There have been differences in temporal trends of asthma prevalence by geographic region and economic prosperity. The aim of this study was to assess temporal trends in asthma prevalence among young adolescents in Skopje, Republic of North Macedonia as a developing country with a low asthma prevalence.
Subjects and methodsData were obtained from three cross-sectional surveys (2002, 2006, and 2016) of adolescents (12–15 years) from randomly selected schools in Skopje. Trends in the prevalence of asthma and asthma-like symptoms were investigated descriptively and using multiple logistic regression to adjust for potential confounding factors.
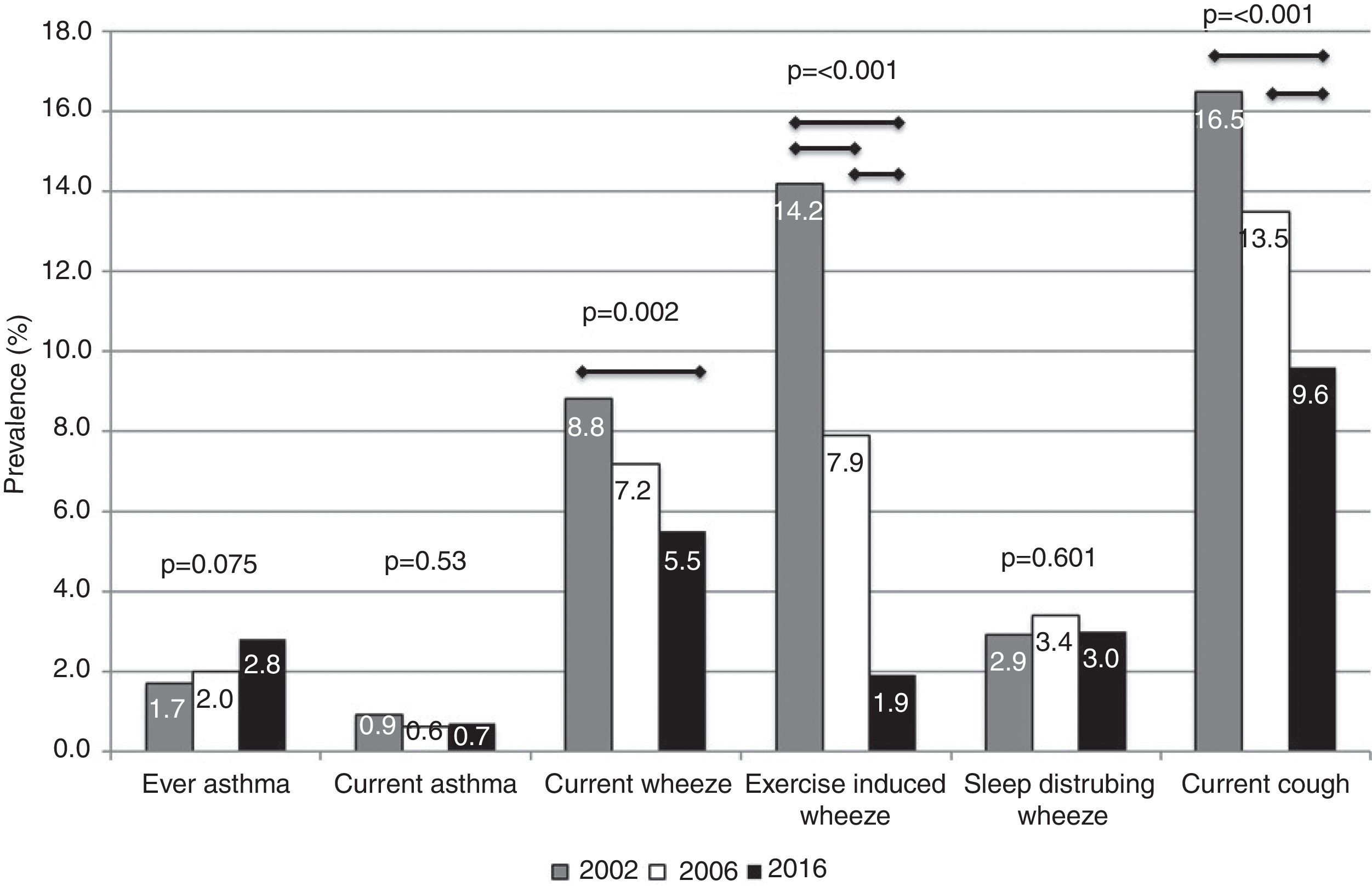
ResultsThe prevalence of asthma increased, although the changes were not statistically significant (2002: 1.7%; 2006: 2.0%; 2016: 2.8%; p=0.075). Statistically significant (p<0.05) reductions in wheeze prevalence over time (2002, 2006, 2016) were observed for current wheeze (8.8%, 7.2%, 5.5%), exercise-induced wheeze (14.2%, 7.9%, 1.9%), and night dry cough (16.5%, 13.5%, 9.6%). After adjustment for potential confounding factors, there was an increase in asthma likelihood by year compared to 2002 (2006: OR=1.22, 95%CI=0.67–2.22; 2016: OR=2.45, 95%CI=1.24–4.84). In the adjusted analyses, associations between year and the asthma-like symptoms confirmed the descriptive results, except for current wheeze, where statistical significance disappeared.
