Methemoglobinemia has been reported to be associated with severe food protein-induced enterocolitis syndrome (FPIES). However, no reports have evaluated methemoglobin (MHb) levels in FPIES without symptomatic methemoglobinemia or the usefulness of MHb measurement for the diagnostic prediction of FPIES. To evaluate the MHb levels of patients with neonatal-onset FPIES and determine whether MHb levels are higher in FPIES than in other gastrointestinal diseases.
Patients and methodsEleven neonates with severe acute FPIES (FPIES group) and 139 neonates with other gastrointestinal diseases (non-FPIES group) were included in this study. Patient characteristics, symptoms, and venous blood test values (MHb, pH, HCO3−, and C-reactive protein) were evaluated.
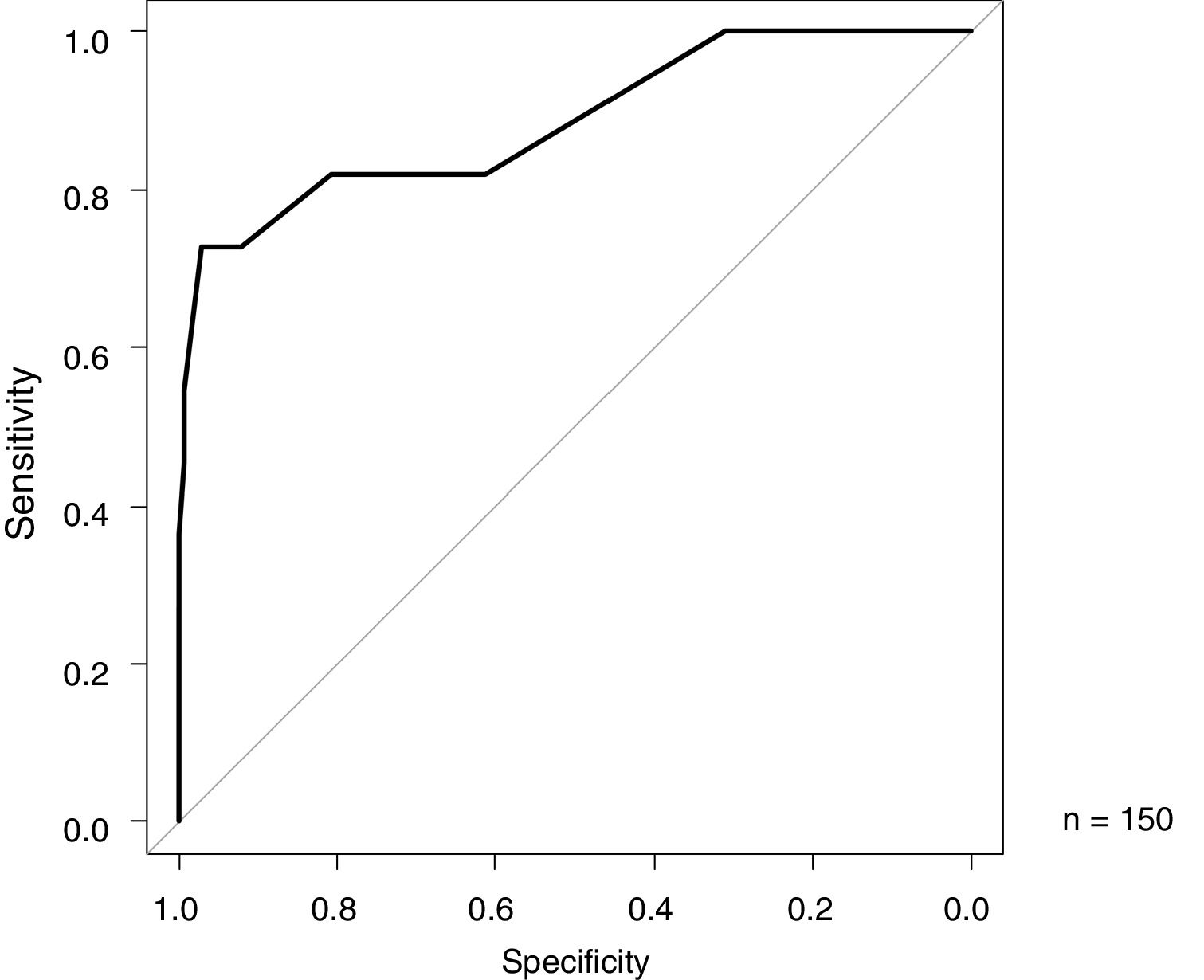
ResultsThe median age at onset was 16 days vs. 1 day; males comprised 64% vs. 46%, the median gestational age was 38 weeks vs. 38 weeks, the median birth weight was 2710g vs. 2880g, and the median hospitalization duration was 31 days vs. 6 days for the FPIES vs. non-FPIES groups, respectively. MHb (%) was higher in the FPIES group than in the non-FPIES group [median (range), 1.1 (0.6–10.9) and 0.6 (0.3–1.2), respectively, p<0.001]. There were no differences in terms of pH, HCO3−, and C-reactive protein (p>0.05). In the receiver operating characteristic analysis for FPIES diagnosis based on MHb (%), the area under the curve was 0.885, specificity was 97.1%, and sensitivity was 72.7% at a MHb cutoff of 1.0.
ConclusionHigh MHb levels may help diagnose severe acute FPIES in neonates, but careful evaluation is needed.
Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE-mediated allergic disease accompanied by only gastrointestinal symptoms, such as vomiting and diarrhea.1,2 FPIES is diagnosed based on the presence of symptoms fulfilling the diagnostic criteria or positive results of the oral food challenge test.2 There have been several reports of methemoglobinemia in severe FPIES cases.3–6 However, there are no reports evaluating the methemoglobin (MHb) levels patients with FPIES without symptomatic methemoglobinemia. If patients with FPIES show a tendency toward increased MHb levels, then MHb measurements could be useful for the diagnostic prediction of FPIES.
