Late-onset neutropaenia is defined as an absolute neutrophil count of <1.5×103cells/μL starting>4 weeks after the last dose of rituximab, in the absence of other identifiable causes.
Late-onset neutropaenia is a rare adverse reaction to rituximab (observed in approximately 5% of patients). Rheumatic diseases constitute the main indication for rituximab; in these patients, neutropaenia appears after a mean of>28 days.
Ocrelizumab is another monoclonal antibody that binds to CD20 (a glycosylated phosphoprotein mainly expressed on the membranes of B-lymphocytes); in January 2018, it was approved for the treatment of relapsing-remitting and primary progressive multiple sclerosis.
We present a case of neutropaenia following intravenous infusion of ocrelizumab in a patient with primary progressive multiple sclerosis who presented with neutropaenic fever, herpetic stomatitis, and ecthyma gangrenosum only 20 days after infusion.
La neutropenia de aparición tardía se define como un recuento absoluto de neutrófilos <1,5×103/μl que se produce >4semanas después de la última dosis de rituximab, precedido por un recuento de neutrófilos normal y sin otra causa identificable. Es una complicación rara del tratamiento con rituximab, habiéndose observado en aproximadamente el 5% de los pacientes tratados, siendo las enfermedades reumáticas su principal indicación, con un tiempo medio hasta el desarrollo de la neutropenia de al menos 28 días. El ocrelizumab, al igual que el rituximab, es un anticuerpo monoclonal dirigido a CD20, una fosfoproteína glicosilada de membrana que se encuentra predominantemente en los linfocitos B y que se aprobó en enero de 2018 para el tratamiento de la esclerosis múltiple remitente recurrente y la esclerosis múltiple progresiva primaria. Se describe un caso de neutropenia después de la infusión de ocrelizumab en un paciente con esclerosis múltiple progresiva primaria que presentó neutropenia febril, estomatitis herpética y ectima gangrenoso solo 20 días después de la infusión.
A 44-year-old woman went to the Emergency Department on May 23, 2020 with fever of 38.5°C (725°F), odynophagia and lower right limb weakness. She was under medical follow-up for PPMS diagnosed in 2019 with first Expanded Disability Status Scale (EDSS) of 4.0 (Pyramidal 3, Sensory 1, Ambulation about 300m without aid or rest).