ConclusionsDivergent trends in prevalence with a decrease in asthma-like symptoms and an increase in physician-diagnosed asthma in Skopje during a period of 14 years were established. Improved asthma labelling and effective preventative treatment of symptoms may explain some of these changes, although changes in environment and lifestyle could not be ruled out.
Asthma is the most common chronic disease in childhood. A wide variation in its prevalence has been established worldwide with more prevalent symptoms in more affluent countries. ‘Western lifestyle’ has been suggested to be a factor responsible for a higher prevalence in some locations [1,2].
An increasing trend in asthma prevalence, over the second half of the 20th century, has been reported, especially in westernized societies [3–5]. There has been evidence that asthma prevalence has been declining or has plateaued in some of these countries from the 1990s, contrary to the developing countries where an increasing trend has been found [6,7]. However, in some westernized countries the asthma epidemic is not over in children and adults [8,9] or has shown divergent trends for asthma symptoms and physician-diagnosed asthma [10,11].
The reasons for these differences in the temporal trends of asthma are still not well known. Some research conducted to explain the temporal trends has included investigations into the differences in diagnostic labelling and awareness or management of symptoms, or changes in lifestyle or environmental factors (reduced exposure to microbes and parasites, increased sensitization to indoor allergens due to increased time spent indoors, changes in diet and micronutrient levels, decreasing physical activity, increased rate of preterm birth and caesarean section delivery, medication use by mother and infant, psychosocial environment, or changes in immunity-environment interaction). It is likely that multiple underlying factors with a small individual effect might be responsible for changes in asthma prevalence and incidence [12,13].
Compared to the worldwide asthma prevalence rates, North Macedonia has a moderately low prevalence of current wheeze [1] and a low prevalence of ever-diagnosed asthma [2], which suggest poor asthma labelling or under-diagnosis of the disease.
The aim of this study was to determine temporal trends in the prevalence of asthma-like symptoms and diagnosed asthma among young adolescents at three time points between 2002 and 2016 in Skopje, Republic of North Macedonia, as a developing country with a low asthma prevalence. An additional aim was to include some behavioural and environmental factors as confounding factors to examine their effect on temporal trends in asthma and symptom prevalence. This is the first asthma trends study in the country, which is important for future national health care planning.
Subjects and methodsStudy population and proceduresData for the analyses was obtained from three cross-sectional surveys (2001/2002, 2005/2006, and 2015/2016) of young adolescents aged 12–15 years in Skopje, the capital of the Republic of North Macedonia. Seventeen out of 55 schools in Skopje were randomly sampled in 2001/2002 to approach at least 3000 students as per ISAAC protocol. All 12–15-year-olds attending selected schools were eligible for participation. A random sample of those 17 schools were then approached in the subsequent study periods with an aim of 1000 students and 2000 students in the second and third surveys, respectively. Almost one third of the Macedonian population live in Skopje and no major changes have occurred in the population structure during the study period. All three surveys were performed during the same season from December to February (out of the pollen season), and with the same principle investigator.
The first survey was part of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3, and was performed in Skopje strictly following the ISAAC methodology with details already published previously [14,15]. In brief, the standardized ISAAC Phase 3 written questionnaires on asthma and environmental risk factors were self-completed in the student's classroom by 3026 adolescents 12–15 years of age after informed consent from parents was obtained. The response rate was 90.9%.
The second survey was at a national level and was carried out in eight cities of the Republic of North Macedonia, including Skopje. ISAAC Phase 3 written questionnaires were self-completed in class by 5507 adolescents aged 12–15 years after informed consent from parents was obtained. The selection of participants and data collection were performed according to the ISAAC Phase 3 protocol [14,15]. The response rate was 92%. Out of all respondents, data obtained by 1088 adolescents from Skopje were included in this temporal trend analyses.
The third survey was a part of an International study about geographic variation related to asthma diagnosis, prevalence, and severity in which Canada, Poland, Belarus, Ukraine, Republic of Georgia (Adjara), and the Republic of North Macedonia all took part [16]. In North Macedonia the survey was conducted in Skopje and included 2310 children aged 5–15 years. The study used a standardized questionnaire, which was based on questionnaires including the ISAAC questionnaire, American Thoracic Society Children's Respiratory Disease questionnaire [17] and questionnaires used previously in Canadian lung studies [18–20]. The questionnaire was translated into Macedonian and then back-translated into English by two independent translators familiar with the topic and the area, the same ones previously included in the translation of the ISAAC Phase 3 questionnaires. The translated questionnaires were distributed through the schools to parents for completion. They were then returned to the school and collected by the research team. Written consent was required for each completed survey to be included in the study. The response rate was 38.5%. Out of all respondents, the data obtained from 690 adolescents aged 12–15 years was included in this temporal trend analyses.