If the MHb levels of patients with FPIES are significantly higher than those of patients with other diseases, this may be useful for the differential diagnosis of FPIES. In this study, we retrospectively evaluated the MHb levels of patients with neonatal-onset FPIES, and we examined whether MHb levels were higher in FPIES than in other gastrointestinal diseases and neonatal transient emesis.
Patients and methodsPatientsThis study included 150 neonates admitted to the neonatal intensive care unit (NICU) and pediatric ward of our hospital with the chief complaint of vomiting from April 2010 to November 2018 (onset in premature infants at less than one month corrected age). Eleven neonates were diagnosed with FPIES (FPIES group), 139 neonates were diagnosed with other gastrointestinal diseases (non-FPIES group). Patients with symptoms that fulfilled the diagnostic criteria2 or patients who showed a positive result on an oral food challenge2 performed at a later date were diagnosed as having FPIES. This study was approved by the Ethics Committee of Jichi Medical University Saitama Medical Center (Approval No. S19-049), and the opt-out consent form was published on the home page of our clinical section. The need to obtain written informed consent was waived due to the retrospective design of this study.
Patients’ characteristicsData on sex, gestational age, birth weight, age at onset, duration of hospitalization, existence of bloody stools, vital signs when vomiting occurred, final diagnosis, and family history of methemoglobinemia were collected. The age at which diagnosis was confirmed in FPIES cases was also assessed.
Venous blood measurementsVenous blood was measured using ABL800FLEX® (Radiometer GmbH, Denmark). MHb (%) was measured using the spectrophotometric method (modified Malloy–Evelyn method).7 MHb (%) levels were measured simultaneously by blood gas analyses. We evaluated the MHb peak values (%) from onset to 48h later.
To evaluate metabolic acidosis and the inflammatory response, the lowest pH value, minimum bicarbonate (HCO3−) level, and peak C-reactive protein (CRP) level were evaluated from onset to 48h later.
Statistical analysisPatient characteristics, vital signs at onset (body temperature, heart rate, respiratory rate, blood pressure, and peripheral artery oxygen saturation), and blood test data were evaluated retrospectively, and the FPIES and non-FPIES groups were compared.
