Although food allergy is recognized as a growing worldwide public health problem, there continues to be limited data on prevalence rates in developing and emerging countries. Most prevalence estimates are based on self-reports, with only few studies using objective assessments. The aim was to analyze the frequency of sensitization to food allergens by serum specific IgE in a large group of unselected allergic patients in Mexico.
Materials and methodsWe analyzed data registries from patients of all ages with suspected food allergy referred to a specialized laboratory in Mexico City from January 2016 to April 2018. A descriptive analysis, and an age/food-group comparison were made.
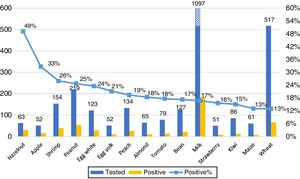
ResultsA total of 2633 subjects tested for food allergy were identified during the study period; 1795 subjects fulfilled the inclusion criteria. The overall positivity (sIgE≥0.35kUA/L) to at least one food was 24%. The most frequently positive foods were hazelnut, apple, shrimp, peanut, egg white, egg yolk, peach, almond, tomato, bean, milk, strawberry, kiwi, maize and wheat. Positivity for some foods was more frequent across different age groups, in young children (≤5 years) milk; in older children (6–17 years): peanut, almond, wheat, soy and maize; in adults: apple. We also found other foods with high positivity but less than 50 samples: rye 60%, mango 42.9%, carrot 37.5%, cashew 27.3%, banana 21.1% and oat 20.6%.
ConclusionOur study reported the presence of a differential regional IgE sensitization pattern as compared with the internationally reported one, highlighting the importance of local staple foods.
Food allergy is an important public health problem that affects children and adults worldwide and its prevalence is increasing.1 More than 170 foods have been reported as triggers of food allergy, but a rather small group is responsible for 90% of the reports: cow's milk, egg, wheat, soy, peanut, tree nuts, fish, shellfish, and seeds.2 Variations exists according to different geographic areas, age groups, races and staple foods.3,4
In 2013, the World Allergy Organization estimated a prevalence in Europe of around 11–26 million people with food allergy, and these data projected on the world's population result in an estimated 220–520 million people potentially suffering from food allergy.5 The rise over the last decades is known as the second wave of the allergy epidemic.6
Although nowadays much is known about food allergy, there are serious knowledge gaps about prevalence rates. Even less evidence is available for developing countries. This is principally due to the wide range of manifestations of food allergy and the diverse methodologies and criteria used for the diagnosis in individual studies, with many of them based on self-reports.7–18
Mexico has a particular climate that influences staple foods, feeding practices and exposure to aeroallergens. This could result in a region-specific pollen-food syndrome, such as birch allergy in Europe being linked to oral allergy to apple, peach and hazelnut.19 This should be accounted for when interpreting serum specific IgE (sIgE) positivity. But also, a higher exposure to some allergens could make people more likely to have a true sensitization to foods not frequently found in other regions.20
In an attempt to have more uniform data about food allergy sensitization, surveys using a standardized method of serum specific IgE determination (ImmunoCAP), have been carried out before in developed countries.20 The measurement of sIgE using native allergen extracts continues to be the most frequently used, especially for initial or screening steps.21 For research purposes, most of the studies use ImmunoCAP-based assays, which use an improved technique, with quantitative results, lack of interference with IgG antibodies, high sensitivity and a wide linear assay range. Moreover, its widespread use enables comparison between studies.22
Our objective was to obtain an initial screening on food allergen sensitization patterns in a large, unselected group of allergic patients in Mexico.
Material and methodsThis is a cross-sectional, observational, descriptive and retrospective trial. We analyzed food allergen serologic testing (ImmunoCAP, Thermo Fisher) registries from a specialized laboratory in Mexico City (Laboratorio de Alergia Molecular). Patients of all gender/ages with suspected clinical allergy, referred by physicians for food allergen sIgE testing were included, as was all data on sIgE to individual foods collected between January 2016 and April 2018. Patients with missing demographic data or who were tested only for allergen mixtures were excluded.
SPSS was used for the analysis, descriptive statistics were calculated, a univariate analysis was conducted to study categorical variables, including demographic characteristics and test positivity. Three cut-off levels were used: sensitization ≥0.1kUA/L, positivity ≥0.35kUA/L, and moderate-high positivity ≥0.71kUA/L. Inter age-group and inter food-group comparisons were made, using non-parametric statistics, Fisher exact test, and Kruskal–Wallis test, respectively.