Analyses focused on the same key outcomes and were based on the same questions used in the three surveys i.e. on the change in 12-month prevalence of wheeze, sleep-disturbing wheeze, exercise-induced wheeze, dry night cough apart from a chest infection, and diagnosed asthma ‘ever’, as they were defined elsewhere [1,14,16]. In addition, we considered current asthma, which was defined by a positive response to the question about a doctor's diagnosis of asthma as well as a positive response to wheeze in the past 12 months. With regard to the use of inhaled corticosteroids (ICS), information was available only for the 2006 and 2016 surveys. In the 2006 survey, the ICS use was defined according to the answer to the question “Have you ever received a breathing medication such as Becomethasone dipropionate, Fluticasone propionate, Budesonide?” while in 2016 the question was based on “Is this child taking breathing medications and if so, please list what they are.” If they had a medicine type listed and it was an ICS medication, the variable was created indicating ICS use.
Gender, age, and the following environmental factors that could have changed over time, as they were defined before [21], were used as potential confounders in adjusted analyses. Current fruit, vegetables, meat, milk, eggs, and fast food intake was categorized into moderate to high (1–2 times weekly and ≥3 times weekly) and low (never/occasionally) categories. Body mass index (BMI) of the respondents was categorized into high (overweight/obesity) and normal/low BMI for gender and age according to the international reference values [22]. Current Paracetamol use was classified as frequent (at least once monthly) and infrequent (at least once yearly/never). Mother's educational level (tertiary and/or secondary vs. primary category) was used as a familiar socioeconomic status indicator. Additionally, passive smoking exposure at home and trucks passage on the street of residence on weekdays were included as confounders to indicate indoor and outdoor air-pollution, while current cat and dog ownership were also included as confounders as indicators of allergen exposure at home. They were all assessed on the basis of yes-no responses. TV-watching/PC playing time (>1h daily vs. less than 1h daily) was included as a confounder as an indicator of sedentary lifestyle.
Ethical approvalThe conducting of the three surveys was approved by The Ethics Committee at the Medical Faculty, University “St. Cyril and Methodius” and The Ministry of Education and Science, Skopje, The Republic of North Macedonia.
Statistical analysesAnalysis was competed using SPSS 25. Descriptive analyses were completed and included comparisons of characteristics of the study population by year. Statistical comparisons were made using the linear trend test to look at overall change over time as well as the chi-squared test with a Bonferonni correction to compare results between years. Descriptive analyses and statistical comparisons looking at the prevalence of diagnosed asthma and asthma-like symptoms were conducted in the same way. For the condition rates, missing or any “other” responses were considered as part of the denominator for the calculation of prevalence by assigning a zero value, which was not the case with respect to potential confounding factors [23].
The association between study year and asthma and asthma-like symptoms was tested using multiple logistic regression analyses to adjust for potential confounders. Potential confounders included age, gender, current Paracetamol use, current cat ownership, dog ownership, trucks passing through the residential street, passive smoking at home, TV watching/PC playing time per day, mother's education level, overweight/obesity, and current dietary variables. These variables were chosen based on their relationships with asthma in the literature and because they were consistently available in each of the three data sets. The strength of association was assessed by the odds ratios (OR) and 95% confidence intervals (CI).
Because the response rate in 2016 was notably lower than in 2002 and 2006, we completed a sensitivity analysis to see if differences in the response rate could modify the observed associations. Sensitivity analyses are an approach to test the robustness of results when there are assumptions or unknowns [24,25]. This was completed by assigning the potential prevalence of a condition to the number of non-participating children then calculating a corrected prevalence for the full 2016 population. We suspect that children were less likely to participate if they did not have a condition [26]. In addition to this, we qualitatively compared ICS use in 2006 to 2016.
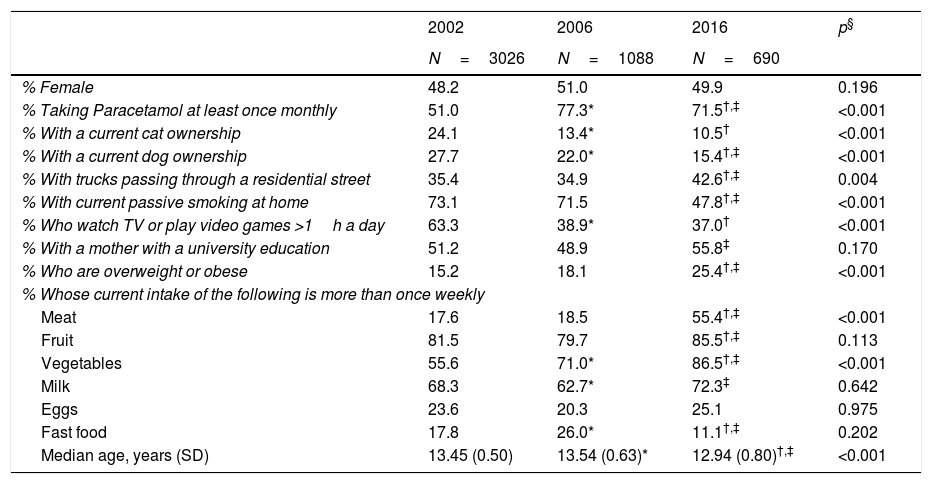
ResultsThere were several differences among the three study years with regard to the personal characteristics of those who took part (Table 1). While the gender distribution was similar across years, there were statistically significant differences in age with the oldest group in 2006 (median age: 13.54 years) and the youngest group in 2016 (median age: 12.94 years). While these differences were statistically significant, the age range included in the study population was the same (12–15 years) and the clinical significance of the difference of approximately 7 months can be questioned. In addition to this, there was a higher proportion of children taking Paracetamol at least once monthly in the later years; there was a decline in cat ownership, dog ownership, passive smoking in the home, and sedentary behaviour over time; a higher proportion with trucks passing in the street, mother having a university education, and being overweight or obese in 2016. Finally, there were also differences in dietary patterns between the 3 years.