Results are expressed as medians (ranges). For statistical comparisons between the FPIES group and the non-FPIES group, we used the Mann–Whitney U test for continuous variables and Fisher's exact test for categorical variables.
For MHb levels, a receiver operating characteristic (ROC) analysis was performed with the differentiation between FPIES and other diseases as the index; the area under the curve (AUC) was also evaluated, and sensitivity and specificity at the cut-off value were determined.
All statistical analyses were performed using EZR 1.33 software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).8
ResultsPatientsFPIES groupEleven patients met the diagnostic criteria for acute FPIES based on the International Consensus Guidelines 20172 (FPIES group). Of the 11 cases, 11 had frequent vomiting, seven had bloody stools, four had diarrhea, 11 had lethargy, four had pallor, three had dehydration, and five had acidemia. The time between antigen intake and onset was one hour in 8 cases, 1.5h in one case, and two hours in two cases. All patients met the symptom criteria of severe FPIES. All 11 patients in the FPIES group were suffering from FPIES due to cow's milk protein (cow's milk formula) and did not display symptomatic methemoglobinemia.
As cow's milk was the cause of FPIES, the patients were preferentially fed breast milk and therapeutic formula milk if needed. Nine patients were fed the extensively hydrolyzed formula [New MA-1® or MA-mi® (Morinaga Milk Industry Co., Ltd. Japan)], and two patients received amino acid-based formula [Elental P® (EA Pharma Co., Ltd., Japan)]. Five patients were diagnosed with FPIES by an oral food challenge approach. For the oral food challenge, a single dose ingestion of cow's milk formula was given. The dose of cow's milk formula was determined by the attending physician. The dose was 5ml in two cases, 20ml in one case, 30ml in one case and 80ml in one case. Symptoms were frequent vomiting, lethargy, and pallor in all five patients. The time from ingestion to symptoms was one hour in four cases and two hours in one case.
In all patients with suspected FPIES due to the ingestion of cow's milk during the neonatal period, avoidance of the use of cow's milk was started. However, the median age at a definitive FPIES diagnosis was five months (range 0–15).
Non-FPIES groupThe 36/139 cases in the non-FPIES group included 15 cases of neonatal melena, five cases of Hirschsprung disease (among them, one case was combined with congestive enteritis), four cases of midgut volvulus, three cases of esophageal atresia, two cases of hypertrophic pyloric stenosis, two cases of duodenal stenosis, and one case each of duodenal atresia, ileal atresia, gastric perforation, gastric volvulus, and necrotizing enterocolitis.
The 103/139 patients in the non-FPIES group underwent a detailed examination to determine the cause of vomiting (blood test, chest–abdominal radiography, head, heart, and abdominal ultrasonography), but the test results were normal. For the patients, small amounts of enteral nutrition (oral feeding or tube feeding) were started after fasting for several hours to one day. The feeding amount was gradually increased, and the patients remained hospitalized until weight gain was confirmed. Because the symptoms gradually improved, the cause of vomiting was considered transient and to be attributed to immaturity in the neonatal period.
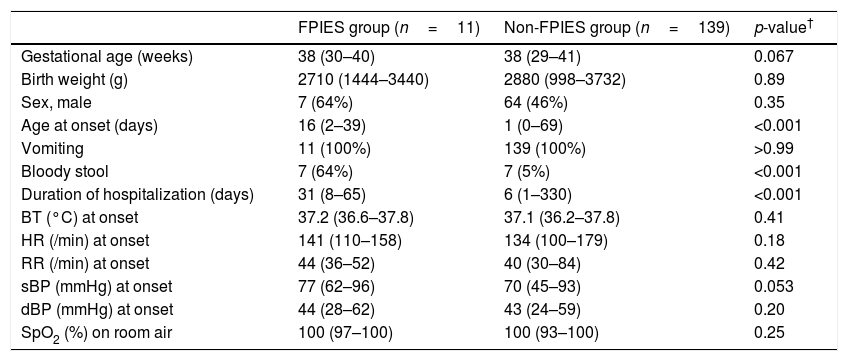
Patient characteristicsPatient characteristics and vital signs at onset in the FPIES group and non-FPIES group are shown in Table 1. The median age at onset was 16 and one days; percentage of males was 64 and 46%; median gestational age was 38 and 38 weeks; median birth weight was 2710 and 2880g; and median duration of hospitalization was 31 and six days for the FPIES group and non-FPIES group, respectively. The age of onset was higher (p<0.001) and the duration of hospitalization was longer (p<0.001) in the FPIES group than in the non-FPIES group; no differences were found between the FPIES and non-FPIES groups for other items (p>0.05). There were also no differences between the FPIES group and the non-FPIES group in terms of vital signs upon the appearance of vomiting (p>0.05). None of the patients had a positive family history of methemoglobinemia or were diagnosed with hemolytic diseases, which can present in combination with methemoglobinemia.
