The present review is focused on literature concerning the relevance of fractional exhaled nitric oxide (FeNO) in clinical practice from a pathophysiological point of view.
There is increasing evidence that asthma is a heterogeneous pathological condition characterised by different phenotypes/endotypes related to specific biomarkers, including FeNO, helpful to predict therapeutic response in selected asthmatic populations. Nowadays FeNO, a non-invasive biomarker, appears to be useful to foresee asthma developing, to recognise specific asthma phenotypes, like the eosinophilic, to ameliorate asthma diagnosis and management in selected populations and to predict standard corticosteroid and biologic therapy efficacy. In addition, FeNO assessment may also be useful in patients with allergic rhinitis in order to detect the potential involvement of eosinophilic bronchial inflammation in “case finding” subjects at risk of asthma diagnosis.
Therefore, it is possible to hypothesise a future with an appropriate use of FeNO by physicians dealing with worrisome clinical issues in specific asthma phenotypes.
The biological messenger nitric oxide (NO) plays a fundamental role in the physiology of several organs and is a mediator in the pathophysiology of different diseases. In the lung, NO at low concentrations acts as a smooth muscle relaxing agent and neurotransmitter of the inhibitory non-adrenergic non-cholinergic nervous (iNANC) system, whereas at high concentrations as an inflammatory mediator.1 In exhaled air of human beings NO has been detected, and higher levels of fractional NO concentration in exhaled breath (FeNO) have been revealed in asthma.2 The role of exhaled NO as an inflammatory biomarker in asthma has been recently investigated and this review will point out the potential use of FeNO in asthma and in allergic rhinitis from a pathophysiological point of view.
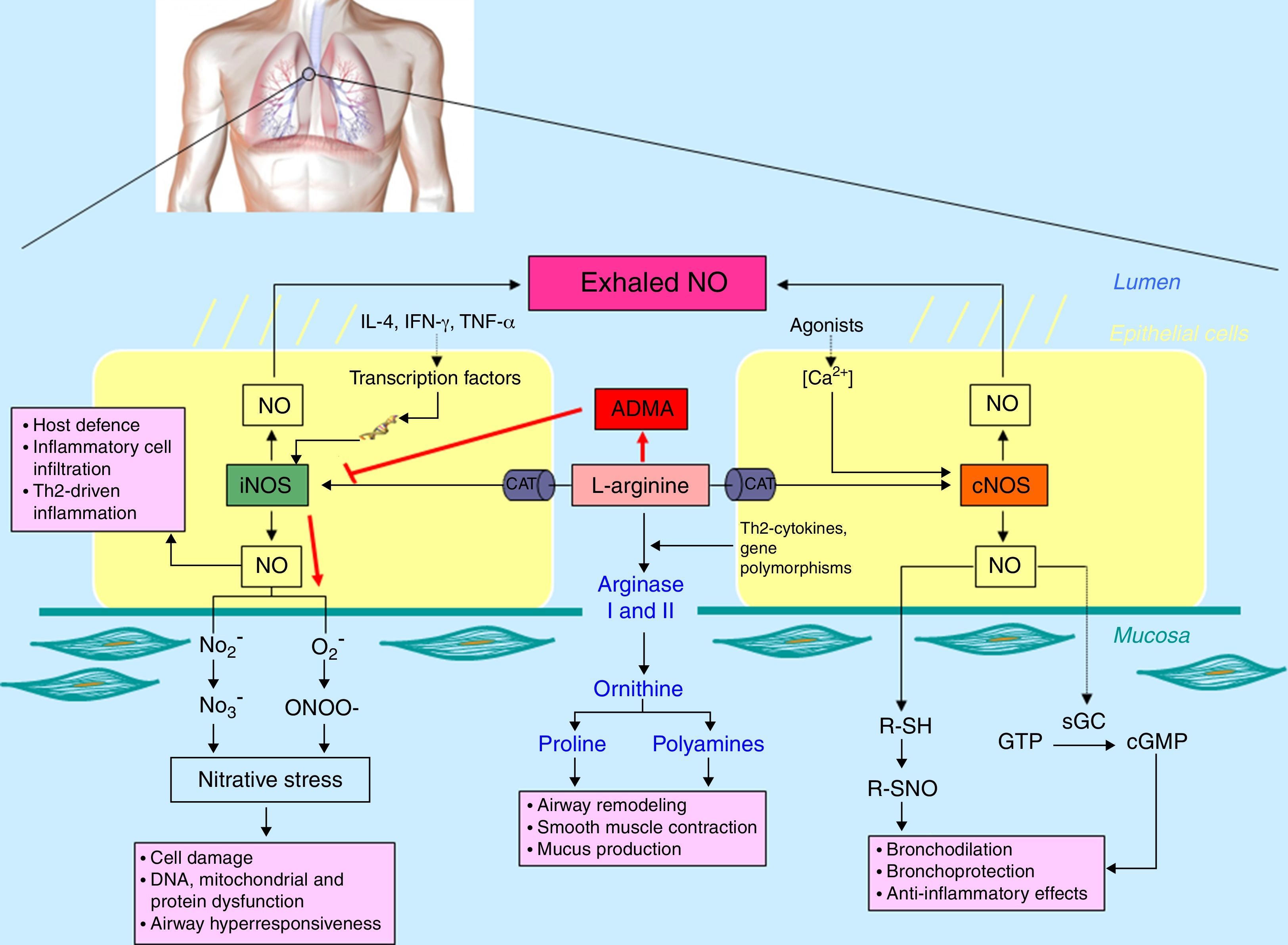