Characteristics of the study population by year of study.
| 2002 | 2006 | 2016 | p§ | |
|---|---|---|---|---|
| N=3026 | N=1088 | N=690 | ||
| % Female | 48.2 | 51.0 | 49.9 | 0.196 |
| % Taking Paracetamol at least once monthly | 51.0 | 77.3* | 71.5†,‡ | <0.001 |
| % With a current cat ownership | 24.1 | 13.4* | 10.5† | <0.001 |
| % With a current dog ownership | 27.7 | 22.0* | 15.4†,‡ | <0.001 |
| % With trucks passing through a residential street | 35.4 | 34.9 | 42.6†,‡ | 0.004 |
| % With current passive smoking at home | 73.1 | 71.5 | 47.8†,‡ | <0.001 |
| % Who watch TV or play video games >1h a day | 63.3 | 38.9* | 37.0† | <0.001 |
| % With a mother with a university education | 51.2 | 48.9 | 55.8‡ | 0.170 |
| % Who are overweight or obese | 15.2 | 18.1 | 25.4†,‡ | <0.001 |
| % Whose current intake of the following is more than once weekly | ||||
| Meat | 17.6 | 18.5 | 55.4†,‡ | <0.001 |
| Fruit | 81.5 | 79.7 | 85.5†,‡ | 0.113 |
| Vegetables | 55.6 | 71.0* | 86.5†,‡ | <0.001 |
| Milk | 68.3 | 62.7* | 72.3‡ | 0.642 |
| Eggs | 23.6 | 20.3 | 25.1 | 0.975 |
| Fast food | 17.8 | 26.0* | 11.1†,‡ | 0.202 |
| Median age, years (SD) | 13.45 (0.50) | 13.54 (0.63)* | 12.94 (0.80)†,‡ | <0.001 |
* p<0.05 comparing 2006 to 2002 after application of a Bonferonni correction.
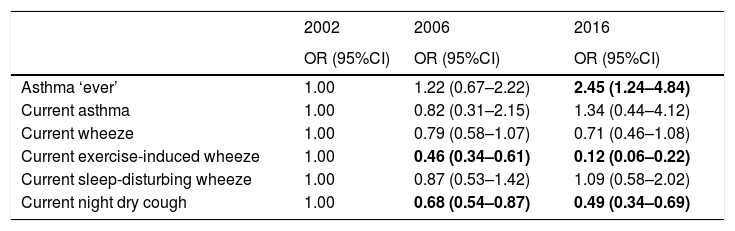
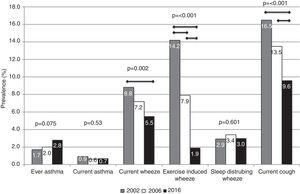
The prevalence of ever asthma increased over time from 1.7% to 2.8%, although the trend was of borderline statistical significance (p=0.075; Fig. 1). There were statistically significant decreases in the prevalence of asthma-like symptoms over time (current wheeze, current exercise-induced wheeze, current night dry cough, Figure 1). In addition to this, the ratio of current wheeze to ever asthma decreased over time (5.2:1 in 2002; 3.6:1 in 2006; 2.0:1 in 2016). There were no differences in current asthma or current wheeze disturbing sleep among the 3 years. After adjusting for potential confounders, asthma prevalence was higher in 2016 (p<0.05) compared to 2002 and current exercise-induced wheeze and current night dry cough both showed statistically significant inverse associations over time (Table 2).
Adjusted associations between year and respiratory outcome.
| 2002 | 2006 | 2016 | |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Asthma ‘ever’ | 1.00 | 1.22 (0.67–2.22) | 2.45 (1.24–4.84) |
| Current asthma | 1.00 | 0.82 (0.31–2.15) | 1.34 (0.44–4.12) |
| Current wheeze | 1.00 | 0.79 (0.58–1.07) | 0.71 (0.46–1.08) |
| Current exercise-induced wheeze | 1.00 | 0.46 (0.34–0.61) | 0.12 (0.06–0.22) |
| Current sleep-disturbing wheeze | 1.00 | 0.87 (0.53–1.42) | 1.09 (0.58–2.02) |
| Current night dry cough | 1.00 | 0.68 (0.54–0.87) | 0.49 (0.34–0.69) |
Current: in the last 12 months; CI: confidence interval; OR: odds ratio.