Characteristics and vital signs in FPIES and non-FPIES groups.
| FPIES group (n=11) | Non-FPIES group (n=139) | p-value† | |
|---|---|---|---|
| Gestational age (weeks) | 38 (30–40) | 38 (29–41) | 0.067 |
| Birth weight (g) | 2710 (1444–3440) | 2880 (998–3732) | 0.89 |
| Sex, male | 7 (64%) | 64 (46%) | 0.35 |
| Age at onset (days) | 16 (2–39) | 1 (0–69) | <0.001 |
| Vomiting | 11 (100%) | 139 (100%) | >0.99 |
| Bloody stool | 7 (64%) | 7 (5%) | <0.001 |
| Duration of hospitalization (days) | 31 (8–65) | 6 (1–330) | <0.001 |
| BT (°C) at onset | 37.2 (36.6–37.8) | 37.1 (36.2–37.8) | 0.41 |
| HR (/min) at onset | 141 (110–158) | 134 (100–179) | 0.18 |
| RR (/min) at onset | 44 (36–52) | 40 (30–84) | 0.42 |
| sBP (mmHg) at onset | 77 (62–96) | 70 (45–93) | 0.053 |
| dBP (mmHg) at onset | 44 (28–62) | 43 (24–59) | 0.20 |
| SpO2 (%) on room air | 100 (97–100) | 100 (93–100) | 0.25 |
Data are presented as median (range) or n (%).
