patients from 25 centers; the safety population comprised 602 patients, and the reference population 565 patients. Patients in the safety population reported 68 NSAEs, 36 being TEAEs. None of the 7 SAEs were TEAEs. TEAEs were experienced by around 6% of the safety population and occurred more frequently in patients experiencing only mild or moderate NSAEs. This shows a satisfactory safety profile of IA-FDC compared to a previous clinical study reporting TEAEs in up to 15.8% of the patients treated for up to 10 weeks.8 Peripheral edema is a known adverse drug reaction of amlodipine and was the most common NSAE in this study and particularly in patients on the higher dose of amlodipine, in line with the literature.10,11 Interestingly, the occurrence of edema in patients treated with amlodipine increases in a dose-dependent manner, with the dose of 10mg being associated with substantially higher risk of peripheral edema.12 In the current study, patients requiring escalation of treatment were more likely to move from the 150/5mg dose to the 300/5mg dose, rather than to the 150/10mg dose or 300/10mg dose (where 10mg is the amlodipine dose). This pattern might be due to the physicians’ concern about peripheral edema, and their attempt to control BP with higher doses of irbesartan rather than amlodipine. A 2015 study reported that combination antihypertensive therapy with amlodipine and irbesartan effectively lowers BP without particular safety problems.13 However, some safety issues have been reported for irbesartan. Photosensitivity reactions have been reported with almost all angiotensin II-receptor blockers14 and the most common AEs reported with irbesartan use were weakness, headaches, dizziness, fatigue, and musculoskeletal pain.15–17 However, while the current study reported an AE incidence of around 12% with the IA-FDC, treatment with other combinations might be associated with higher safety events. For instance, a 2013 study reported that 32.5% of patients on irbesartan/hydrochlorothiazide combination had at least one AE.18 Hyperuricemia was the most frequent of the 77 AEs that were found to be related to the study medication; a total of four SAEs were reported in four patients, including one hemorrhagic stroke, one hypertensive emergency, one hypertensive urgency, and one spinal disc herniation.18 This might suggest that IA-FDC is safer than other commonly used antihypertensive combinations.
Patient characteristics indicate that BP and heart rate are higher in obese patients. This was expected as the effects of obesity on cardiovascular parameters have long been established.19–21 Although increased BMI did not affect the occurrence of AEs, the study suggested that overweight patients experienced more TEAEs. The study also showed that an increased time since diagnosis and a higher SBP recorded prior to study entry increase TEAEs occurrence, which was not previously reported in the literature for this drug combination. Equally of interest, patients who presented TEAEs had a greater decrease in both SBP and DBP at three months.
In terms of efficacy of IA-FDC, the study showed that this drug combination decreased both SBP and DBP, which was noted three months after study initiation and maintained until nine months after study initiation. This effect was previously reported by a study showing a decrease in BP starting four weeks and onward.13 Interestingly, patients aged from 18 to 52 years had the highest decrease in SBP and DBP at the 3-month visit, which was not previously reported for this drug combination.
This national, multicenter, non-interventional, longitudinal product registry evaluated the safety of IA-FDC in the real-life management of hypertension in Lebanon. The safety profile was in line with safety data described in the reference document of IA-FDC. The study documented that IA-FDC effectively reduced BP in hypertensive patients, as shown by a controlled BP in 86.19% of patients at the end of the study.
ConclusionThe data collected from this longitudinal study provides a better understanding of adult hypertensive patients in the Lebanese population treated with IA-FDC and their response to the study treatment over 9 months, in order to plan and implement effective healthcare strategies. The study confirms satisfactory safety and tolerability profiles of this anti-hypertensive treatment.
FundingThis work was supported by Sanofi.
Conflict of interestNo conflicts of interest to disclose.
The study was sponsored by Sanofi including Data Management, Biostatistics and Medical Writing. KBP-Biomak contract research organization provided assistance in data management, analysis and in the writing of the manuscript.