Despite the recommendation against routine use of inhaled bronchodilators in infants with viral bronchiolitis given in the main clinical practice guidelines (CPGs) on viral bronchiolitis, albuterol is widely prescribed to patients with this disease. The aim of this study was to identify predictors of prescription of albuterol in a population of infants hospitalized for viral bronchiolitis.
Material and methodsAn analytical cross-sectional study performed during the period from March 2014 to August 2015, in a random sample of patients <2 years old hospitalized in the Fundacion Hospital La Misericordia, a hospital located in Bogota, Colombia. After reviewing the electronic medical records, we collected demographic, clinical, and disease-related information, including prescription of albuterol at any time during the course of hospitalization as the outcome variable.
ResultsFor a total of 1365 study participants, 1042 (76.3%) were prescribed with albuterol therapy. After controlling for potential confounders, it was found that age (OR 1.11; CI 95% 1.08–1.15; p<0.001), and a prolonged length of stay (LOS) (OR 1.93; CI 95% 1.44–2.60; p<0.001) were independent predictors of prescription of albuterol in our sample of patients. By contrast, albuterol prescription was less likely in the post-guideline assessment period (OR 0.41; CI 95% 0.31–0.54; p<0.001), and in infants with RSV isolation (OR 0.71; CI 95% 0.52–0.97; p=0.035).
ConclusionsAlbuterol was highly prescribed in our population of inpatients with the disease. The independent predictors of prescription of albuterol in our sample of patients were age, implementation of a CPG on viral bronchiolitis, RSV isolation, and LOS.
Viral bronchiolitis is the most important cause of lower respiratory tract infection in children during the first 2 years of life and is the leading cause of hospitalization among infants younger than 1 year.1 In addition to the significant clinical burden of viral bronchiolitis on patients,2 the disease is usually associated with substantial direct and indirect costs, not only for healthcare systems but also for families and society as a whole,3 mainly in middle income countries (MICs).4
For decades, it has been a well-established position that bronchiolitis treatment is mostly supportive, focusing only on observation, hydration, and oxygen supplementation. Although prior evidence-based medicine (EBM) clinical practice guidelines (CPGs), such as the 2006 American Academy of Pediatrics (AAP) bronchiolitis CPGs, recommended the use of bronchodilators on a trial basis,5 the latest EBM-based CPGs on viral bronchiolitis no longer recommend a trial of bronchodilators.6,7 The most commonly argued reasons for this new recommendation are the greater strength of the evidence demonstrating no benefit in the bronchiolitis population as a whole and that there is no well-established way to determine an “objective method of response”.6 However, there is increasing evidence showing that this recent new recommendation is not being followed by a large number of pediatric care providers, suggesting that treatment recommendations from recent CPGs have not yet had a major impact on all physicians’ behavior, not necessarily due to a lack of awareness of these guidelines.8 Specifically, there are reports showing rates of use of bronchodilators ranging between 18% and 90%,9–11 with substantial differences between different countries and even between different hospitals in the same country.12 Although at first glance this use of bronchodilators might appear inadequate, it is important to take into consideration and highlight two important issues: first, although EBM has been considered the gold standard for clinical practice for many years, its limitations such as a lack of an individualized diagnosis and treatment approach of diseases through genetic, biomarker, phenotypic, and psychosocial characteristics (as with personalized medicine),13 have recently been recognized.14 Second, and related to the first, although substantial variation of bronchodilators use has been widely reported in the literature, there are few reports addressing patient characteristics associated with the prescription of albuterol and other bronchodilators in patients with viral bronchiolitis. A recent prospective, multicenter, multiyear study of infants hospitalized for bronchiolitis showed that in addition to local practice as a cause of variation in albuterol use, patient characteristics such as age, presence of wheezing, and previous use of a bronchodilator were independently associated with pre-admission albuterol use.9 Additionally atopic dermatitis, length of stay (LOS), and number of siblings were found to be independently associated with inadequate adherence to an EBM-based CPG on viral bronchiolitis (with a rate of use of bronchodilators of 89.7%).11 The identification of factors associated with the prescription of albuterol in infants with viral bronchiolitis could be useful for targeting future studies aimed at defining a possible subgroup of bronchodilator responders.9
Accordingly, the aim of this study is to identify predictors of prescription of albuterol in a population of infants hospitalized for viral bronchiolitis in a MIC, and to judge, if based on current knowledge this prescription of albuterol could be considered as potentially appropriate, or if otherwise, it was used indiscriminately without plausible logic behind it.
