Various inflammatory biomarkers have been used in asthma cases for evaluating inflammation, however it has been determined that the majority of these biomarkers are insufficient for putting forth the course and severity of the disease. Osteoprotegerin is a glycoprotein mediator in the lung and macrophages. As far as we know, there are no studies about the role played by osteoprotegerin in child patients with asthma.
ObjectiveIt was planned to examine the relationship between osteoprotegerin levels in childhood asthma and respiratory functions and airway inflammation and to assess its use as a biomarker.
MethodsThe study included patients aged 6–16 years who were diagnosed with asthma at the pediatric allergy outpatient clinic of Bagcilar Training and Research Hospital in Turkey. The correlation analyses for the osteoprotegerin levels of asthma patients and their respiratory functions were examined.
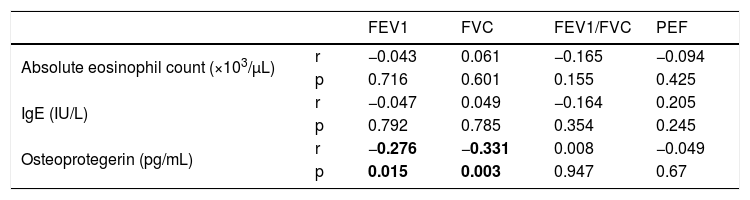
ResultsThe age average of asthma cases was 10.61±3.04 years and 51.2 % were female. No statistically significant difference was observed between the osteoprotegerin levels of the groups (p>0.05). A negative and statistically significant correlation was observed between the FEV1 and FVC values and osteoprotegerin levels (p=0.015, p=0.003).
ConclusionsThis was the first study to examine the relationship between osteoprotegerin levels and airway inflammation in children with asthma. We believe that there is a need for wider scale studies in which clinical symptoms and more parameters are evaluated for defining the role played by osteoprotegerin level in children with asthma and for determining its usability as a biomarker.
Asthma is a heterogenic, chronic inflammatory disease characterized by reversible obstruction and airway hypersensitivity.1 The identification of similar biological biomarkers which play a role in inflammation in asthma patients is important with regard to both diagnosis and the developments it will provide for treatment. The identification of special non-invasive biomarkers that will enable monitoring and follow up is of greater importance, especially in childhood asthma cases. Spirometric measurements used frequently for this purpose require effective cooperation and therefore cannot be sufficiently evaluated in children. Similarly, current methods such as bronchoalveolar lavage cannot be routinely used because they are invasive.2–5 Hence, there is a need for non-invasive methods that may be used for the follow up of these diseases.
Initially, osteoprotegerin (OPG) is a polypeptide synthesized with 401 amino acids. A mature protein composed of 380 amino acids is formed after removing the 21 amino acid propeptide section. It is secreted extracellularly as a homodimeric, soluble glycoprotein with 60kDa monomeric and 120kDa disulfite bond. OPG is a member of the tumor necrosis factor receptors (TNFR) super family but does not contain transmembrane and cytoplasmic sections like the other receptors of the TNFR super family.6,7 OPG is synthesized by many different tissues, including hematopoietic and immune cells such as osteoblasts, bone marrow, kidney, spleen, brain, lung and cardiovascular system (heart, arteries and veins).8,9 The primary function of OPG is known as osteoclast differentiation and activation inhibition, however the role played by OPG synthesized in other tissues is not known for sure. OPG synthesis in large arteries’ media layers and different vascular cell types such as coronary artery smooth muscles and endothelium cells indicate its function at the vascular bed.10
The role played by OPG, a glycoprotein mediator in the lung and macrophages, in childhood asthma and the relationship between its level and disease severity is not known. The number of studies with invasive bronchoscopy techniques in children is limited, hence studies have focused more on identifying various markers for determining airway inflammation. In this study it was planned to evaluate the relationship between OPG levels and airway inflammation in childhood asthma and the assessment of its usability as a biomarker.
Methods and materialsPatient populationPatients aged 6–16 years diagnosed with asthma in pediatric allergy outpatient clinic of Bagcilar Training and Research Hospital in Turkey were included in the study. Global Initiative for Asthma guidelines were used as the basis for diagnosis and treatment.1 Skin prick tests were carried out on all patients for the same allergens. Asthma cases were classified according to the state of atopy as atopic (n=25) and non-atopic cases (n=14). Full blood parameters and osteoprotegerin levels of healthy children (n=40) were compared among the groups. Respiratory functions tests for asthma cases and correlation analyses with osteoprotegerin levels were also carried out.