Lung synthesis of NOIn the lung, different cell types (including epithelial cells, nerves, vascular endothelial cells and inflammatory cells) are able to produce NO.2l-Arginine is transported into the cell via the cationic amino acid transport (CAT) system and can be metabolised by both NO synthases (NOS) and arginases. There are three NOS isoforms expressed in the airways including constitutive neural NOS (NOS-I or nNOS), inducible NOS (NOS-II or iNOS) and constitutive endothelial NOS (NOS-III or eNOS). In the respiratory tract, NO at low concentration (picomolar range) derived from constitutive isoforms mediates a plethora of physiological responses, including lung development, airway smooth muscle relaxation, bronchoprotection against bronchoconstrictor stimuli and ciliary motility.1,2 Conversely, an elevated release of NO (nanomolar range) from iNOS is involved in innate immunity against pathogens and malignant cells, together with chronic inflammatory diseases. Most of these effects are dependent on the reaction between NO and superoxide anion (O2−) formed in the airways during inflammation that generates peroxynitrite (ONOO−), a highly reactive oxidant specie (Fig. 1).1,2 Arginases (arginases I and II) are also able to metabolise l-arginine by catalysing the synthesis of polyamines and l-proline via the conversion of l-arginine to l-ornithine.1,3 In addition, asymmetric dimethyl arginine (ADMA) is an endogenous inhibitor of NO formation by arginine/NOS pathway reducing intracellular l-arginine availability. ADMA competitively inhibits NOS by interfering in its binding to l-arginine, so that high levels of ADMA reduce the synthesis of NO and augment the formation of superoxide and peroxynitrite.4 Thus, the “nitrative-oxidative stress” (responsible for protein dysfunction and cellular damage) might be facilitated by ADMA (Fig. 1).
Schematic representation of nitric oxide (NO) metabolism in the airways. l-Arginine is transported into the cell via the cationic amino acid transport (CAT) system and can be metabolised by both nitric oxide synthases [constitutive NOS (cNOS) and inducible NOS (iNOS)] and arginases (I and II). Moreover, asymmetric dimethyl arginine (ADMA), an l-arginine analogue, can competitively inhibit NOS isoforms that, in uncoupling conditions, generate O2− and, as consequence, “nitrative stress”.
