The aim of the study was to investigate the diagnostic value of an interferon gamma release assay (IGRA) - QuantiFERON-TB GOLD in-tube (QFT-GIT) - to identify new cases of active pulmonary tuberculosis (PTB) in Quzhou City, and to assess possible factors associated with false-negative IGRA results in bacteriologically confirmed PTB.
MethodsThis study collected clinical data of suspected adult PTB patients who underwent QFT-GIT in Quzhou People's Hospital between January 1, 2020 and December 31, 2020, and calculated the total sensitivity, specificity of QFT-GIT in new case of active PTB patients were 90.9% (401/448) and 72.9%(929/1275), respectively. Univariate and multivariate logistic regression were used to analyze the influencing factors of the false-negative results of QFT-GIT.
ResultsA total of 1723 patients were enrolled, including 448(448/1723,26.0%) with new case of active PTB (389 cases were bacteriologically confirmed and 59 cases were clinically diagnosed) and 1275 non-PTB patients. There were 747(747/1723,43.4%) patients with positive results of QFT-GIT, among whom 401 were new case of active PTB patients (347 cases were bacteriologically confirmed and 54 cases were clinically diagnosed) and 346 were non-PTB patients. The total sensitivity and specificity of QFT-GIT in the diagnosis of new case of active PTB patients were 90.9% and 72.8% respectively. In bacteriologically comfirmed PTB patients, multivariate conditional logistic regression analysis showed that BMI and diabetes, malignant tumors and severe infections were all independent risk factors for the false-negative results of QFT-GIT.
ConclusionQFT-GIT showed high sensitivity and relatively low specificity in the diagnosis of new case of active PTB patients. In clinical practice, we should analyze the suspected PTB patients comprehensively in terms of whether they are overweight or complicated with diabetes, malignant tumors or severe infections, and avoid missing the diagnosis of PTB.
El objetivo del estudio fue investigar el valor diagnóstico de un ensayo de liberación de interferón gamma (IGRA) - QuantiFERON-TB GOLD in tube (QFT-GIT) - para identificar nuevos casos de tuberculosis pulmonar activa (TBP) en la ciudad de Quzhou, y evaluar los posibles factores asociados con resultados falsos negativos de IGRA en un TBP bacteriológicamente confirmado.
MétodosEste estudio recogió datos clínicos de pacientes adultos sospechosos de TPT que se sometieron a QFT-GIT en el Hospital popular de Quzhou entre el 1 de enero de 2020 y el 31 de diciembre de 2020, y calculla sensibilidad total, la especificidad de QFT-GIT en un nuevo caso de pacientes activos de TPT fueron el 90.9% (401/448) y el 72.9% (929/1275), respectivamente. Se utilizó la regresión logística univariable y multivariable para analizar los factores que influyeron en los resultados falsos negativos del QFT-GIT.
ResultadosSe incluyeron un total de 1723 pacientes, incluidos 448(448/1723, 26.0%) con un nuevo caso de TBP activo (389 casos confirmados bacteriológicamente y 59 casos diagnosticados clínicamente) y 1275 pacientes no TBP. Hubo 747(747/1723, 43.4%) pacientes con resultados positivos de QFT-GIT, de los cuales 401 fueron nuevos casos de pacientes con TBP activos (347 casos confirmados bacteriológicamente y 54 casos diagnosticados clínicamente) y 346 eran pacientes sin tpt. La sensibilidad total y la especificidad del QFT-GIT en el diagnóstico de un nuevo caso de pacientes con TBP activos fueron del 90.9% y el 72.8%, respectivamente. En pacientes con TPT con confirmación bacteriológica, el análisis multivariado de regresión logística condicional mostró que el IMC y la diabetes, los tumores malignos y las infecciones graves eran factores de riesgo independientes para los resultados falsos negativos de la QFT-GIT.
ConclusiónEl QFT-GIT mostró una alta sensibilidad y una especificidad relativamente baja en el diagnóstico de un nuevo caso de pacientes con TPT activos. En la práctica clínica, debemos analizar exhaustivamente a los pacientes sospechosos de TBP en términos de si tienen sobrepeso o son complicados con diabetes, tumores malignos o infecciones graves, y evitar perder el diagnóstico de TBP.