Patients with asthma exacerbations who received systemic steroids within the last month, those with acute/chronic infection, and any other systemic disease such as hepatic, renal, cardiovascular diseases, diabetes mellitus, cancer, sepsis and systemic inflammatory disorders were excluded from the study. Informed consent was acquired from the parents of all patients which were then approved by the Research Ethics Committee of Bagcilar Training and Research Hospital. The study was carried out in accordance with all ethical issues specified in the Helsinki declaration.
Skin prick testSkin prick tests were carried out on the anterior surface of the forearm when patients were not under the effect of anti-histamines. Skin prick tests were performed using a Stallerpoint device to test for common aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae), a mixture of grass pollens (Lolium perenne, Dactylis glomerata, Phleum pratense, Anthoxanthum odoratum, Poa pratensis, Festuca elatior, Agrostis vulgaris, Holcus lanatus, Cynodon dactylon, Avena sativa, Avena fatua, Lotus Corniculatus), a mixture of grain pollens (oats, wheat, barley, corn), a mixture of tree pollens (Acer pseudoplatanus, Aesculus hippocastanum, Robinia pseudoacacia, Tilia platyphyllos, Platanus vulgaris), weed-mix pollens (Medicago sativa, Trifolium pratense, Brassica nigra, Urtica dioica, Rumex acetosa), Alternaria alternaria, cockroaches (Blattella germanica), and cat and dog dander (Stallergenes SA, 92160 Antony, France). Histamine (10mg/mL) and physiological saline were used as positive and negative references, respectively. Skin reactions were evaluated after 20min. Wheal diameters of ≥ 3mm were considered as a positive reaction. At least one positive reaction in the skin test was classified as atopy.
Counting blood samplesPeripheral venous blood samples were drawn after an overnight fasting period lasting for 10h in order to measure OPG levels. These samples were centrifuged at 1500 rcf for 10min, and the isolated plasma was stored at −80°C until assayed. OPG (Affymetrix eBioscience, San Diego, CA 92121, USA, Human OPG kit, Cat no: BMS2021) measurements were carried out using an enzyme linked immunosorbent assay (ELISA). An intra-assay coefficient of variation of the ELISA system was 3–9% with inter-assay coefficient of variation ranging between 4–10.2%.
SpirometrySpirometry was applied on groups I and II for a minimum of three times using a ZAN 100 (Germany) at the spirometry laboratory. Bronchodilator reversibility was accepted as more than 12%, or 200ml change from the FEV1 baseline. The measurements for these parameters were carried out in accordance with GINA criteria.1
Statistical analysisStatistical analyses were conducted with the 2007 program Number Cruncher Statistical System (NCSS) (Utah, USA). In addition to the descriptive statistics (average, standard deviation), unidirectional variation analysis was used for multiple group comparisons of normally distributed variables. Tukey’s multiple comparison tests were used in subgroup comparisons, independent t-tests were used in the paired-group comparisons, Kruskal-Wallis tests were used in multiple-group comparisons of variables not distributed normally, Dunn’s multiple comparison tests were used in subgroup comparisons, and chi-square tests were used to compare qualitative data. Significance level was considered as p<0.05 for the evaluation of the results.