FeNO can be measured by spectroscopic and electroanalytic methods. Among spectroscopic methods the chemiluminescence is the most used and is based on the measurement, by a sensitive photomultiplier tube, of the fluorescent radiation intensity emitted after chemical oxidation of NO by ozone. The product of this reaction (NO2*) emits a photon, and the total number of photons produced is proportional to the NO concentration. Electrochemical methods for NO determination are based on the electrochemical oxidation of NO on solid electrodes. If the current generated during its oxidation is linearly proportional to the concentration, the oxidation current can be used as an analytic signal. Recently, a new electrochemical sensor has been developed, based on the amperometric technique (the production of a current when a potential is applied between two electrodes), which is suitable for NO analysis in exhaled breath.5
Comparing the stationary chemiluminescence analysers, including Sievers NOA280i (GE Analytical Instruments, Boulder, CO, USA), and the electrochemical analysers like NIOX MINO (NIOX MINO, Aerocrine AB, Sweden) a significant correlation, but only a moderate agreement, emerged between FeNO values measured with the two devices showing significant lower values detected with the electrochemical device. These differences should be taken into account when interpreting the results of FeNO.6
FeNO concentrations from the lower respiratory tract exhibit significant expiratory flow dependence.7,8 This variation in exhaled NO has been attributed to faster flows minimising the transit time of alveolar gas in the airway. The rate of NO output, however, is greater at higher flow rates, but not in direct proportion; this is analogous to respiratory heat loss.7 In view of this flow dependency, the use of constant expiratory flow rates is emphasised in standardised techniques. A flow rate of 0.05L/s (BTPS) was chosen for the 1999 ATS statement to be a reasonable compromise between measurement sensitivity and patient comfort. In general one exhalation is considered adequate if the mean exhalation flux velocity is 0.05L/s (±10%) during the generation time of a NO plateau and the instant flux is included between 0.045L/s and 0.055L/s at any time throughout exhalation.9 FeNO at 0.05L/s mostly reflects large airways NO flux, but the single constant expiratory flow rate is not able to discriminate the individual contributions of large airways NO flux versus small airways/alveolar production sites. A two-compartment lung (alveolar and bronchial) model was suggested to interpret the different components that participate in exhaled NO generation.10 In this review, unless otherwise mentioned, FeNO was considered at 0.05L/s.
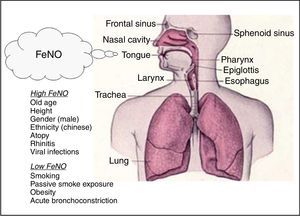
FeNO in health and diseaseFeNO reference values and factors affecting FeNOSeveral studies concentrated on derivation of reference values in both adults and children considering the common factors affecting FeNO. On the basis of population studies in non-asthmatic subjects, the demographic factors age, height and gender and also other factors like viral infections and cigarette smoking can be considered key determinants of FeNO values.11
In adults, the Travers’ study on healthy subjects showed that FeNO levels are significantly influenced by atopy, current and past smoking status, and gender, indicating for instance that the highest “upper limit of normal” was observed in atopic never smoker males (56.5ppb), whereas the lowest was observed in non-atopic smoker females (30.5ppb).12 In another study on healthy non-smoking subjects, the key factors considered were age and height: older and taller subjects had higher FeNO levels with “upper limit of normal” ranging from 24ppb (age 25–34 years; height<160cm) to 54ppb (age 65–75 years; height>190cm).13 A study on 405 healthy children showed that FeNO increases with age (mean FeNO value 9.7ppb, upper 95% CI: 25.2ppb ranging from 15ppb at 4 years old to 22.4ppb at 14 to 17 years old),14 while in a study on 531 schoolchildren aged 11–18 years and enrolled from five schools (three of which were international schools) ethnicity emerged as a factor affecting FeNO, demonstrating that Chinese children had higher FeNO levels compared with Caucasians.15 The relationship with age and obesity in adults is still controversial16,17 and different studies indicate higher FeNO levels in men.12,18 Increased FeNO levels are positively associated with atopy,12,13 and viral infections,19 whereas both current and, to a lesser extent, previous smoking and passive smoke exposure, decrease FeNO levels (Fig. 2).12,13,20,21
Finally, FeNO levels appear to be lower in circumstances of smaller airway diameter. In particular, women showed reduced FeNO levels compared to matched men probably due to reduced geometry of the airways.18 Hence, within a subject, FeNO levels may be decreased during bronchoconstriction. This may be caused by increased airflow velocity in constricted airways when the exhalation rate is kept constant.22
FeNO in rhinitisRhinitis per se, mainly if allergic, is the most important risk factor for asthma onset and worsening.