Adjusted OR for year of study, age, gender, current Paracetamol use, current cat ownership, current dog ownership, frequency of trucks passing, passive smoking at home, TV watching time, mother's education level, being overweight, meat and fish, fruit, vegetables, milk, eggs, and fast food intake.
Bold indicates a statistically significant association (p<0.05).
Because the response rate was lower in 2016 compared to 2002 and 2006, we conducted a sensitivity analysis to assess the impact of non-participation on the prevalence estimates. If we assumed that the prevalence of asthma in non-participants was 50% of the observed prevalence (i.e. 1.4%), the overall corrected prevalence of asthma would be 1.9%. If we assumed that the prevalence of asthma among non-participants was the same as it was in 2002 and 2006, when there were large response rates, the overall corrected asthma prevalence would be 2.1% and 2.3%, respectively. Because the prevalence of asthma-like symptoms tended to decrease over time and typically participants are more likely to have a condition over non-participants, we only considered a 50% prevalence of a condition among non-participants when looking at asthma-like symptoms. In these scenarios, the overall corrected prevalence in 2016 would be 3.8% (current wheeze), 1.3% (current exercise-induced wheeze), 2.1% (current sleep-disturbing wheeze), and 6.6% (current cough). Each of these situations would make the prevalence of the condition lower in 2016, strengthening the observed patterns over time.
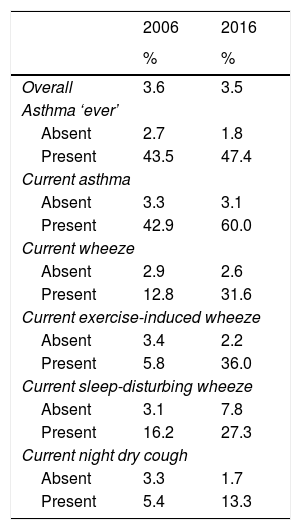
Using information available from 2006 and 2016 regarding breathing medication use, we found that overall there was a negligible difference in the proportion of children using ICS (3.6% vs. 3.5%; Table 3). However, when comparing ICS use among those with a condition, a higher proportion of children with a condition used ICS in 2016 compared to 2006, with very large differences among those with asthma symptoms.
Proportion of children using inhaled corticosteroids by study year.
| 2006 | 2016 | |
|---|---|---|
| % | % | |
| Overall | 3.6 | 3.5 |
| Asthma ‘ever’ | ||
| Absent | 2.7 | 1.8 |
| Present | 43.5 | 47.4 |
| Current asthma | ||
| Absent | 3.3 | 3.1 |
| Present | 42.9 | 60.0 |
| Current wheeze | ||
| Absent | 2.9 | 2.6 |
| Present | 12.8 | 31.6 |
| Current exercise-induced wheeze | ||
| Absent | 3.4 | 2.2 |
| Present | 5.8 | 36.0 |
| Current sleep-disturbing wheeze | ||
| Absent | 3.1 | 7.8 |
| Present | 16.2 | 27.3 |
| Current night dry cough | ||
| Absent | 3.3 | 1.7 |
| Present | 5.4 | 13.3 |
Current: in the last 12 months.
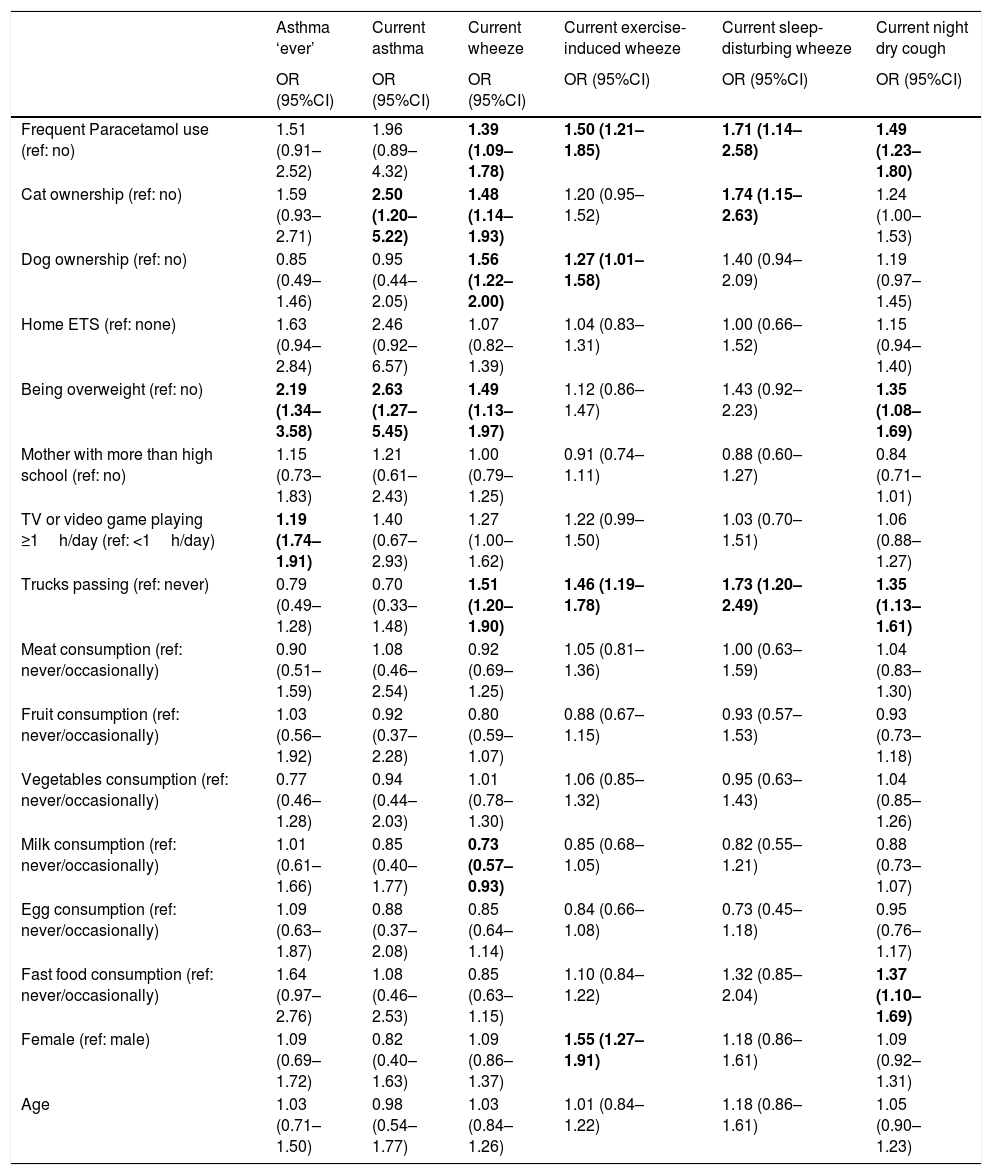
Finally, we investigated the association between various personal, environmental, and behavioural characteristics with the conditions of interest after adjustment for potential confounders (Table 4). Paracetamol use and trucks passing in residential streets were consistently associated with an increased risk of each asthma symptom. Aside from this, being overweight and more sedentary behaviour was associated with an increased risk of asthma; cat ownership and being overweight were associated with an increased risk of current asthma; cat ownership, dog ownership, and being overweight were associated with an increased risk of current wheeze while higher milk consumption was associated with less likelihood of current wheeze; dog ownerships and being female were associated with a higher likelihood of exercise-induced wheeze; cat ownership increased the risk of sleep-disturbing wheeze; and being overweight and fast food consumption increased the risk of current night dry cough.