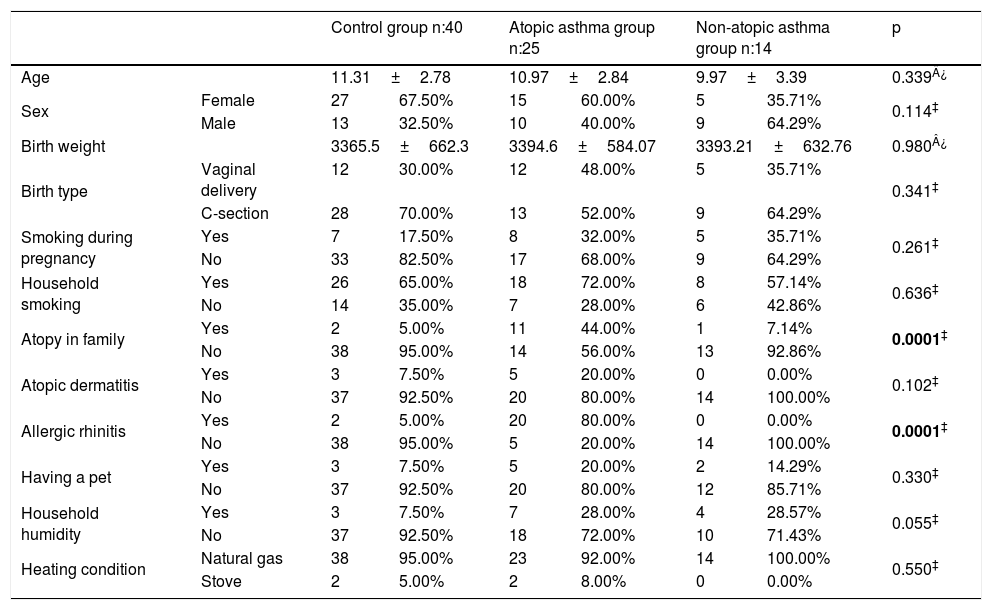
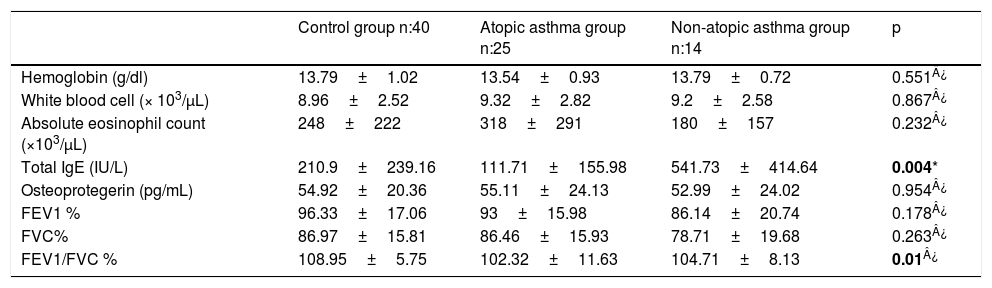
ResultsThe average age of asthma patients was 10.61±3.04 years. 51.2% were female and 64.1% were atopic. Dust mite sensitivity was present in all patients. Statistically significant differences were not observed between the average ages, gender distributions, birth weight, birth week, birth type, smoking during pregnancy, household smoking, atopic dermatitis, having a pet, household humidity and heating distributions of healthy cases and cases with asthma (p>0.05). A statistically significant difference was observed in the atopic asthma group regarding atopy in the family and allergic rhinitis (p<0.001) (Table 1). A statistically significant difference could not be observed between the serum OPG levels of the groups (p>0.05) (Table 2). No statistically significant difference was observed between the PEF, FEV1/FVC measurements and IgE, eosinophil count and OPG levels of asthma patients upon evaluating the spirometric measurements and correlation analyses of asthma patients (p>0.05). A negative and statistically significant correlation was observed between the FEV1 and FVC values and OPG levels (p=0.015, p=0.003) (Table 3).
Comparison of the demographic parameters of the groups.