Ethical considerationsThe present study was conducted following the principles of the Declaration of Helsinki and the local Regulations of the General Law of Health in matter of health research, Title second, chapter I, Article 17, section I. The study did not require IRB approval, because we documented retrospective lab results using an anonymous database. No subjects’ identification data were provided by the laboratory to the researchers.
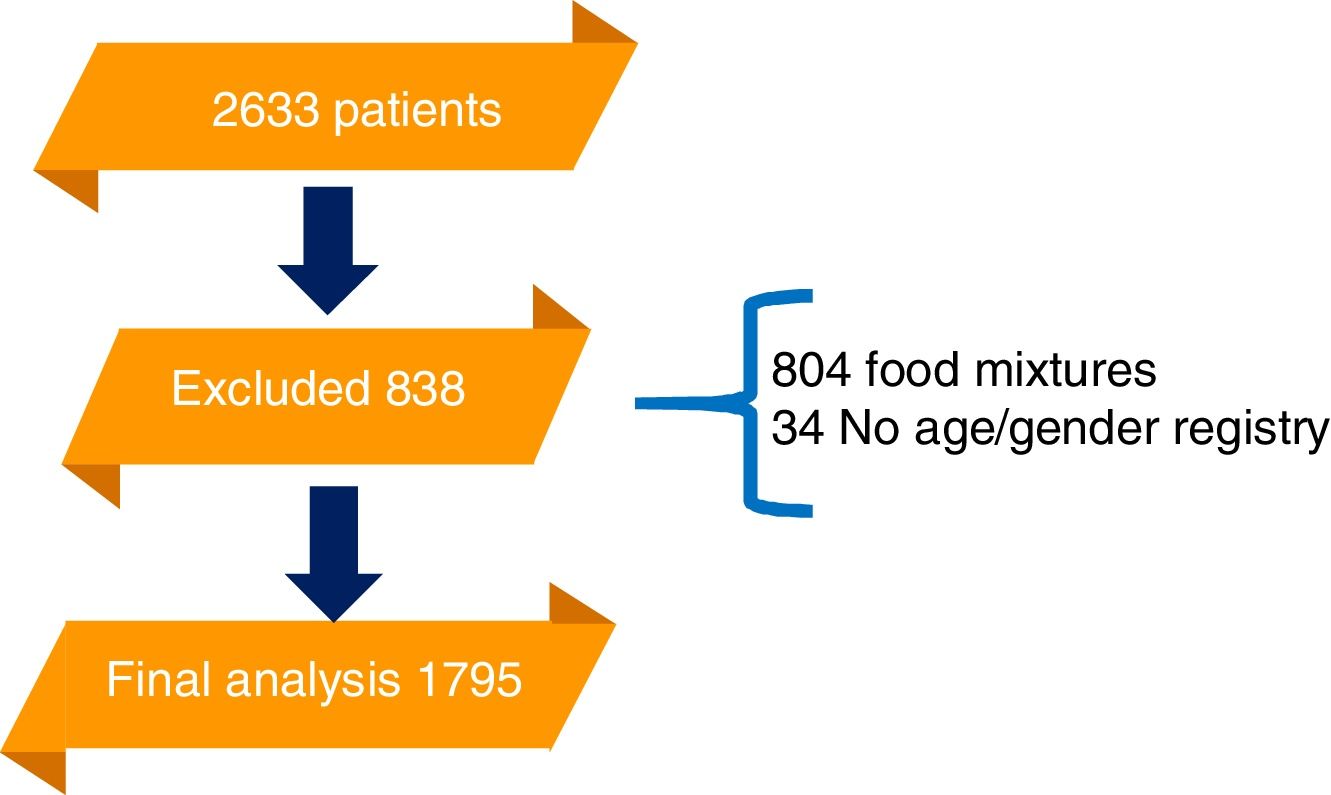
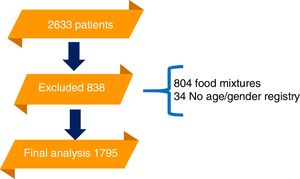
ResultsA total of 2633 patients were referred to the laboratory for food allergen serologic testing by ImmunoCAP during the time frame of the study. Of those, 838 had an exclusion criterium. The final analysis was conducted on 1795 samples. 764 patients were included from the first year, 790 from the second and 241 the last four months (Fig. 1).