Adjusteda associations between personal, environmental, and behavioural variables with each of the outcomes.
| Asthma ‘ever’ | Current asthma | Current wheeze | Current exercise-induced wheeze | Current sleep-disturbing wheeze | Current night dry cough | |
|---|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Frequent Paracetamol use (ref: no) | 1.51 (0.91–2.52) | 1.96 (0.89–4.32) | 1.39 (1.09–1.78) | 1.50 (1.21–1.85) | 1.71 (1.14–2.58) | 1.49 (1.23–1.80) |
| Cat ownership (ref: no) | 1.59 (0.93–2.71) | 2.50 (1.20–5.22) | 1.48 (1.14–1.93) | 1.20 (0.95–1.52) | 1.74 (1.15–2.63) | 1.24 (1.00–1.53) |
| Dog ownership (ref: no) | 0.85 (0.49–1.46) | 0.95 (0.44–2.05) | 1.56 (1.22–2.00) | 1.27 (1.01–1.58) | 1.40 (0.94–2.09) | 1.19 (0.97–1.45) |
| Home ETS (ref: none) | 1.63 (0.94–2.84) | 2.46 (0.92–6.57) | 1.07 (0.82–1.39) | 1.04 (0.83–1.31) | 1.00 (0.66–1.52) | 1.15 (0.94–1.40) |
| Being overweight (ref: no) | 2.19 (1.34–3.58) | 2.63 (1.27–5.45) | 1.49 (1.13–1.97) | 1.12 (0.86–1.47) | 1.43 (0.92–2.23) | 1.35 (1.08–1.69) |
| Mother with more than high school (ref: no) | 1.15 (0.73–1.83) | 1.21 (0.61–2.43) | 1.00 (0.79–1.25) | 0.91 (0.74–1.11) | 0.88 (0.60–1.27) | 0.84 (0.71–1.01) |
| TV or video game playing ≥1h/day (ref: <1h/day) | 1.19 (1.74–1.91) | 1.40 (0.67–2.93) | 1.27 (1.00–1.62) | 1.22 (0.99–1.50) | 1.03 (0.70–1.51) | 1.06 (0.88–1.27) |
| Trucks passing (ref: never) | 0.79 (0.49–1.28) | 0.70 (0.33–1.48) | 1.51 (1.20–1.90) | 1.46 (1.19–1.78) | 1.73 (1.20–2.49) | 1.35 (1.13–1.61) |
| Meat consumption (ref: never/occasionally) | 0.90 (0.51–1.59) | 1.08 (0.46–2.54) | 0.92 (0.69–1.25) | 1.05 (0.81–1.36) | 1.00 (0.63–1.59) | 1.04 (0.83–1.30) |
| Fruit consumption (ref: never/occasionally) | 1.03 (0.56–1.92) | 0.92 (0.37–2.28) | 0.80 (0.59–1.07) | 0.88 (0.67–1.15) | 0.93 (0.57–1.53) | 0.93 (0.73–1.18) |
| Vegetables consumption (ref: never/occasionally) | 0.77 (0.46–1.28) | 0.94 (0.44–2.03) | 1.01 (0.78–1.30) | 1.06 (0.85–1.32) | 0.95 (0.63–1.43) | 1.04 (0.85–1.26) |
| Milk consumption (ref: never/occasionally) | 1.01 (0.61–1.66) | 0.85 (0.40–1.77) | 0.73 (0.57–0.93) | 0.85 (0.68–1.05) | 0.82 (0.55–1.21) | 0.88 (0.73–1.07) |
| Egg consumption (ref: never/occasionally) | 1.09 (0.63–1.87) | 0.88 (0.37–2.08) | 0.85 (0.64–1.14) | 0.84 (0.66–1.08) | 0.73 (0.45–1.18) | 0.95 (0.76–1.17) |
| Fast food consumption (ref: never/occasionally) | 1.64 (0.97–2.76) | 1.08 (0.46–2.53) | 0.85 (0.63–1.15) | 1.10 (0.84–1.22) | 1.32 (0.85–2.04) | 1.37 (1.10–1.69) |
| Female (ref: male) | 1.09 (0.69–1.72) | 0.82 (0.40–1.63) | 1.09 (0.86–1.37) | 1.55 (1.27–1.91) | 1.18 (0.86–1.61) | 1.09 (0.92–1.31) |
| Age | 1.03 (0.71–1.50) | 0.98 (0.54–1.77) | 1.03 (0.84–1.26) | 1.01 (0.84–1.22) | 1.18 (0.86–1.61) | 1.05 (0.90–1.23) |
Current: in the last 12 months; CI: confidence interval; ETS: environmental tobacco smoke; OR: odds ratio.
Adjusted OR for year of study, age, gender, current Paracetamol use, current cat and dog ownership, frequency of trucks passing, passive smoking at home, TV watching time, mother's education level, being overweight, meat and fish, fruit, vegetables, milk, eggs, and fast food intake.
Bold indicates a statistically significant association (p<0.05).