Represents the statistical significance between the FPIES group and the non-FPIES group. Differences were evaluated using the Mann–Whitney test for continuous variables or the Fisher's exact test for categorical variables.
BT: body temperature; dBP: diastolic blood pressure; FPIES: food protein-induced enterocolitis syndrome; HR: heart rate; RR: respiratory rate; sBP: systolic blood pressure; SpO2: oxygen saturation of peripheral artery.
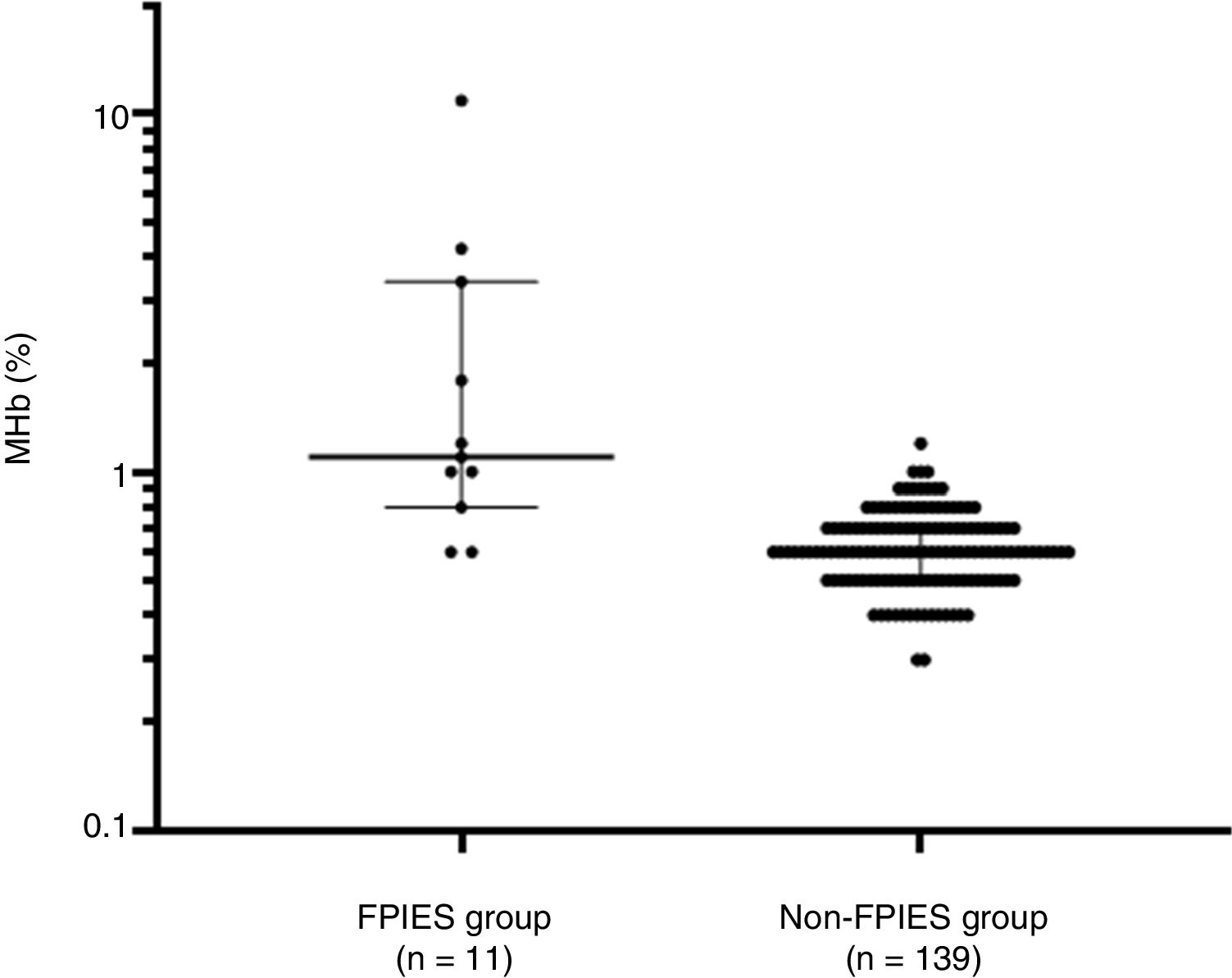
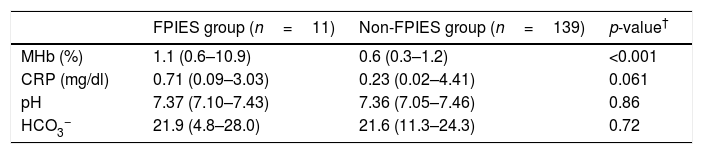
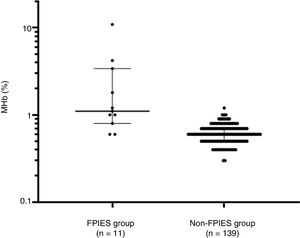
Laboratory test results of the FPIES group and non-FPIES group are shown in Table 2. The median (range) of MHb (%) was 1.1 (0.6–10.9) and 0.6 (0.3–1.2); that of CRP (mg/dl) was 0.71 (0.09–3.03) and 0.23 (0.02–4.41); that of pH was 7.37 (7.10–7.43) and 7.36 (7.05–7.46); and that of HCO3- was 21.9 (4.8–28.0) and 21.6 (11.3–24.3) for the FPIES group and non-FPIES group, respectively. MHb levels were higher in the FPIES group than in the non-FPIES group (p<0.001) (Fig. 1). With regard to CRP, pH, and HCO3-, no differences were found between the FPIES group and the non-FPIES group (p>0.05).