Fifty-three percent (952) were male; 30.6% (550) were under two years of age, 31.3% (561) between 2 and 5 years, 17.4% (312) between 6 and 17 years and 20.7% (372) were eighteen or above.
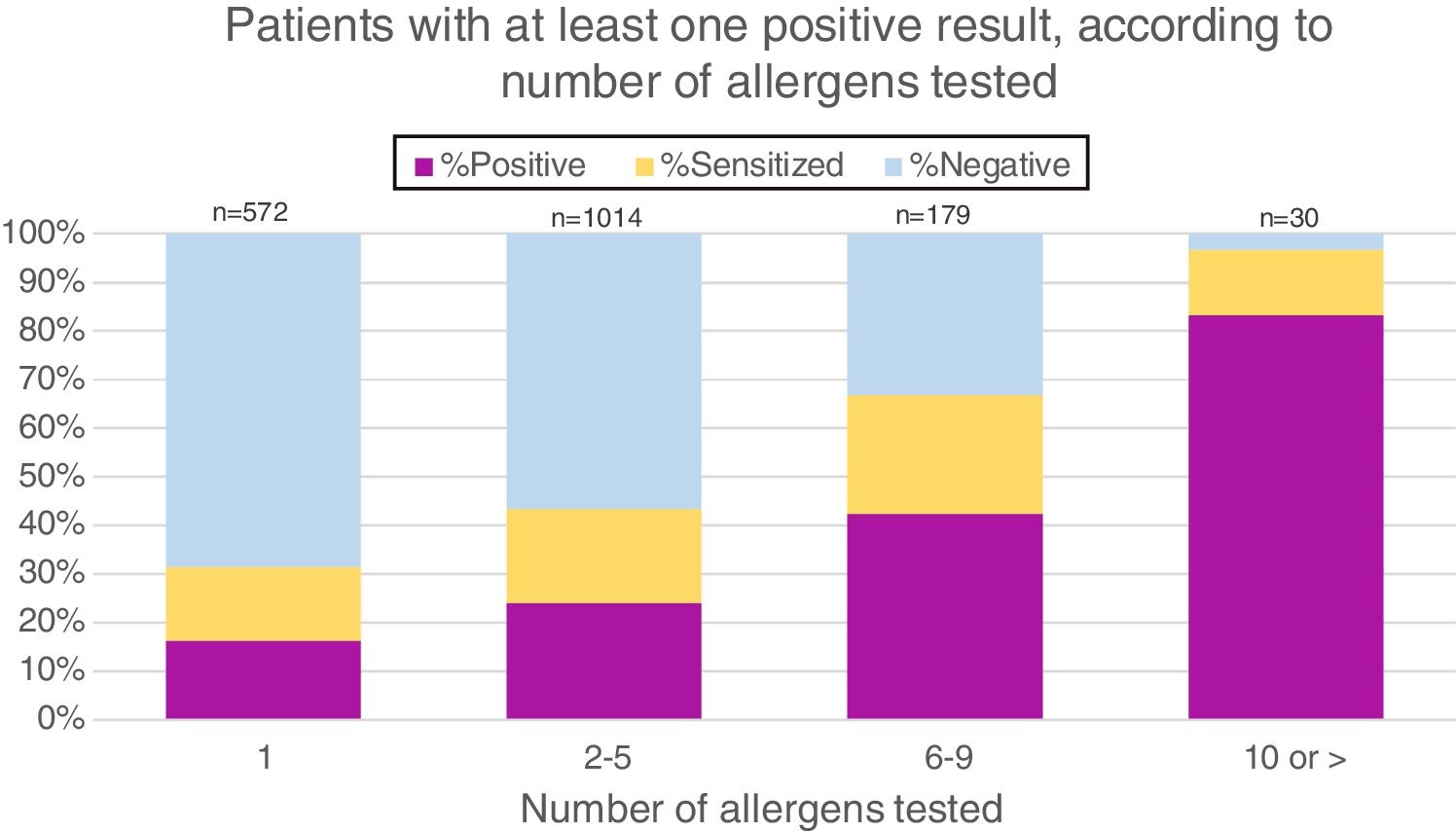
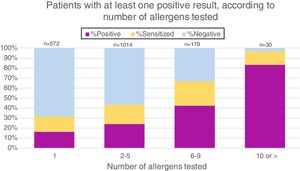
As patients with suspected food allergies were sent by individual doctors for sIgE testing, the number of allergens tested per patient varied widely. More than half (56.5%) were tested for 2–5 food extracts, 31.9% for only one extract, 9.9% for 6–9 extracts and 1.7% for 10 or more. Fig. 2 demonstrates that the likelihood of having at least one positive result increases with the number of allergens tested.