| Control group n:40 | Atopic asthma group n:25 | Non-atopic asthma group n:14 | p | |||||
|---|---|---|---|---|---|---|---|---|
| Age | 11.31±2.78 | 10.97±2.84 | 9.97±3.39 | 0.339¿ | ||||
| Sex | Female | 27 | 67.50% | 15 | 60.00% | 5 | 35.71% | 0.114‡ |
| Male | 13 | 32.50% | 10 | 40.00% | 9 | 64.29% | ||
| Birth weight | 3365.5±662.3 | 3394.6±584.07 | 3393.21±632.76 | 0.980¿ | ||||
| Birth type | Vaginal delivery | 12 | 30.00% | 12 | 48.00% | 5 | 35.71% | 0.341‡ |
| C-section | 28 | 70.00% | 13 | 52.00% | 9 | 64.29% | ||
| Smoking during pregnancy | Yes | 7 | 17.50% | 8 | 32.00% | 5 | 35.71% | 0.261‡ |
| No | 33 | 82.50% | 17 | 68.00% | 9 | 64.29% | ||
| Household smoking | Yes | 26 | 65.00% | 18 | 72.00% | 8 | 57.14% | 0.636‡ |
| No | 14 | 35.00% | 7 | 28.00% | 6 | 42.86% | ||
| Atopy in family | Yes | 2 | 5.00% | 11 | 44.00% | 1 | 7.14% | 0.0001‡ |
| No | 38 | 95.00% | 14 | 56.00% | 13 | 92.86% | ||
| Atopic dermatitis | Yes | 3 | 7.50% | 5 | 20.00% | 0 | 0.00% | 0.102‡ |
| No | 37 | 92.50% | 20 | 80.00% | 14 | 100.00% | ||
| Allergic rhinitis | Yes | 2 | 5.00% | 20 | 80.00% | 0 | 0.00% | 0.0001‡ |
| No | 38 | 95.00% | 5 | 20.00% | 14 | 100.00% | ||
| Having a pet | Yes | 3 | 7.50% | 5 | 20.00% | 2 | 14.29% | 0.330‡ |
| No | 37 | 92.50% | 20 | 80.00% | 12 | 85.71% | ||
| Household humidity | Yes | 3 | 7.50% | 7 | 28.00% | 4 | 28.57% | 0.055‡ |
| No | 37 | 92.50% | 18 | 72.00% | 10 | 71.43% | ||
| Heating condition | Natural gas | 38 | 95.00% | 23 | 92.00% | 14 | 100.00% | 0.550‡ |
| Stove | 2 | 5.00% | 2 | 8.00% | 0 | 0.00% | ||
*Kruskal Wallis test.
Comparison of the laboratory and spirometric measurements of the cases.
| Control group n:40 | Atopic asthma group n:25 | Non-atopic asthma group n:14 | p | |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 13.79±1.02 | 13.54±0.93 | 13.79±0.72 | 0.551¿ |
| White blood cell (× 103/μL) | 8.96±2.52 | 9.32±2.82 | 9.2±2.58 | 0.867¿ |
| Absolute eosinophil count (×103/μL) | 248±222 | 318±291 | 180±157 | 0.232¿ |
| Total IgE (IU/L) | 210.9±239.16 | 111.71±155.98 | 541.73±414.64 | 0.004* |
| Osteoprotegerin (pg/mL) | 54.92±20.36 | 55.11±24.13 | 52.99±24.02 | 0.954¿ |
| FEV1 % | 96.33±17.06 | 93±15.98 | 86.14±20.74 | 0.178¿ |
| FVC% | 86.97±15.81 | 86.46±15.93 | 78.71±19.68 | 0.263¿ |
| FEV1/FVC % | 108.95±5.75 | 102.32±11.63 | 104.71±8.13 | 0.01¿ |
| Multiple comparison tests | Tukey | Dunn’s |
|---|---|---|
| Fev1/FVC | IgE | |
| Control group/atopic asthma group | 0.048 | 0.579 |
| Control group/non-atopic asthma group | 0.613 | 0.026 |
| Atopic asthma group/non-atopic asthma group | 0.624 | 0.003 |
Correlation distributions between the spirometric measurements and biomarkers of asthma cases.
| FEV1 | FVC | FEV1/FVC | PEF | ||
|---|---|---|---|---|---|
| Absolute eosinophil count (×103/μL) | r | −0.043 | 0.061 | −0.165 | −0.094 |
| p | 0.716 | 0.601 | 0.155 | 0.425 | |
| IgE (IU/L) | r | −0.047 | 0.049 | −0.164 | 0.205 |
| p | 0.792 | 0.785 | 0.354 | 0.245 | |
| Osteoprotegerin (pg/mL) | r | −0.276 | −0.331 | 0.008 | −0.049 |
| p | 0.015 | 0.003 | 0.947 | 0.67 |
Pearson correlation test.
In this study examining the relationship between serum OPG levels and respiratory functions and airway inflammation in children with asthma and assessing its use as a biomarker, the OPG levels of asthma cases measured during the stable period were determined to be similar with those of the healthy group.
OPG was originally discovered as an inhibitor of bone resorption, and various cytokines and hormones regulate its expression and production.11 Besides the significant role it plays with regard to the regulation of bone metabolism, osteoprotegerin has important anti-inflammatory and anti-apoptotic effects.12 It neutralizes the effect of receptor activator of nuclear factor-κ B ligand (RANKL) and TNF-related apoptosis-inducing ligand (TRAIL) thus inhibiting the activation of specific pro-inflammatory and pro-apoptotic signaling pathways.13 Studies put forth that dendritic cells have abnormal activation in asthma patients.14–16 However, the impact of osteoprotegerin on inflammation in asthma could not be explained clearly since there is not sufficient knowledge of its impacts on RANK/RANKL signal inhibition and the life and function of the dendritic cells in the lungs. Yang et al.17 carried out related studies on rat models with asthma and showed that OPG as a RANK/RANKL signal inhibitor may reduce the inflammatory reaction in asthma by preventing the life and function of dendritic cells.