No previous disease modifying treatment or concomitant medications. She had no other medical illnesses. She received her first Ocrelizumab dose (600mg iv) on October 28, 2019 administered as two separated infusions over two weeks period and second dose was administered on May 04, 2020, without incidents with previous laboratory variables within normality (Table 1).
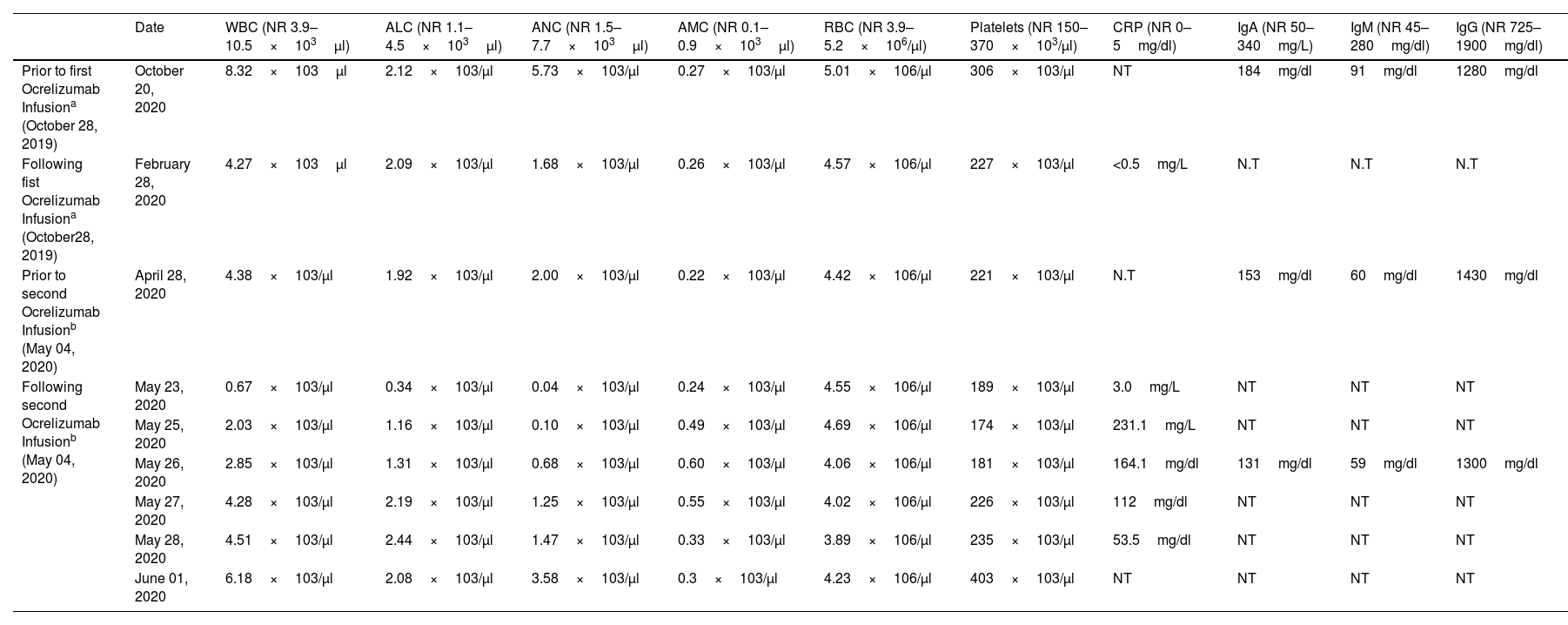
Serial blood levels prior and following Ocrelizumab infusions.
| Date | WBC (NR 3.9–10.5×103μl) | ALC (NR 1.1–4.5×103μl) | ANC (NR 1.5–7.7×103μl) | AMC (NR 0.1–0.9×103μl) | RBC (NR 3.9–5.2×106/μl) | Platelets (NR 150–370×103/μl) | CRP (NR 0–5mg/dl) | IgA (NR 50–340mg/L) | IgM (NR 45–280mg/dl) | IgG (NR 725–1900mg/dl) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior to first Ocrelizumab Infusiona (October 28, 2019) | October 20, 2020 | 8.32×103μl | 2.12×103/μl | 5.73×103/μl | 0.27×103/μl | 5.01×106/μl | 306×103/μl | NT | 184mg/dl | 91mg/dl | 1280mg/dl |
| Following fist Ocrelizumab Infusiona (October28, 2019) | February 28, 2020 | 4.27×103μl | 2.09×103/μl | 1.68×103/μl | 0.26×103/μl | 4.57×106/μl | 227×103/μl | <0.5mg/L | N.T | N.T | N.T |
| Prior to second Ocrelizumab Infusionb (May 04, 2020) | April 28, 2020 | 4.38×103/μl | 1.92×103/μl | 2.00×103/μl | 0.22×103/μl | 4.42×106/μl | 221×103/μl | N.T | 153mg/dl | 60mg/dl | 1430mg/dl |
| Following second Ocrelizumab Infusionb (May 04, 2020) | May 23, 2020 | 0.67×103/μl | 0.34×103/μl | 0.04×103/μl | 0.24×103/μl | 4.55×106/μl | 189×103/μl | 3.0mg/L | NT | NT | NT |
| May 25, 2020 | 2.03×103/μl | 1.16×103/μl | 0.10×103/μl | 0.49×103/μl | 4.69×106/μl | 174×103/μl | 231.1mg/L | NT | NT | NT | |
| May 26, 2020 | 2.85×103/μl | 1.31×103/μl | 0.68×103/μl | 0.60×103/μl | 4.06×106/μl | 181×103/μl | 164.1mg/dl | 131mg/dl | 59mg/dl | 1300mg/dl | |
| May 27, 2020 | 4.28×103/μl | 2.19×103/μl | 1.25×103/μl | 0.55×103/μl | 4.02×106/μl | 226×103/μl | 112mg/dl | NT | NT | NT | |
| May 28, 2020 | 4.51×103/μl | 2.44×103/μl | 1.47×103/μl | 0.33×103/μl | 3.89×106/μl | 235×103/μl | 53.5mg/dl | NT | NT | NT | |
| June 01, 2020 | 6.18×103/μl | 2.08×103/μl | 3.58×103/μl | 0.3×103/μl | 4.23×106/μl | 403×103/μl | NT | NT | NT | NT |
Abbreviations: NR: normal range, WBC: white blood cells, ALC: absolute lymphocyte count, ANC: absolute neutrophil count, AMC: absolute monocyte count, RBC: red blood cells, CRP: C-reactive protein, N.T: not tested.