Pulmonary tuberculosis (PTB) is an airborne infectious disease of major public health concern caused by Mycobacterium tuberculosis (MTB). It seriously threatens human health and has been listed as one of the major infectious diseases in China. According to the 2021 Global Tuberculosis Report,1 China has returned to second place in the world in the number of TB cases. Therefore, early and rapid diagnosis of PTB will help to reduce its infection rate and incidence in the population, which is also an important mean to prevent and control the spread of PTB.2
The diagnosis of tuberculosis in primary hospitals mainly depends on clinical symptoms, chest imaging, the purified protein derivative (PPD) test, and the sputum smear/culture, etc. In recent years, interferon-γ release assay (IGRA) has been considered an important supplement or auxiliary approach for the diagnosis of MTB infection, and which in the diagnosis of tuberculosis has been of widespread concern.3 However, in clinical practice, patients with bacteriologically comfirmed PTB (BC-PTB) or clinically diagnosed PTB (CD-PTB) may possibly present with negative results of IGRA, resulting in missed diagnosis and delayed treatment, and even affecting the prognosis of PTB.4 Thus, the aim of the study was to investigate the diagnostic value of an interferon gamma release assay (IGRA) - QuantiFERON-TB GOLD in-tube (QFT-GIT) - to identify new cases of active pulmonary tuberculosis (PTB) in Quzhou City, and to assess possible factors associated with false-negative IGRA results in bacteriologically confirmed PTB, so as to provide theoretical support for the standardized diagnosis of PTB in this region.
Objects and methodsStudy objectsThis study includes the continuous collection of clinical data of adult patients with suspected PTB who underwent QFT-GIT in Quzhou People's Hospital between January 1, 2020 and December 31, 2020.The clinical data including their general information (gender, age, height, weight, underlying diseases), underlying diseases (diabetes, long-term use of hormones, non-steroidal anti-inflammatory drugs and immunosuppressants, malignant tumors, severe infections, AIDS) and various laboratory tests (sputum smear, GeneXpert, culture of MTB, QFT-GIT, peripheral blood lymphocyte count, CD4+T lymphocyte count).
A patient with BC-PTB was defined as someone whose biological specimen is positive by smear microscopy, culture or WHO-approved rapid diagnostic test (such as Xpert MTB/RIF). A patient with CD-PTB was defined as someone with PTB who does not meet the above criteria for BC-PTB but has been diagnosed with active TB by a clinician or other medical practitioner who has decided to give the patient a full course of TB treatment. This definition includes cases diagnosed on the basis of X-ray abnormalities or suggestive histology and pulmonary cases without laboratory confirmation.Patients who met any of the following criteria were excluded as non-PTB patients: (1) Patients with confirmed tumors, inflammation or other diseases based on pathological results, and who had excluded the combined MTB infection; (2) Patients who had been clinically diagnosed with other diseases, and excluded MTB infection after corresponding treatment or following up.
Patients who met any of the following criteria were included as new patients with PTB: (1) Patients who had never been treated with anti-tuberculosis drugs; (2) Tuberculosis patients who were adhering to standard chemotherapy regimens but had not completed the course of treatment; (3) Tuberculosis patients who had been undergoing irregular chemotherapy for less than 1 month. Patients who met any of the following were defined as the retreatment patients with PTB: (1) Patients who have been treated irrationally or irregularly for one month or more with anti-TB drugs in the past; (2) Patients who failed from initial treatments and they had a positive sputum smear or culture result again.
Patients who met any of the following criteria were excluded: (1) Patients who were defined as the nontuberculous mycobacterial pulmonary disease (NTM-PD); (2) Patients who were defined as the retreatment patients with PTB; (3) Patients who were excluded with incomplete information.
MethodsQFT-GIT: The whole-blood assay of QFT-GIT was performed according to the manufacturer's instructions (Wantai Biotechnology Co., LTD). Fasting venous blood (5ml) was collected early in the morning and stored in a lithium heparin vacuum blood collection tube, 3ml of which was distributed into three tubes (1ml per tube): N tube for negative control, T tube for tests, and P tube for positive control. The tubes were incubated at 37°C for 22 ± 2 hours, following which the supernatant was collected after 10min of centrifugation. IFN-γ in the supernatant was measured by ELISA according to the manufacturer’s instructions.