Anand et al.18 carried out a study on 510 patients with type 2 diabetes for examining the relationship between OPG, inflammation biomarkers of hs-CRP, IL-6 and coronary artery calcification and cardiovascular disease as a result of which a strong relationship was put forth between the plasma OPG levels and increased coronary artery calcification. It is also reported that OPG is involved in lipopolysaccharide-induced acute inflammation in animal models.19 13 Coronary artery disease, rheumatoid arthritis, and some other diseases cause an increase in serum OPG levels and thus it has been used as a biomarker.20,21
As far as we know, there is no study in the literature which examines the relationship between OPG levels and airway inflammation in children with asthma. Masako et al.22 carried out a study on patients with chronic obstructive pulmonary disease (COPD) and showed that phlegm OPG levels were observed to be higher in patients with COPD in comparison to healthy controls and those of smokers were observed to be higher in comparison to asthma patients. OPG levels of COPD patients were similar compared with the control group. They believe the reason for these results is because OPG is the biomarker of a local inflammation instead of a systemic inflammation. They also reported that phlegm OPG concentrations in COPD patients are negatively correlated with FEV1, which may be related with the course and severity of the disease. In conclusion, the authors put forth that OPG levels in the phlegm of patients with COPD are correlated with the lung function changes indicating parenchymal damage but that there is a need for more comprehensive studies in order for OPG to be used as a bio-biomarker for emphysema. In accordance with the related literature, in our study, the serum OPG levels of patients with asthma were determined to be at similar levels with the serum OPG levels of healthy cases. Similarly, a negative and statistically significant correlation was observed between the FEV1 and FVC values and osteoprotegerin levels.
Some authors reported an increase in serum IgE levels whilst a decrease in FEV1 levels.23,24 Kumar et al.23 stated a statistically significant weak negative correlation between serum IgE and FEV1 levels (p=0.04) (r=−0,23). Di Lorenzo et al.24 suggested a positive correlation between both serum IgE and eosinophil numbers, and bronchial hyperactivity in patients with Parietaria pollen hypersensitivity. They also stated that beside eosinophils, allergen types and IgE levels could play a role in the development of bronchial hypersensitivity and asthma progression in allergic rhinitis patients. In the same research mentioned above, Kumar et al.23 showed that, while there is no significant correlation between serum eosinophil numbers and respiratory function tests (r=-0.95), there is a weak negative correlation between sputum eosinophil and FEV1, FVC levels (p=0.011) (p=0.015). Our study shows no statistically substantial relation between FEV1, FVC, IgE and serum eosinophil levels. The reason for the obtained result might be the lack of severe asthma patients.
We are of the opinion that the limitations of our study are the low number of patients, the fact that the patients with asthma have been evaluated during the stable period and that there are no severe asthma cases included in the study.
Our study is the first study on the relationship between osteoprotegerin levels and airway inflammation in children with asthma. We believe that there is a requirement for wider scale studies in which clinical symptoms and more parameters are evaluated preferably with phlegm and/or BAL evaluations for defining the role played by osteoprotegerin in children with asthma and for determining its usability as a marker.
Trial registrationNot applicable.
Contributors’ statement pageNacaroglu, Hikmet Tekin: literature search, study design, data collection, manuscript preparation/editing, final manuscript approval
Büke Övgü: literature search, study design, data collection, manuscript preparation/editing, final manuscript approval
Bostan Gayret Ozlem: literature search, manuscript preparation/editing, data analysis
Erol Meltem: literature search, data collection, data analysis, data interpretation
Zengi Oguzhan: literature search, data collection, data analysis, data interpretation
Financial disclosureThe authors have no financial relationships relevant to this article to disclose.
Funding sourceNo external funding was secured for this study.
Conflict of interestThe authors have no conflicts of interest to disclose.
We thank Bagcilar training and research hospital. This study was reviewed and approved by the review board of Bagcilar training and research hospital.