Laboratory findings in FPIES and non-FPIES groups.
| FPIES group (n=11) | Non-FPIES group (n=139) | p-value† | |
|---|---|---|---|
| MHb (%) | 1.1 (0.6–10.9) | 0.6 (0.3–1.2) | <0.001 |
| CRP (mg/dl) | 0.71 (0.09–3.03) | 0.23 (0.02–4.41) | 0.061 |
| pH | 7.37 (7.10–7.43) | 7.36 (7.05–7.46) | 0.86 |
| HCO3− | 21.9 (4.8–28.0) | 21.6 (11.3–24.3) | 0.72 |
Data are presented as median (range).
Comparison of methemoglobin (%) between the food protein-induced enterocolitis syndrome (FPIES group) and other gastrointestinal diseases (non-FPIES group). Median and interquartile range are indicated by horizontal lines. Differences between the two groups were evaluated using the Mann–Whitney test.
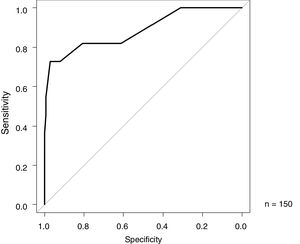
The ROC analysis results of MHb (%), for the differentiation between FPIES and other diseases as the index, are shown in Fig. 2. The AUC was 0.885 (95% CI 0.758–1), with a specificity of 97.1% and sensitivity of 72.7% at a cutoff value of 1.0 (Youden index) (Fig. 2).
DiscussionThis investigation is the first study to evaluate the MHb levels of neonates with FPIES without symptomatic methemoglobinemia. FPIES is a non-IgE-mediated allergic disease that presents with only gastrointestinal symptoms, such as vomiting and diarrhea. There have been several reports on the presence of methemoglobinemia in severe cases of FPIES.3–6
Normal hemoglobin carries divalent iron; when oxidized, it changes into MHb, which carries trivalent iron. The latter lacks oxygen transport function. Increased blood MHb concentration is termed methemoglobinemia. Although MHb is found under normal conditions, it is usually maintained at less than 1%.9 When MHb levels are less than 10–15%, it is usually asymptomatic. However, a higher percentage is associated with hypoxemia, and tachycardia, fatigue, confusion, and tachypnea are associated with levels above 30%; convulsion and lethargy are associated with levels above 50%; and death is associated with levels above 70%.10
This study was conducted with the hypothesis that MHb levels in FPIES cases are increased even in the absence of symptoms due to methemoglobinemia. As a result, the MHb levels in the neonatal period were higher in the FPIES group than in the non-FPIES group.
There are two different mechanisms for the pathogenesis of acquired methemoglobinemia. According to the first mechanism, methemoglobinemia is due to sepsis and/or significant acidosis; when the general health condition is poor, a large amount of nitric oxide (NO) is released into the blood, and hemoglobin is oxidized by NO, causing an increase in MHb concentration.10–12 According to the second mechanism, NO3−, which is increased in the intestinal tract due to enteritis, is restored to NO2− by intestinal anaerobes, NO2− is transported to the intestinal epithelia, hemoglobin is oxidized by NO2− flowing into the blood, and the MHb concentration increases. In other words, the balance of enzymatic activities of oxidase in the intestinal epithelia and intestinal bacterial flora changes, resulting in increased nitrate and subsequent MHb oxidation. This mechanism is considered to result in intestinal methemoglobinemia; there are also reports on the presence of transient methemoglobinemia in patients with enteritis.13,14 In our study, the FPIES group had significantly higher MHb levels than the non-FPIES group, but there was no significant difference in pH value and HCO3− level. This suggests that, in the FPIES patients in our study, intestinal methemoglobinemia is more important than acidosis as a mechanism by which MHb increases.
MHb levels tend to increase during the neonatal period due to the following reasons. First, the pH of gastric fluid is less acidic and allows for the proliferation of bacteria, such as Escherichia coli, which restore nitrate. Second, MHb reductase activity of neonates is low, at approximately 60% of that of adults. Third, the level of HbF, which is more easily oxidized than HbA, is high.10,15 Based on these factors, mild pathological changes may be sharply reflected by the MHb level during the neonatal period. It has also been reported that 13–18% of patients less than two months of age with FPIES have methemoglobinemia.4,5 Therefore, in neonates with FPIES, it would be beneficial to monitor any increase in MHb level.