The minimum detection amount was 2pg/mL, ranging from 2 to 400pg/mL. Result judgement: Positive (N ≤ 400pg/mL, P-N is any value, T-N ≥ 14pg/mL and ≥N/4), negative (N ≤ 400pg/mL, P-N ≥ 20pg/mL, T-N ≤ 14pg/mL or T-N ≥ 14pg/mL but ≤N/4). The positive results might imply the presence of TB infection.
Statistical analysisThe data were recorded in Epidata 3.1, and analyzed using SPSS 24.0 (IBM Corporation). According to the results of QFT-GIT, patients were divided into a positive group and a negative group. The normally-distributed data were described as mean ± standard deviation (SD) and analyzed using the two-sample t-test. The skewedly-distributed data were described as median (inter-quartile range) [Median (IQR)] and analyzed using the rank-sum test. The qualitative data were described in percentage (%) form and analyzed by using the chi-squared test or Fisher's exact test. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and a 95% confidence interval (CI) were calculated according to binomial distribution. Factors with statistically significant differences in univariate analysis were incorporated into the multivariate logistic regression analysis to construct a prediction model for analysis. P < 0.05 was considered to be statistically significant. The receiver operator characteristic (ROC) curve was used to evaluate the prediction performance of the model.
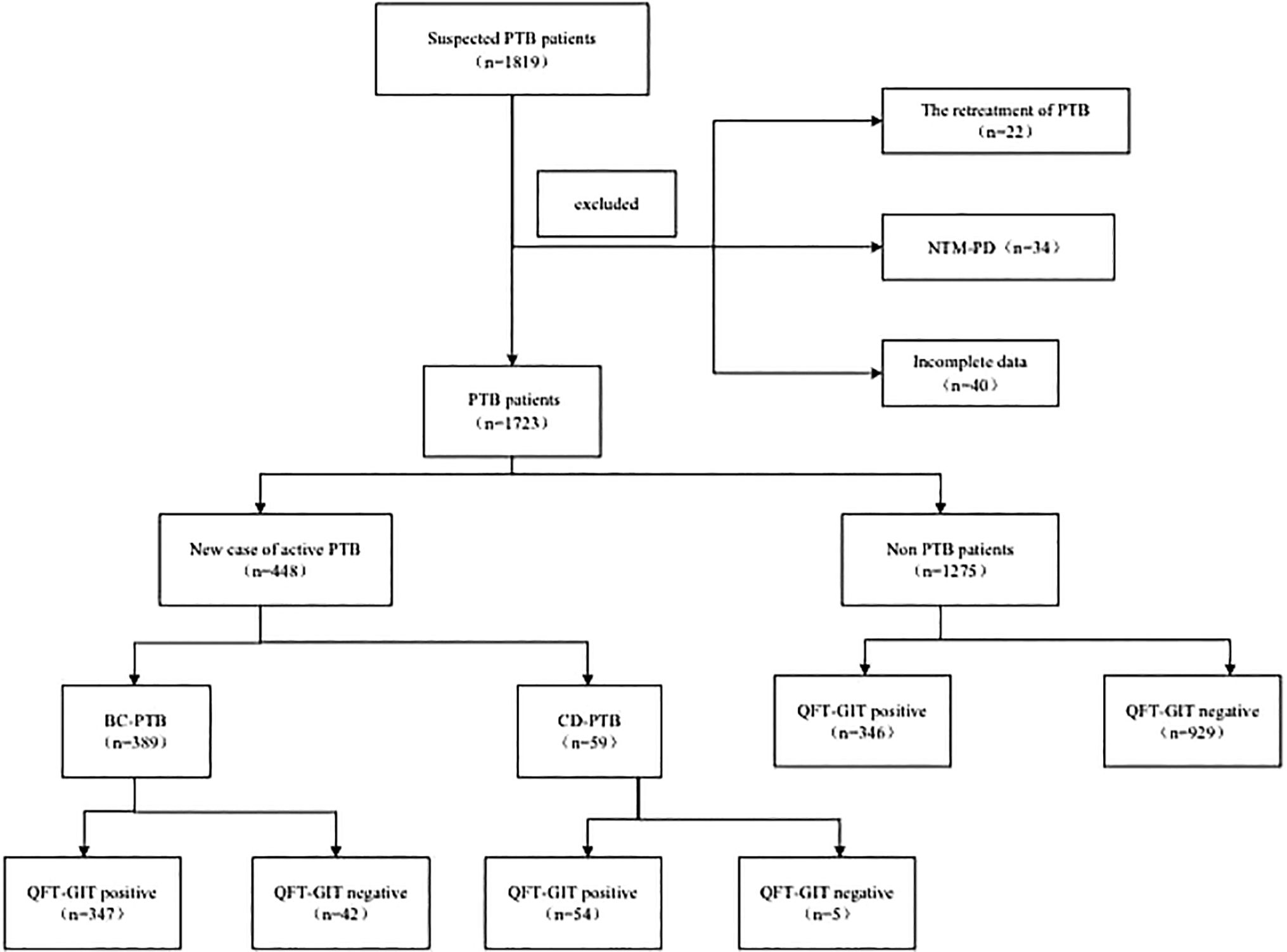
ResultsGeneral informationDuring the study period, a total of 1,819 suspected PTB patients who had undergone QFT-GIT were enrolled, among whom 22 had recurrent PTB, 34 had pulmonary non-MTB, and 40 had incomplete data were excluded. Finally, 1,723 patients were included, among whom 448 were new case of active PTB patients (389 with BC-PTB and 59 with CD-PTB) and 1,275 were non-PTB patients. There were 747 patients with positive results of QFT-GIT, among whom 401 were new case of active PTB patients (347 with BC-PTB, 54 with CD-PTB) and 346 were non-PTB patients (Fig. 1).