In general, the prevalence rates of all asthma-like symptoms were found to be significantly higher compared to those of ever-diagnosed asthma in the three surveys, and the overall prevalence rates of ICS use in both surveys, 2006 and 2016, were higher compared to those of ever-diagnosed asthma, which suggest a high likelihood of asthma under-diagnosis in young adolescents in North Macedonia. This is further supported by the established high current wheeze/ever-diagnosed asthma ratios by year as in 12–15-year-olds current wheeze is rarely due to conditions other than asthma. In an international comparison about asthma, wheeze, and breathing medication use, it has been documented that only 14.9% of 5–15-year-old children with a history of current wheeze in North Macedonia had a diagnosis of asthma and that 73.6% of current wheezers reported current breathing medication use [16]. Similarly, in Ukraine and Belarus 19.6% and 8.1%, respectively of those who currently wheezed had an asthma diagnosis in contrast to Canada and Poland where 65.8% and 61.4%, respectively of current wheezers were diagnosed as asthma [16]. These suggest that besides asthma under-diagnosis, Macedonian children may be labelled with another condition such as recurrent obstructive bronchitis and treated with breathing medications, similar to children in some other countries of Central and Eastern Europe [27] and Turkey [28]. In our country doctors are reticent to formally diagnose asthma without lung function evidence and patients do not readily accept and use this diagnosis.
We have found a consistent trend of a decline in current asthma symptoms and a trend of an increase in ever physician-diagnosed asthma, although of borderline statistical significance, over a 14-year period. Regarding ICS use, although there was no difference in overall prevalence of use in 2016 vs. 2006, among those with ever asthma and with asthma-like symptoms their use was higher in 2016. The ICS use among those without a condition was relatively consistent and, except for sleep-disturbing wheeze, was lower in 2016. This explains why there was no difference in overall ICS use. With a large number of children in the condition absent groups, the overall proportion of ICS use would be heavily weighted to the proportion of use in those groups as opposed to the condition present groups and patterns overall would reflect the condition absent group.
Our findings have followed the same pattern as in Belarus children (7–15 years) over a 5-year period (2009–2014) [16,29]. Additionally, similar low prevalence of current wheeze and diagnosed asthma, as well under-diagnosis of asthma have been documented in Belarus, as in the present study. Divergent asthma trends in the present study were also in line with those reported for Brazilian young adolescents, although their prevalence of asthma symptoms and diagnosed asthma was much higher [30]. The authors, as the most likely explanation for the paradoxical increase in the prevalence of labelled asthma, have supposed that it was due to a greater knowledge on the disease through asthma guidelines and the asthma treatment availability with a greater control of the disease. Over a period of 20 years Kälvesten et al. [10] also have reported divergent trends for wheeze or three or more asthma-like symptoms and physician-diagnosed asthma in Swedish children, suggesting an increased community awareness of asthma and changed diagnostic habit.