The overall sensitization (≥0.1kUA/L) to at least one food was 43%, ≥0.35kUA/L positivity to at least one food was 24%, and moderate-high positivity ≥0.71kUA/L was found in 17%.
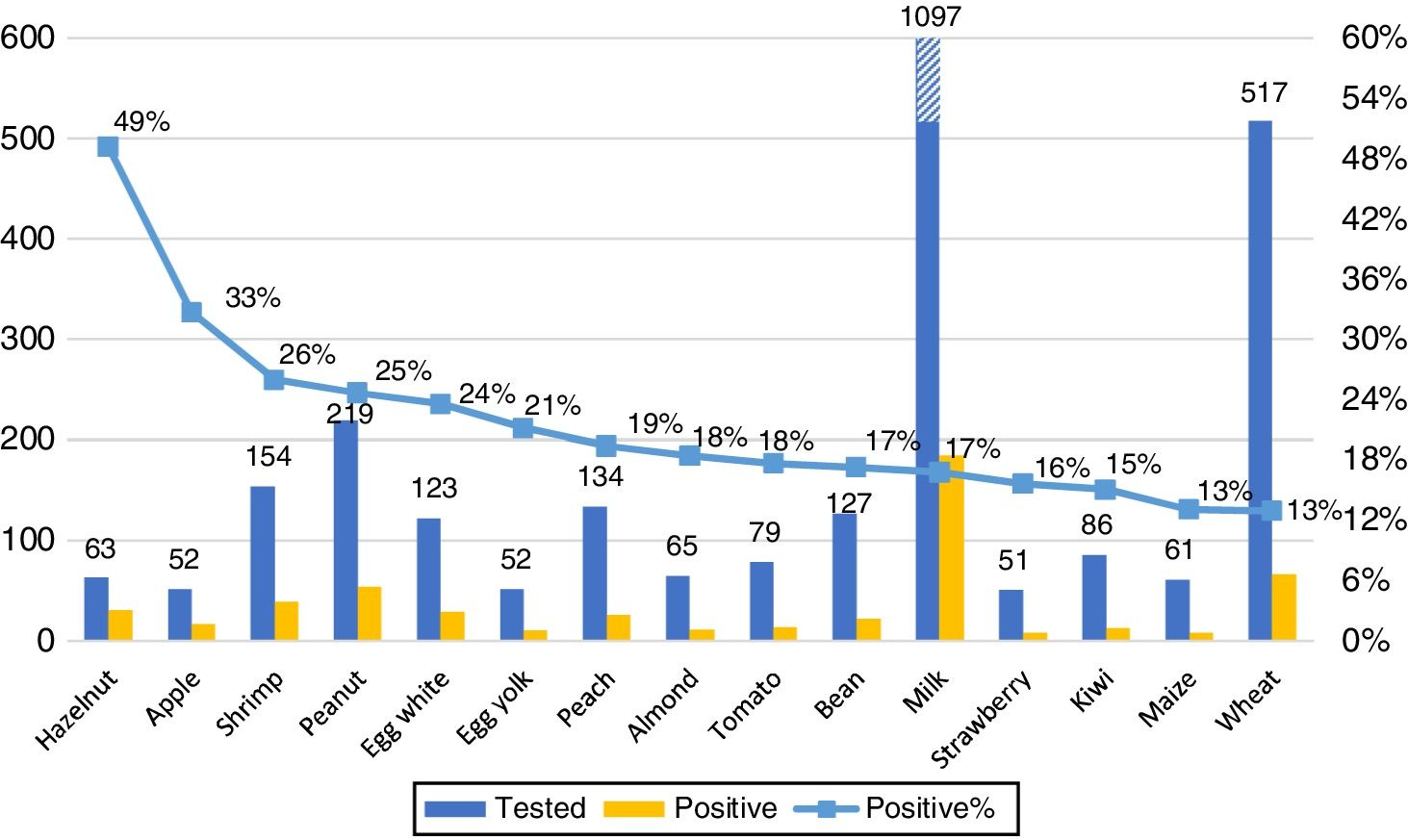
Our primary analysis was conducted on foods with at least 50 samples. The 15 foods with most frequent ImmunoCAP positivity are shown in Fig. 3. No gender differences in the frequency of positive results for any food were observed.