Diagnostic value of QFT-GIT in new case of active PTB patientsThe total sensitivity, specificity, PPV, NPV, and QFT-GIT in new case of active PTB patients were 90.9% (401/448, 95%CI 0.893-0.969), 72.9% (929/1275, 95%CI 0.665-0.757), 54.1% (401/747, 95% CI 0.471-0.645), and 95.2% (929/976, 95% CI 0.951-0.992), respectively. PLR and NLR were 3.16 and 0.08, respectively.
The sensitivity of QFT-GIT was 89.2% (347/389, 95% CI 0.861-0.997) in BC-PTB patients and 91.5% (54/59, 95% CI 0.872-0.983) in CD-PTB patients, and the difference between the two groups was not statistically significant (χ2=0.000, P > 0.05).
Clinical characteristics and influencing factorsThe results of the clinical characteristics showed that there were 42 BC-PTB patients with negative results of QFT-GIT, including 32 males and 10 females. For the 32 males, the average age was 70 (54,77) years old and the average Body mass index [BMI is calculated by taking a person's weight, in kilograms, divided by their height, in meters squared, or BMI = weight (in kg)/ height^2 (in m^2)] was 19.15±2.52. Among the 42 patients, 28 (28/42, 66.7%) had additional underlying diseases in the 3 months prior, including nineteen with diabetes (19/42, 45.2%), nine with malignant tumors (9/42, 21.4%), four with severe infections (4/42, 9.52%), one using immunosuppressants (1/42, 2.4%), one using glucocorticoids (1/42, 2.4%), one using non-steroidal anti-inflammatory drugs (NSAIDs) (1/42, 2.4%) and one with acquired immune deficiency syndrome (AIDS) (1/42, 2.4%).
Univariate analysis showed that BMI, CD4+T lymphocyte count, lymphocyte count, complicated with diabetes, malignant tumors and severe infections were significant influencing factors for false-negative results of QFT-GIT in BC-PTB patients (Table 1).