In contrast to the findings in the present study, in a 25-year general-population study with a large age range from childhood to the elderly, still increasing trends of current asthma attacks and asthma diagnosis have been documented in Central Italy [9]. The authors have assumed that this might be due to cumulative exposures to various risk factors over a long time period. Parallel increasing trends in the prevalence of asthma-like symptoms and physician-diagnosed asthma in four time points have also been found in Polish children [31], suggesting a real increase in the occurrence of asthma. Other international studies from developed or developing countries in children or general population have confirmed this rising asthma trend observed in Europe [32–34].
Contrary to the present study, no further increase in the parent-reported asthma prevalence, which was quite stable and more frequent in boys, for the period from 2004 to 2012 has been reported in Bavarian preschool children [35]. We did not analyze temporal trends in our male and female adolescents separately due to their very low prevalence of asthma. However, gender and age were included in the analyses as confounding factors. A stabilization of wheeze and asthma prevalence between 2001 and 2010 has also been observed in Dutch children, after a decline in wheeze prevalence between 1989 and 2001 [36]. On the other hand, some studies in western populations have reported a downward trend in asthma incidence in children [37] or doctor-diagnosed current asthma prevalence in children and adults under 45 years of age [38]. The decline in current asthma prevalence in the latter study has been associated with increases in ICS prescriptions, add-on asthma therapies and treatment of exacerbations in primary care. As potential explanations for the findings, a more aggressive management of the disease within primary care and better controller treatments, as well as a possible reticence of giving a formal asthma diagnosis until a certain level of disease severity in the UK, in the investigated period (2006–2016) have been suggested [38].
We believe that our results of divergent asthma trends could be due, in part, to an increased awareness and improved diagnostic shift from recurrent obstructive bronchitis to asthma as a result of the introduction of international and national guidelines on asthma. This is a reason to suspect that a true increase in asthma is based on a higher proportion of children with asthma using ICS in 2016 vs. 2006. The much higher ICS use for asthma-like symptoms in comparison to that of ever asthma in 2016 vs. 2006 could mean that the asthma labelling is still poor and that the diagnosis of recurrent obstructive bronchitis is still employed and treated with ICS with subsequent reductions in asthma-like symptoms. Besides the improved diagnostic shift, change over time in the counterbalance of protective and risk environmental and behavioural factors could not be ruled out as a reason for the increased asthma prevalence.
There are several limitations regarding the present study that need to be considered. Because this study was not initially designed to be an investigation of temporal tends, the third survey was not identical to the previous two surveys. This included differences in questionnaires. In the 2001/2002 and 2005/2006 studies, we did not have information on parental history of asthma, dampness at home, or breastfeeding history so we were unable to control for these important confounders. Therefore, it is possible that some of the associations observed could be due to residual confounding. However, all the key outcomes were the same, as they were based on the ISAAC questionnaire, except for the definition of ICS use. Regarding the data collection, the initial two surveys were self-completed while the third survey was parent completed. There is evidence that respiratory symptoms have been accurately reported by adolescents and strongly agree with parental reports regarding asthma diagnosis [39]. Additionally, the response rate in 2016 was low, which could bias the results with an overestimated asthma prevalence. However, based on the sensitivity analysis, the prevalence in 2016 was consistently higher than in 2002 regardless of which imputed value we used. Thus, while the magnitude of difference might vary slightly, there was still a trend showing an increase in asthma prevalence. This was even true when we compared 2016 to 2006 – except for the 50% imputed prevalence, the other corrected prevalence estimates were still higher than the 2006 estimate. Although these were not statistically compared, patterns could be observed. While there might be a slight overestimate (by up to 0.9% – i.e. 2.8–1.9%) this was not large and our trends were, for the most part, consistent.
This is the first investigation of temporal trends in asthma prevalence from the Republic of North Macedonia, as a developing country with a low prevalence of asthma. There are a limited number of published asthma trends studies from developing countries, especially in regard of three time-points and with adjustment for potential behavioural and environmental protective/risk factors.
ConclusionsA decreasing trend in asthma-like symptoms contrary to an increasing trend in ever-diagnosed asthma in young adolescents in Skopje during a period of 14 years was established, which support the international evidence of asthma increases in developing countries. Improved diagnostic labelling and effective treatment with inhaled steroids in children may explain some of these changes, although changes in environment and lifestyle could not be ruled out and should be further investigated. Efforts should be made to reduce under-diagnosis of asthma and to improve asthma labelling in our country.
FundingThis work was financially supported by The Ministry of Science and Education of the Republic of North Macedonia to conduct the first survey, GlaxoSmithKline North Macedonia to conduct the second survey, and the University of Saskatchewan and Saskatchewan Health Research Foundation, Canada to conduct the third survey.
Conflict of interestThe authors have no conflict of interest to declare.
We are grateful to Lidija Sechkova, Rozalinda Isjanovska, Angel Sazdovski, Ilija Kirovski, Arben Iseni, Vesna Micevska and Tara Ristevska for their participation in data collection and data entry in the data set related to the surveys that have given rise to this article.