MethodsStudy design and proceduresAn analytical cross-sectional study was performed during the period from March 2014 to August 2015, in a random sample of patients <2 years old hospitalized in the Fundación Hospital La Misericordia with a diagnosis of viral bronchiolitis (ICD-10 codes J21, J21.0, J21.1, J21.8, and J21.9). The Fundacion Hospital La Misericordia is a tertiary care university-based children's hospital located in the metropolitan area of Bogota, the capital city of Colombia, a tropical MIC located in South America. The hospital serves the city of Bogota, as well as other cities of the country, functioning as a referral center. An institutional EBM-based CPG on viral bronchiolitis previously developed by a multidisciplinary team was implemented by means of individual and group standardized educational strategies in the period between February and March 2015. This CPG recommends against routine use of inhaled bronchodilators, but a carefully monitored trial of their use is an option (recommending to continue their administration only if there is a documented positive clinical response to the trial). Therefore, the implementation of the above-mentioned EBM-based CPG on viral bronchiolitis was included as one potential predictor of prescription of albuterol in the population analyzed.
Viral bronchiolitis was defined as the first acute episode of viral infection of the lower respiratory tract with wheezing preceded by a cold-like clinical picture of the upper respiratory tract, so we excluded patients with previous wheezing episodes. In our institution, patients with viral bronchiolitis who are sick enough to be admitted to the hospital are tested only for respiratory syncytial virus (RSV) using a rapid immuno chromatographic test Method (RSV Respi-Strip; Coris BioConcept, Gembloux, Belgium).
After reviewing the electronic medical records (EMRs) we collected the following demographic, clinical, and disease-related information in a standardized data collection form: month of the year in which patients were hospitalized, age, gender, assessment period (pre-guideline vs. post-guideline), presence of underlying disease conditions (prematurity, pre-existing respiratory conditions, bronchopulmonary dysplasia, congenital heart disease, and pulmonary hypertension), RSV isolation, LOS, personal history of atopic dermatitis, first-degree family history of asthma, prescription of albuterol (either as metered dose inhaler – MDI – or as nebulization), and if albuterol was prescribed as a therapeutic trial.
The study protocol was approved by the local ethics board.
Outcome definitionAs the primary outcome of interest, we defined prescription of albuterol as a categorical binary variable based on the prescription of albuterol either as MDI or as nebulization, at any time during the course of hospitalization.
Statistical analysisContinuous variables are presented as mean±standard deviation (SD) or median (interquartile range – IQR), whichever is appropriate. Categorical variables are presented as numbers (percentage). Differences in continuous variables between patients with prescription of albuterol or the lack thereof were analyzed using the unpaired t test or Wilcoxon's signed rank test, whichever was appropriate. Associations between categorical variables and the outcome variable were analyzed using Chi-square test or Fisher's exact test, whichever was appropriate. To identify factors independently associated with prescription of albuterol, we adjusted logistic regression models. Predictive variables that were considered to be able to influence prescription of albuterol, such as age, gender, assessment period (pre-guidelines vs. post-guidelines), hospitalization during the 3-month period from March to May (the first rainy season of the year in the city, with the greatest RSV circulation through the year), prolonged LOS (defined as at least one hospital stay of 5 or more days), RSV isolation, personal history of atopic dermatitis, and first-degree family history of asthma, were selected a priori for inclusion as predictor variables in multivariable models. The final model was chosen on the basis of those variables for which p≤0.05. Regression results are reported as ORs and their respective 95% CIs. All statistical tests were two-tailed, and the significance level used was p<0.05. The data were analyzed with the Statistical Package Stata 12.0 (Stata Corporation, College Station, TX, USA).
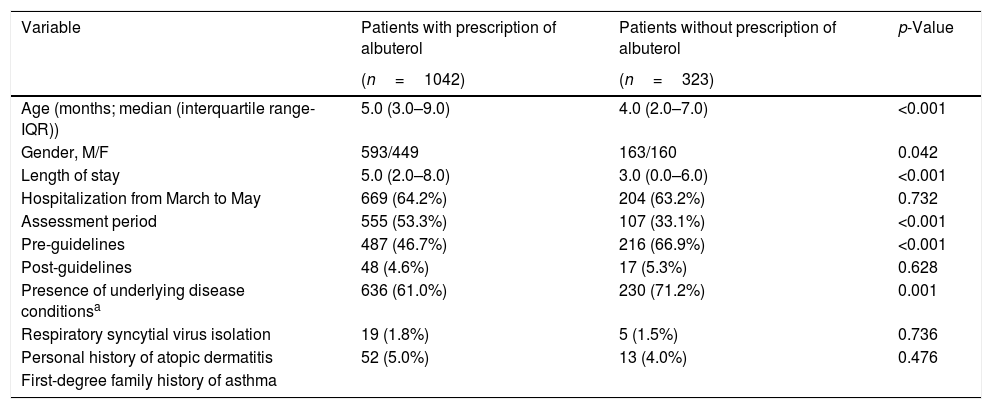
ResultsStudy populationA total of 1365 infants with a diagnosis of viral bronchiolitis were analyzed during the study period. Of the 1365 included patients, 756 (55.4%) were males, and the median (IQR) age was 5.0 (2.0–9.0) months. The age group distribution was 756 (55.4%) less than 6 months, 468 (34.3%) between 6 and 12 months, and the remaining 141 (10.3%) between 13 and 24 months. The demographic and clinical variables measured, according to the prescription or lack thereof of albuterol, are presented in Table 1.