The diagnosis of FPIES is made if the major criterion (vomiting in the 1- to 4-h period after ingestion of the suspected food combined with the absence of classic IgE-mediated allergic skin or respiratory symptoms) and more than three minor criteria are confirmed.1,2 If the symptoms and causative antigen are unclear, an oral food challenge is recommended. However, there is the risk that the oral food challenge may provoke severe symptoms.2 Although FPIES cannot be diagnosed based on test values other than an oral food challenge, a high probability of FPIES could be predicted in advance, based on test values, and the risk of symptom provocation due to an oral food challenge could be estimated. In the case of a high risk of symptom provocation, the risk of this being severe can be minimized by setting a low antigen load in the oral food challenge.
Between 2% and 20% of FPIES cases are atypical, where a specific IgE of causative antigens is present during follow-up.2 Typical cases are IgE negative, and it is difficult to diagnose FPIES by IgE measurements. Whereas neutrophil left shift, eosinophilia, and metabolic acidosis were confirmed in studies that evaluated blood test data from patients with FPIES, there have been no studies evaluating their utility for the diagnosis of FPIES.4,6,16 In addition, although the usefulness of the κ-casein lymphocyte stimulation test for the diagnosis of FPIES has been reported, it is time-consuming and requires a specialized technique and equipment.17 However, MHb levels can be examined in real time using common measuring instruments.
In our study, patients with high MHb levels were found to be those with severe FPIES. However, it is necessary to exclude causes other than FPIES that cause MHb. Also, the ROC analysis showed high specificity but not high sensitivity; therefore, low MHb values could not exclude FPIES. In particular, in the case of patients with mild or moderate FPIES, MHb may be more difficult to increase. That is to say that high MHb levels may help diagnose severe acute FPIES if other causative diseases are ruled out, but careful evaluation is needed.
LimitationsOur study has several limitations. First, there were no cases of infectious gastroenteritis in the non-FPIES group. It has been previously reported that infectious enteritis causes methemoglobinemia in infants.13,14 However, this study focused on neonates, and infectious gastroenteritis is extremely rare among patients in the NICU. In addition, MHb levels were not elevated in one case of necrotizing enterocolitis and one case of Hirschsprung disease combined with congestive enteritis in the non-FPIES group. Thus, it is speculated that FPIES is a disease that is often associated with MHb elevation compared to other causes of enterocolitis.
Second, patients were not tested for congenital methemoglobinemia. Even though reductase activity measurement and genetic screening were not performed, none of the patients had a family history of methemoglobinemia, and it was confirmed that the minimum MHb of all patients was approximately 0–1% during hospitalization.
Third, this study is a retrospective investigation, and the period and frequency of MHb measurement were not determined. However, as MHb levels in this study were measured at the same time during each blood gas analysis, at the frequency of more than once daily, the overall trend could be recognized.
Fourth, our study evaluated only severe acute FPIES. Since there were no patients with mild or moderate acute FPIES or chronic FPIES, future research on the evaluation of MHb levels in these cases is desired.
In conclusion, high MHb levels could help the clinician diagnose FPIES in a very select group of patients (those with neonatal severe FPIES) once the other potential causes of high MHb levels have been excluded.
In the future, larger-scale studies, as well as the investigation into the pathogenesis of elevated MHb in FPIES, are warranted.
Unblinded ethical approval statementThis study was approved by the Ethics Committee of Jichi Medical University Saitama Medical Center (Approval No. S19-049), and the opt-out consent form was published on the home page of our clinical section. The need to obtain written informed consent was waived due to the retrospective design of this study.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone declared.
We thank all the pediatricians and nurses at Saitama Medical Center Jichi Medical University. We would like to thank Editage (www.editage.jp) for English-language editing.