Influencing factors of false-negative results of QFT-GIT in BC-PTB patients.
| Influencing factors | Univariate analysis | |||
|---|---|---|---|---|
| QFT-GIT Negative | QFT-GIT Positive | Statistical quantity | P-value | |
| n=42 | n=347 | |||
| Age[years,M(Q1,Q3)] | 70(54,77) | 64(46,73) | Z = −1.915 | 0.056 |
| BMI(Mean±SD) | 19.15±2.52 | 20.33±2.89 | t = 2.511 | 0.012 |
| CD4+T lymphocyte count [×106/L ,M(Q1,Q3)] | 238.85(98.35,387.63) | 347.50(192.50,519.80) | Z = −3.082 | 0002 |
| Lymphocyte count [×109/L ,M(Q1,Q3)] | 0.87(0.56,0.87) | 1.08(0.80,1.52) | Z = −2.481 | 0.013 |
| Sex,male[n,(%)] | 32(76.20) | 239(68.88) | 0.948 | 0.330 |
| Underlying diseases B | ||||
| Diabetes[n,(%)] | 19(45.24) | 52(14.99) | 22.981 | 0.000 |
| Immunosuppressants exposure within 3 months | 1(2.38) | 9(2.59) | 0.000 | 1.000 |
| Glucocorticoids exposure within 3 months | 1(2.38) | 6(1.73) | 0.000 | 1.000 |
| Non-steroidal anti-inflammatory drugs exposure within 3 months | 2(4.76) | 12(3.46) | 0.000 | 1.000 |
| Malignant tumors | 9(21.43) | 18(5.19) | 12.889 | 0.000 |
| Severe infections | 4(9.52) | 8(2.31) | 4.338 | 0.037 |
| AIDS | 1(2.38) | 1(0.29) | 0.421 | 0.516 |
QFT-GIT, QuantiFERON-TB GOLD in-tube; BMI, body mass index; SD, standard deviation; M, median; Q1, 1st quartile; Q3, 3rd quartile; AIDS, acquired immunodeficiency syndrome;
Malignant tumors (lung cancer, rectal cancer, gastric cancer, esophageal cancer, liver cancer, prostate cancer, thyroid cancer, throat cancer);
Severe infections (When the SOFA score ≥ 2 and procalcitonin ≥ 2ng/ml, the patient was considered to have serious infection).
The significant influencing factors in univariate analysis were included in multivariate logistic regression analysis, and the results showed that BMI, diabetes, malignant tumors and severe infections were independent risk factors for false-negative results of QFT-GIT in BC-PTB patients (Table 2).