In an urban population of non-asthmatic children (mean age 10 years old) FeNO level was elevated in atopic compared to non-atopic children and in rhinitic children compared to non-rhinitic children, suggesting that atopy and rhinitis could be factors influencing FeNO.23 In particular, it was also demonstrated that allergic rhinitis is able to increase FeNO levels compared to atopic controls without rhinitis,12,24,25 and that the group of asthma with allergic rhinitis did not show an enhancement of FeNO level compared to allergic rhinitis alone.23,24 This indicates that inflammation in upper airways by allergic rhinitis is able to modulate per se NO production by lower airways in line with the postulated concept of “united airways.”26
A confounding factor could be chronic rhinosinusitis (CRS) which is able to increase FeNO level compared to controls independently of atopy and, of note, CRS patients with lower respiratory symptoms either or not asthma-like significantly showed increased FeNO level compared to CRS patients with rhinitis symptoms, only pointing out that CRS per se may contribute to iNOS-induced lower airway inflammation.27 Finally, patients with CRS have to be differentiated on the basis of eosinophilic and non-eosinophilic infiltration into the paranasal sinus mucosa. Patients with eosinophilic CRS showed higher levels of FeNO in comparison with non-eosinophilic CRS and normal subjects suggesting that eosinophilic inflammation in paranasal sinus mucosa is able to affect the production of NO from lower airways.28
A paediatric study in children with allergic rhinitis (AR) reported that FeNO is negatively related to PD20 methacholine (r=−0.61); thus higher FeNO values correspond to a more severe bronchial hyperresponsiveness (BHR); a FeNO cut-off of 32ppb was a predictive factor for BHR.29 One study, conducted in adults with AR, confirmed moderate and negative correlation between FeNO levels and BHR severity (r=−0.58); 37ppb was found to be the best cut-off to predict the presence of BHR in AR patients with a specificity equal to 90.5%, a sensitivity equal to 79.1% and an AUC (area under the ROC curve) of 0.90.30 These studies suggest that FeNO may potentially be a relevant predictive marker for BHR in AR patients; FeNO measurement could be thereafter useful for the screening of AR subjects in order to identify the ones at risk of developing asthma. Similarly, Skiepko et al. observed increased FeNO levels in AR patients with BHR compared to healthy subjects during the pollen season; a correlation was also found between increased BHR to histamine and increased FeNO.31 Another study demonstrated significantly higher FeNO levels in young adults with AR, in the presence or absence of asthma, compared to subjects with non-allergic rhinitis alone; in addition, perennial sensitisation caused higher FeNO levels than seasonal one.32 Strong correlation was also demonstrated between FeNO and ΔFEV1 after bronchodilation (BD) testing; FeNO values >34ppb were predictors of bronchial reversibility (ORAdj 1.9); thus FeNO may predict positive BD in AR children.33 Finally, in a group of allergic patients suffering from rhinitis alone it has been shown that a BMI value >25, such as overweight, is a risk factor (OR 1.96) for high FeNO levels in AR patients.34