Demographic characteristics and clinical information of the patients included in the study, according to the prescription of albuterol.
| Variable | Patients with prescription of albuterol | Patients without prescription of albuterol | p-Value |
|---|---|---|---|
| (n=1042) | (n=323) | ||
| Age (months; median (interquartile range-IQR)) | 5.0 (3.0–9.0) | 4.0 (2.0–7.0) | <0.001 |
| Gender, M/F | 593/449 | 163/160 | 0.042 |
| Length of stay | 5.0 (2.0–8.0) | 3.0 (0.0–6.0) | <0.001 |
| Hospitalization from March to May | 669 (64.2%) | 204 (63.2%) | 0.732 |
| Assessment period | 555 (53.3%) | 107 (33.1%) | <0.001 |
| Pre-guidelines | 487 (46.7%) | 216 (66.9%) | <0.001 |
| Post-guidelines | 48 (4.6%) | 17 (5.3%) | 0.628 |
| Presence of underlying disease conditionsa | 636 (61.0%) | 230 (71.2%) | 0.001 |
| Respiratory syncytial virus isolation | 19 (1.8%) | 5 (1.5%) | 0.736 |
| Personal history of atopic dermatitis | 52 (5.0%) | 13 (4.0%) | 0.476 |
| First-degree family history of asthma |
Overall, among the total of 1365 study participants, 1042 (76.3%) were prescribed with albuterol therapy. Remarkably, no patient who was prescribed albuterol was in a monitored trial. The rate of prescription of albuterol was significantly lower in the post-guideline assessment period when compared to the pre-guideline assessment period (46.7 vs. 53.3%, p<0.01).
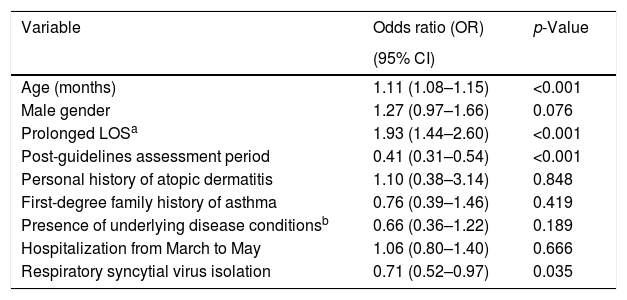
Predictors of prescription of albuterol determined through multivariate analysisMultivariate analyses were conducted to determine independent factors associated with prescription of albuterol among the patients analyzed. The predictor variables included in the multivariate models were age, gender, assessment period (pre-guidelines vs. post-guidelines), hospitalization during the 3-month period from March to May, presence of underlying disease conditions, prolonged LOS, RSV isolation, personal history of atopic dermatitis, and first-degree family history of asthma. After controlling for these potential confounders, it was found that age, and a prolonged LOS were independent predictors of prescription of albuterol in our sample of patients. By contrast, albuterol prescription was less likely in the post-guideline assessment period, and in infants with RSV isolation (Table 2).
Predictors for the prescription of albuterol in infants hospitalized for viral bronchiolitis determined through multivariate analysis.
| Variable | Odds ratio (OR) | p-Value |
|---|---|---|
| (95% CI) | ||
| Age (months) | 1.11 (1.08–1.15) | <0.001 |
| Male gender | 1.27 (0.97–1.66) | 0.076 |
| Prolonged LOSa | 1.93 (1.44–2.60) | <0.001 |
| Post-guidelines assessment period | 0.41 (0.31–0.54) | <0.001 |
| Personal history of atopic dermatitis | 1.10 (0.38–3.14) | 0.848 |
| First-degree family history of asthma | 0.76 (0.39–1.46) | 0.419 |
| Presence of underlying disease conditionsb | 0.66 (0.36–1.22) | 0.189 |
| Hospitalization from March to May | 1.06 (0.80–1.40) | 0.666 |
| Respiratory syncytial virus isolation | 0.71 (0.52–0.97) | 0.035 |
The present study shows that albuterol was highly prescribed in our population of inpatients with viral bronchiolitis, despite recommendations against its routine use in infants with the disease. While age and prolonged LOS were identified as independent predictors of prescription of albuterol, its prescription was less likely in the post-guideline assessment period and in infants with RSV isolation in our sample of patients.