Multivariate logistic regression of false-negative results of QFT-GIT in BC-PTB patients.
| Factors | β | s‾x | Wald χ2 | OR | 95%CI | P-value |
|---|---|---|---|---|---|---|
| BMI | 0.186 | 0.070 | 7.114 | 1.204 | 1.051∼1.381 | 0.008 |
| CD4+T lymphocyte count | 0.002 | 0.001 | 3.006 | 1.002 | 1.000∼1.005 | 0.083 |
| Lymphocyte count | −0.119 | 0.469 | 0.064 | 0.888 | 0.354∼2.228 | 0.800 |
| Diabetes | −1.895 | 0.393 | 23.287 | 0.150 | 0.070∼0.325 | 0.000 |
| Severe infections | −1.645 | 0.685 | 5.763 | 0.193 | 0.050∼0.739 | 0.016 |
| Malignant tumors | −1.525 | 0.502 | 9.209 | 0.218 | 0.081∼0.583 | 0.002 |
OR, odds ratio;BMI, body mass index
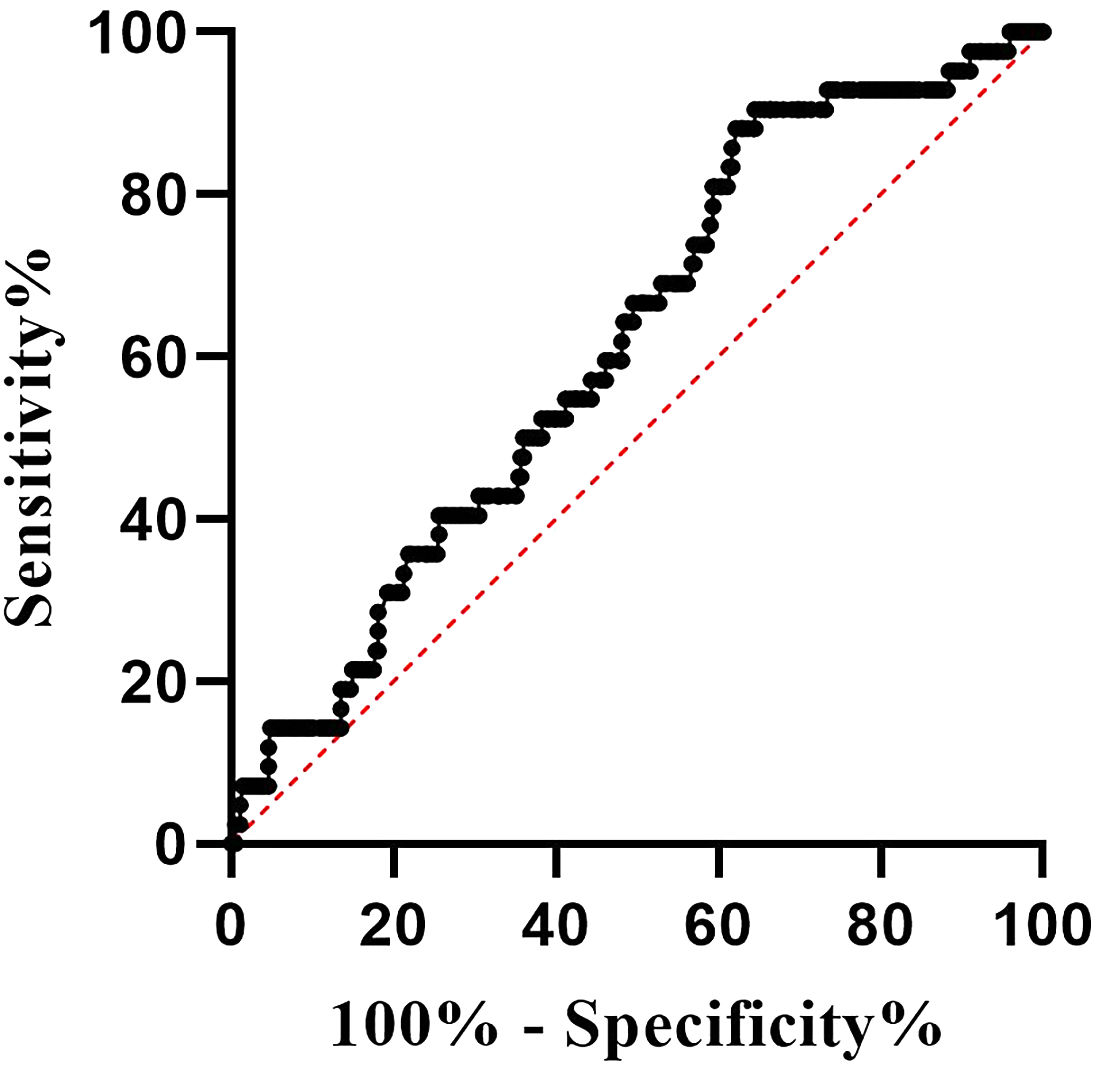
The ROC curve was used to assess the value of BMI in predicting the false-negative results of QFT-GIT in BC-PTB patients, and the results showed that the area under the curve (AUC) was 0.614 (Standard error: 0.042, 95% confidence interval [CI]: 0.531-0.697, P=0.016), and the best cut-off value was 21.244 (sensitivity: 35.4%, specificity: 90.5%, Yoden’s index: 0.259) (Fig. 2).
DiscussionQFT-GIT, which measures the level of IFN-γ released after a specific MTB antigen stimulates sensitized T lymphocytes, has been introduced to determine whether there exists MTB infection, and has been widely used in clinical practice.4 The results of this study showed that the total sensitivity and specificity of QFT-GIT in the diagnosis of new case of active PTB patients were 90.9% and 72.9%, respectively, among which the sensitivity was significantly higher than past results, and the specificity was similar to those reported in other studies previously.5 From 1990-2019, the prevalence rate of LTBI decreased in China, ranging from 34.28% to 30.73%. However, based on the huge population base, China was still the country with the most LTBI cases in 2019.6 The reason for the relatively low specificity of IGRA in this study might lie in the large population of our country and the high prevalence rate of LTBI.