FeNO as biomarker of airway inflammation in asthmaFeNO is a biomarker of atopy and allergic airway inflammation in subjects with respiratory symptoms.35 Jouaville et al.23 found that FeNO level increased with the number of positive skin prick tests (SPTs) in asthmatics together with non-asthmatic subjects. FeNO was always more elevated in asthmatic compared to non-asthmatic children with equal levels of positive SPTs. In children without asthma, FeNO increased only in atopic subjects with rhinitis, whereas in children with asthma FeNO enhanced in atopic subjects (Fig. 2) both in the absence or presence of rhinitis. These data indicate that allergic rhinitis should be considered in the assessment of FeNO in conjunction with atopy and asthma.36 Among allergic mild-intermittent young asthmatics in stable condition blood eosinophilia significantly correlated with FeNO levels only in allergic polysensitised individuals, suggesting that in mild asthmatic children an association between a comparable disease severity and a greater inflammatory component may be hypothesised in allergic compared to non-allergic subjects.37 In asthmatic children sensitised to airborne allergens the exposure to pollens during the grass pollen season led to an increase of FeNO concentrations that returned to pre-seasonal values thereafter.38 Young adults with eczema showed on average significantly higher levels of FeNO compared to those without eczema, as well as those with versus without allergic rhinitis and atopic versus non-atopic asthmatics, suggesting an independent association among eczema, allergic rhinitis, or atopic status and elevated FeNO values.39
There is evidence showing the correlation between FeNO and the eosinophilic phenotype in asthma.40 The relationship between eosinophilic airway inflammation and high FeNO in childhood and adult asthma with different severity, before and after steroid treatment, was found in studies on sputum,41 bronchoalveolar lavage fluid,42 and bronchial biopsies.43
In the study by Berry et al.44 a FeNO of 36ppb (at a flow rate of 50ml/s) had a sensitivity and specificity for sputum eosinophilia of more than 3% (the cut-off point deemed by the authors to be clinically significant) of 78% and 72%, respectively. Shaw et al.45 demonstrated that a FeNO below 26ppb had a negative predictive value of 85% for sputum eosinophils less than 3%. Thus, a low FeNO is worthy to ascertain the absence/low levels of eosinophilic airway inflammation. Interestingly, in severe asthmatic children FeNO is more strongly correlated with BAL than induced sputum eosinophilia and there was no correlation between eosinophils in BAL than in subepithelial mucosal infiltration, indicating that in BAL and bronchial biopsies inflammation is measured in different compartments. In that study, normal levels of FeNO are good predictors of the absence of submucosal eosinophilia (negative predictive value: 83%).46
A positive correlation between mucosal eosinophil number and FeNO was also reported in bronchial biopsies from allergic adult asthmatics before and after allergen challenge.43
Furthermore, cluster analysis identified different phenotypes with high FeNO, including the early onset atopic and the inflammation predominant, and with low FeNO, such as obese non-allergic and adult-onset asthma.47 In late onset asthmatics obesity with uncontrolled respiratory symptoms, less allergy and lower lung volume is associated with a decreased l-arginine/ADMA ratio, explaining why FeNO is negatively related to BMI in obese late onset asthmatics with lowered l-arginine/ADMA ratio, and suggesting the relation between obesity and asthma worsening in subjects with low FeNO48 (Fig. 2).