The present findings could be a starting point for future studies aimed at improving our understanding of possible subgroups of bronchodilator responders among infant with viral bronchiolitis. This is because, based on current knowledge on immune responses and molecular immune signatures, the identified predictors of prescription of albuterol in infants with viral bronchiolitis are deemed as potentially appropriate, and they could help to guide future targeted randomized clinical trial (RCTs) and research of albuterol for bronchiolitis, making it increasingly possible to identify patients most likely to benefit from this therapy.
As expected, and in line with previous studies reported in the literature,15–18 implementation of an EBM-based CPG on viral bronchiolitis was associated with a significant reduction in the use of bronchodilators for patients with the disease. However, even after the implementation of the CPG, about two thirds of patients were still prescribed with albuterol, and in no patient was it prescribed in the form of a monitored trial, as is recommended in the CPG implemented.
In agreement with our findings, the age of patients itself has been shown to independently predict the prescription of albuterol in infants with viral bronchiolitis, with the older patients being more likely to be prescribed with this bronchodilator therapy.9 Likewise, our results align well with those obtained in previous studies in which the type of respiratory virus identified was associated with the appropriateness of the management of patients with viral bronchiolitis (with the use of albuterol included in the definition of appropriate management of the disease), with patients with RSV bronchiolitis being more likely to be treated in accordance with the recommendations given in a EBM-based CPG on viral bronchiolitis when compared with patients with non-RSV bronchiolitis.15 Our findings are also consistent with previous studies which have also identified prolonged LOS as an independent predictor of greater adherence to the recommendations given in a EBM-based CPG on viral bronchiolitis, that include a recommendation against the routine use of albuterol and other inhaled bronchodilators.11 Although the above-identified set of predictors could simply reflect an intuitive decision of pediatric providers to prescribe albuterol in patients with less typical clinical presentations of the disease, such as a small infant with a demonstrated infection by RSV which usually requires a short-term stay when hospitalization is necessary,19 some of them could be deemed as potentially appropriate based on current knowledge on immune responses and molecular immune signatures. Specifically, it has been reported that the age of presentation of bronchiolitis is linked to inflammatory (atopy-related) and virologic risk factors of asthma (rhinovirus (RV)-associated disease), with an greater occurrence of RV and prevalence of many atopic characteristics with increased age.20 The greater probability of RV infection with increased age is particularly relevant in patients with viral bronchiolitis because there is convincing evidence showing that bronchiolitis occurring during RV-predominant months has been associated with an estimated 25% increased risk of early childhood asthma compared with RSV-predominant months.21 Multi-omic analysis of nasopharyngeal airway samples shows pathobiological differences between RSV and RV bronchiolitis, with different mechanisms that involve a complex interplay between virus, microbiome, and host.22 RSV and RV infections have different nasal airway microRNA profiles associated with a nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) signaling.23 In addition, recent evidence shows that compared to infants with RSV bronchiolitis, those with RV bronchiolitis display a predominant Th2-oriented immune response, with significantly higher Th2 cells frequencies, Th2 index,24 and increased airway interferon lambda receptor 1 (IFNL1R) transcript levels.25 Although unfortunately we did not test for RV, it is probable that a significant proportion of patients with non-RSV bronchiolitis in our sample have had RV-bronchiolitis.26 For all the above-mentioned reasons, it is plausible to consider that, similar to our study, but contrary to what is recommended in the main EBM-based CPG on viral bronchiolitis, prescribing albuterol to older infants with non-RSV bronchiolitis (specifically RV-bronchiolitis), could be deemed as potentially appropriate, and therefore could be routinely recommended. However, it still must be demonstrated in future targeted RCTs.
We are aware that our research may have three limitations. First, viral diagnostic was focused only on RSV, and importantly in the light of the results of recent studies, we did not test for RV. Second, our results were derived from a single Center and limited only to hospitalized patients, so they could not be generalizable to all bronchiolitis patients. Finally, our results need to be interpreted with caution, because residual confounding cannot be excluded. However, we are confident that our results represent an excellent initial step toward improving our understanding of possible subgroups of bronchodilator responders among infant with viral bronchiolitis.
In conclusion, the findings of the present study show that despite the recommendation against routine use of inhaled bronchodilators in infants with viral bronchiolitis, albuterol was highly prescribed in our population of inpatients with the disease. Age, implementation of an EBM-based CPG on viral bronchiolitis, type of respiratory virus isolated, and LOS were independent predictors of prescription of albuterol in our sample of patients. Some of these predictors of prescription of albuterol are deemed as potentially appropriate and could be the starting point for future targeted RCTs on the use of albuterol among a subset of infants with bronchiolitis.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank Mr. James Donelly for his editorial assistance.