Up to now, China has remained one of the countries with the highest TB burden worldwide, and in this study patients with LTBI and inactive PTB7,8 were not separated from non-PTB patients, which may also have contributed to the low specificity of QFT-GIT.
In this study, several factors related to the false-negative results of QFT-GIT were reported, which helped to explain why some patients presented with characteristics of BC-PTB in clinical practice but obtained negative results of QFT-GIT to some extent. The state of severe immunosuppression, long-term use of immunosuppressants or glucocorticoids, diabetes or malignant tumors, age, gender, BMI and other factors were demonstrated to be reasonable for the false-negative results of QFT-GIT.5,9–14 In our study,overweight, complicated with diabetes, malignant tumors, or severe infections were found to be independent risk factors of false-negative results of QFT-GIT.
Being overweight may influence the QFT-GIT results in PTB patients. In fact, there is no unified standard for the specific value of BMI to cause false negative results, past studies proved that BMI ≥ 25kg/m2 was an influential factor for false-negative IGRA results in active PTB patients.Considering its effect on the immune functions of T cells, being overweight may cause abnormal secretion of IFN-γ and further influence the result of IGRA.13 It has also been reported that BMI < 16.0 kg/m2 is independently associated with false-negative IGRA results, and low BMI may be associated with malnutrition, progressive disease consumption and severe disease condition.15 In our study, the average of BMI was 19.15, and BMI was found to be associated with the false-negative results of QFT-GIT, following which its value in predicting the false-negative outcome in BC-PTB patients was further evaluated. Based on the analysis of the ROC curve, the predicted AUC was 0.614 and the best cut-off value was 21.244, with sensitivity, specificity and Jorden’s index totaling 35.4%, 90.5% and 0.259, respectively, thus indicating that the value of BMI alone in predicting false-negative results of QFT-GIT was not high enough.
There were several limitations in this study. We aimed to compare the results of QFT-GIT and PPD at first. Unfortunately, it was found that the results of PPD could not be truly counted in the process of data collection and were not included in the statistical analysis of this study. In addition, latent TB infection (LTBI) and inactive PTB patients were not separated from non-PTB patients, which might have exerted an influence on the specificity of the prediction model in this study.
In conclusion, QFT-GIT showed high sensitivity and relatively lower specificity for the diagnosis of new case of active PTB. In BC-PTB patients, being overweight, complicated with diabetes, malignant tumors or severe infections were the risk factors for false-negative results of QFT-GIT. Therefore, in clinical practice, we should analyze the suspected PTB patients comprehensively in terms of whether they are overweight or dealing with complicatied with diabetes, malignant tumors or severe infections, so as to avoid missing the diagnosis of PTB.
Ethical issuesThis study was approved by the Medical Ethics Committee of Quzhou People's Hospital (ethical code: Ethics Y (Real 2018-080))
Consent of publicationAuthors agree to allow the publication and declare that the work described was original research that has not been published previously, and not under consideration for publication elsewhere. Also, the authors agree to allow the publication and distribution of the materials submitted in all available forms, without limiting territory or language, provided that the material is accepted for publication. Authors confirm that all information is original and free from plagiarism.
Conflicts of interestThe authors declare no conflicts of interest.
Authors’ contributionsConception and design: Tao Lu, Chunxian Peng; Provision of study materials or patients: Weili Lu, Shun Wang, Zhiyu Wu, Shungen Wu; Collection and data analysis: Ling Ye, Jianhua Lan; Manuscript writing: Tao Lu; Final approval of manuscript: All authors
FundingThis work was supported by the Project of Science and Technology Plan in Quzhou (2018072)
The authors are very grateful to the participants.We thank all staf of the Quzhou People's Hospital and the research staf involved in the work. Also, we would like to thank Ms. Min Fang for helping in data analysis.