Because of the heterogeneous response to asthma therapy, to identify biomarkers assessing treatment effectiveness of specific medications could improve the likelihood of successful treatment. The EXTRA study evaluated in severe allergic asthma three biomarkers involved in Th2-driven inflammation (such as FeNO, blood eosinophil, and serum periostin) as potential predictors of omalizumab efficacy, estimated in terms of protocol-defined asthma exacerbations during the 48-week treatment period; dividing patients into low- and high-biomarker subgroups.49 After 48 weeks of omalizumab, there was significant percentage reduction in exacerbations in all the three higher biomarker than the lower biomarker subgroups, suggesting that in these patients omalizumab may provide greater advantages.49
FeNO and pulmonary functionMeasurement of lung function by spirometry is crucial in asthma management. Initially, it was thought that forced respiration for spirometry could affect FeNO outcome.50 Actually, it has been evidenced that both in children and adults FeNO values are not modified by a previous spirometry if the FeNO measurement is performed 10min after baseline spirometry or spirometry pre- and post-bronchodilators, indeed even if repeated spirometry has an immediate effect on FeNO,36 an interval of 10min seems to be sufficient to revert the values to pre-spirometric levels.51
A study focused on a paediatric cohort of allergic subjects with asthma or rhinitis provided evidence that FeNO was strongly related with the response to bronchodilation testing and could predict bronchial reversibility, suggesting that a simple FeNO measurement could offer relevant information about bronchial reversibility.33
As previously described in FeNO reference values and factors affecting FeNO section, FeNO can also be influenced by airway calibre, thus clinicians should interpret FeNO value changes over time in asthmatic patients with consideration of concomitant changes in airway obstruction.52 Some reports showed that in mild-to-moderate asthma FeNO levels can be modulated not only by changes in airway inflammatory status but also by bronchial tone related to acute bronchoconstriction induced by direct and indirect stimuli (Fig. 2)53–55 or lung function changes commonly seen in the course of asthma,52 suggesting that airway calibre should be taken into account when monitoring exhaled NO in asthma and that FeNO should be considered a biomarker that integrates both airway inflammation and lung function changes.52
On the other hand, a moderate negative correlation (r=−0.44) was found between FeNO levels and FEF25–75 in allergic rhinitic adolescents.56 A strong negative relationship (r=−0.70) between FeNO and FEF25–75 was observed in children with asthma; impaired FEF25–75 values (<65% of predicted) were significantly related to high FeNO levels (like >34ppb); thus this study evidenced that impaired FEF25–75 may predict high FeNO levels (ORAdj 3.9) in allergic children.57
In a prospective study on 44 clinically stable, treated, non-smoking asthmatics it was shown that FeNO≥28ppb, independent of FEV1, heightened the relative risk for exacerbation by 3.4 (p=0.007), and that combining FeNO≥28ppb and FEV1≤76% predicted increased the probability (85%) for future exacerbation, while in asthmatics with a combination of FEV1>76% and FeNO<28ppb the probability of asthma exacerbation within 18 months was zero; these data suggest that the combination of FeNO and FEV1 percentage of predicted can stratify risk for asthma exacerbation.58
BHR to non-specific bronchoconstrictor stimuli is a key feature of asthma in conjunction with the inflammatory process of the disease and can be considered a marker of disease severity and control.59 Several studies evidenced the relation between FeNO levels and degree of BHR, bronchodilator reversibility or atopy.60,61 Recently, a strong and negative association between FeNO and BHR in asthmatic children with allergic rhinitis29 and in adults with allergic rhinitis30 has been demonstrated, suggesting for FeNO the role as surrogate marker for the BHR test and as diagnostic marker for bronchial impairment in allergic patients. A significant relationship between BHR to bradykinin, but not to methacholine, and eosinophilic inflammation was previously highlighted in mild-to-moderate asthmatics treated or not with corticosteroids62 proposing BHR to bradykinin as a clinical marker to assess asthma severity. Ricciardolo et al.54 firstly demonstrated that allergen exposure is able to raise FeNO during late asthmatic response and to worsen BHR to bradykinin63; then they reported the direct association between high FeNO levels and increased number of eosinophils or potentiated BHR to bradykinin in atopic asthmatics after allergen exposure indicating that FeNO can reflect the loss of asthma control.43