On examination, she was found to have erythematous lesions on hard palate and a painful, papulose erythematous-violaceus lesion with minimal central crust on the back of the distal interphalangeal joint of the right index finger.
The neurological examination was normal except for mild paresis on lower right limb.
Laboratory test showed 0.34×103/μl absolute lymphocyte count (ALC) and 0.04×103/μl ANC (Table 1).
She was hospitalized because of febril neutropenia, mucositis and possible gangrenous ecthyma. Empirical treatment with prophylactic cefepime iv (2g/8h), acyclovir iv (500mg/8h) and voriconazol iv (200mg/12h) were started. Investigations for infection including chest X-ray, cultures of blood, throat, urine and molecular studies for viruses were negative as well as SARS CoV2 Polymerase Chain Reaction (PCR) test. Symptoms started improving 24h after treatment started, her ALC and ANC rose without requiring colony stimulatin factor and C-reactive protein (CRP) was in decline (Table 1). She was discharged on June 1, 2020 without further symptoms and final diagnosis of febrile neutorpenia relative to Ocrelizumab with secondary herpetic stomatitis and gangrenous ecthyma resolved.
Rituximab is a chimeric monoclonal anti CD20 antibody for treatment of rheumatologic disorders and hematologic malignancies. It has been also used as an off-label treatment of Multiple Sclerosis (MS).4 Late-onset neutropenia is a rare complication of Rituximab treatment defined as a total number of neutrophils<1.5×109/L that develops after 4 weeks of the last dose without any other identifiable cause and preceded by a normal neutrophil count.1,6 LON ranged between 3 and 27% in various studies6 and grade IV neutropenia was reported in <1% of riruximab treated patients with average time to LON of 90–154 days.1,5 Those patients who have been retreated with Rituximab, more often, did not experience recurrence of neutropenia.1-4
Physiopathological theories includes: emergent antibodies against neutrophils, aberrant reconstitution of B lymphocyes, IgG Fc receptor polymorphism and viral infections (PVB19).5,7,8
Ocrelizumab, just like Rituximab, is a monoclonal antibody directed at CD20, a membrane glycosylated pshosphoprotein found predominately on B-lymphocytes and has been approved in January 2018 for treatment of relapsing-remitting and primary progressive multiple sclerosis.
In the period of active control treatment for RRMS (OPERA I and OPERA II-NCT01247324 and NCT01412333)7,8 a decrease in neutrophils<LIN (lower limit of normal) was observed in 14.7% of patients treated with Ocrelizumab vs 40.9% of patients treated with interferón beta-1a In the placebo-controlled clinical trial on PPMS (ORATORIO-NCT01194570)9 neutropenia was transiently noted in 12.9% of patients with Ocrelizumab vs 10.0% on placebo patients and without associated infection. Most of them were transient and grade 1 (<1500/mm3) or grade 2 (1000–1500/mm3) 1000/mm3) and one patient with grade 4 neutropenia Only one patient with grade 3 neutropenia (500–1000/mm3) and one grade 4 (<500/mm3) required specific treatment with granulocyte colony stimulating factor (G-CSF) and they remained in the ocrelizumab group after resolution.10
There have been reports in other autoimmune diseases of G-CSF triggering or worsening immune phenomena. Therefore it should only be used if strictly necessary in MS patients.
After bibliographic research only three published cases of neutropenia after infusion of ocrelizumab have been found. All of them three months after the second ocrelizumab infusion and one of them in a patient primarily treated with Rituximab.11–13
For ocrelizumab only few cases of LON have been reported and it is a rare adverse event following treatment with anti-CD20 antibodies.
To our knowledge, this is the first reported case of neutropenia after only 20 days of ocrelizumab infusion and highlights the importance of serial monitoring after ocrelizumab infusion, probably monthly at least the first year of treatment.
Taking into account previous experience with Rituximab and possible recurrence of neutropenia, readministration of treatment should be evaluated individually.
Conflict of interestElda Mª Alba Suárez received compensation for speaking activities, and/or consulting services from Sanofi-Genzyme and Roche.
Antonio Tallón Barranco received compensation for speaking activities, and/or consulting services from Biogen and Roche.
Inmaculada Puertas Muñoz has nothing to disclose.
Ángel Robles Marhuenda has nothing to disclose.
No targeted funding reported. Patient gave informed consent for personal and clinical data publication.