FeNO and asthma controlThe gold standard for asthma treatment is to achieve and maintain asthma control.64,65 Among common markers of airway inflammation and clinical parameters FeNO showed the ability to predict and diagnose poorly-controlled asthma66 and, during inhaled corticosteroid (ICS) therapy, to be the fastest marker responding to treatment67 guiding adjustments in ICS dosage and assessing the risk of worsening asthma.68 Zeiger et al.69 proved that a FeNO value higher than 300% of predicted was able to identify patients at risk of excessive use of rescue medication and needing oral corticosteroids within 1 year. Although some studies which evaluated regular FeNO measurements for ICS therapy adjustments to achieve asthma control failed to show important benefits,70,71 a FeNO-based asthma-management strategy significantly reduced exacerbation rate in children with moderate-severe asthma72; likewise in adult stable asthmatics titration of anti-inflammatory therapy in relation to both FeNO and sputum eosinophilia determined a ICS dose reduction and a progressive symptoms score improvement.73
In a population of pregnant asthmatic women monitored for ICS therapy, Powell et al. showed that patients with low FeNO and asthma symptoms received LABA and the minimum ICS dose, while patients with high FENO and asthma symptoms received higher ICS doses by step-wise titration. In the latter group, exacerbations were significantly reduced and although the number of patients using ICS was superior compared to the control group, the mean ICS dose was significantly reduced, indicating that FeNO should be used in selected rather than in general asthmatic populations.74
A recent report demonstrated that in a real-world setting not only high but also intermediate FeNO levels are associated with a significant improvement in asthma control after starting ICS treatment, challenging the current clinical guidelines statement which affirms that only high FeNO levels predict response to ICS therapy.75 Another study showed that a FeNO-guided anti-inflammatory treatment significantly improved asthma-related quality of life and asthma symptom control and reduced exacerbations in atopic asthmatics within primary care76 as well as in a non-blind, pragmatic, cluster-randomised trial on asthmatic adults the symptom-plus FeNO-driven strategy reduced asthma medication use while sustaining asthma control in conjunction with quality of life, and was considered the preferred strategy for adult asthmatic patients in primary care.77
ConclusionsFeNO is a non-invasive biomarker for allergic and/or eosinophilic airway inflammation in patients with asthma. Several non-disease related factors like anthropometric characteristics, obesity and smoking habits are able to influence FeNO levels and should be accurately considered in the interpretation of FeNO in asthma. AR influences FeNO levels and in these subjects FeNO may be predictive of bronchial hyperresponsiveness (BHR) and bronchial reversibility.
Factors affecting airway calibre such as bronchoconstriction and bronchodilation could affect FeNO levels in asthmatics, suggesting that they should be measured in parallel with FeNO.
Asthma is a multifaceted disease with a plethora of phenotypes, which cannot advocate the use of a single biomarker in order to monitor it and determine different responses to treatments. At present, FeNO is the best non-invasive biomarker able to predict corticosteroid responsiveness in asthma and is clinically relevant in identification of various severe asthma phenotypes and in prediction of biologic therapy efficacy.
A correct use of FeNO, combined with other diagnostic instruments (pulmonary function test including reversibility or bronchial challenge and sputum analysis), might be useful for physicians in the management of asthma control and in “case finding” of allergic rhinitis subjects at risk of developing asthma. In conclusion, FeNO measurement might be fruitful in achieving asthma control in patients with high FeNO.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.




![Schematic representation of nitric oxide (NO) metabolism in the airways. l-Arginine is transported into the cell via the cationic amino acid transport (CAT) system and can be metabolised by both nitric oxide synthases [constitutive NOS (cNOS) and inducible NOS (iNOS)] and arginases (I and II). Moreover, asymmetric dimethyl arginine (ADMA), an l-arginine analogue, can competitively inhibit NOS isoforms that, in uncoupling conditions, generate O2− and, as consequence, “nitrative stress”. Schematic representation of nitric oxide (NO) metabolism in the airways. l-Arginine is transported into the cell via the cationic amino acid transport (CAT) system and can be metabolised by both nitric oxide synthases [constitutive NOS (cNOS) and inducible NOS (iNOS)] and arginases (I and II). Moreover, asymmetric dimethyl arginine (ADMA), an l-arginine analogue, can competitively inhibit NOS isoforms that, in uncoupling conditions, generate O2− and, as consequence, “nitrative stress”.](https://static.elsevier.es/multimedia/03010546/0000004300000006/v1_201511050434/S0301054615000270/v1_201511050434/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)