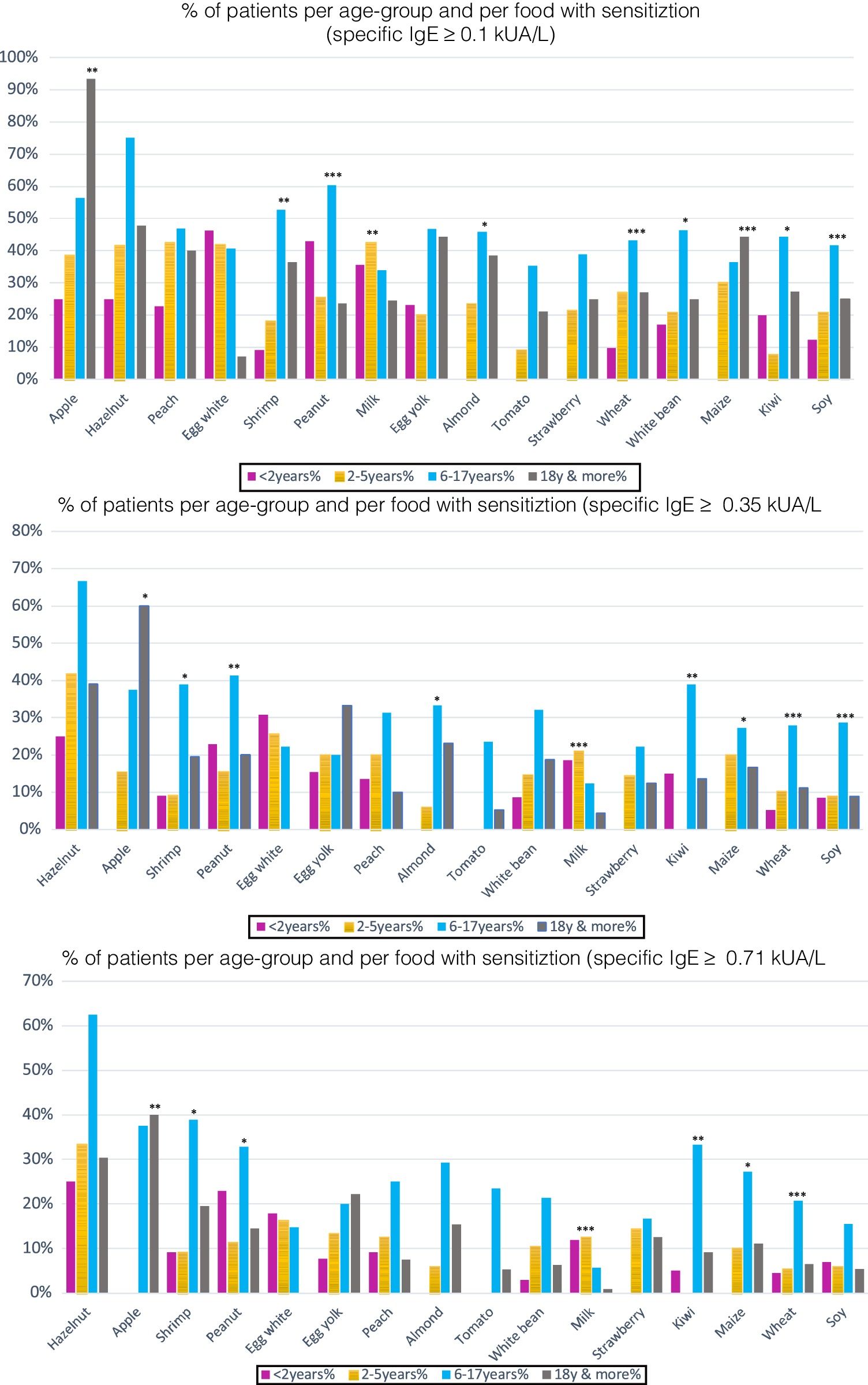
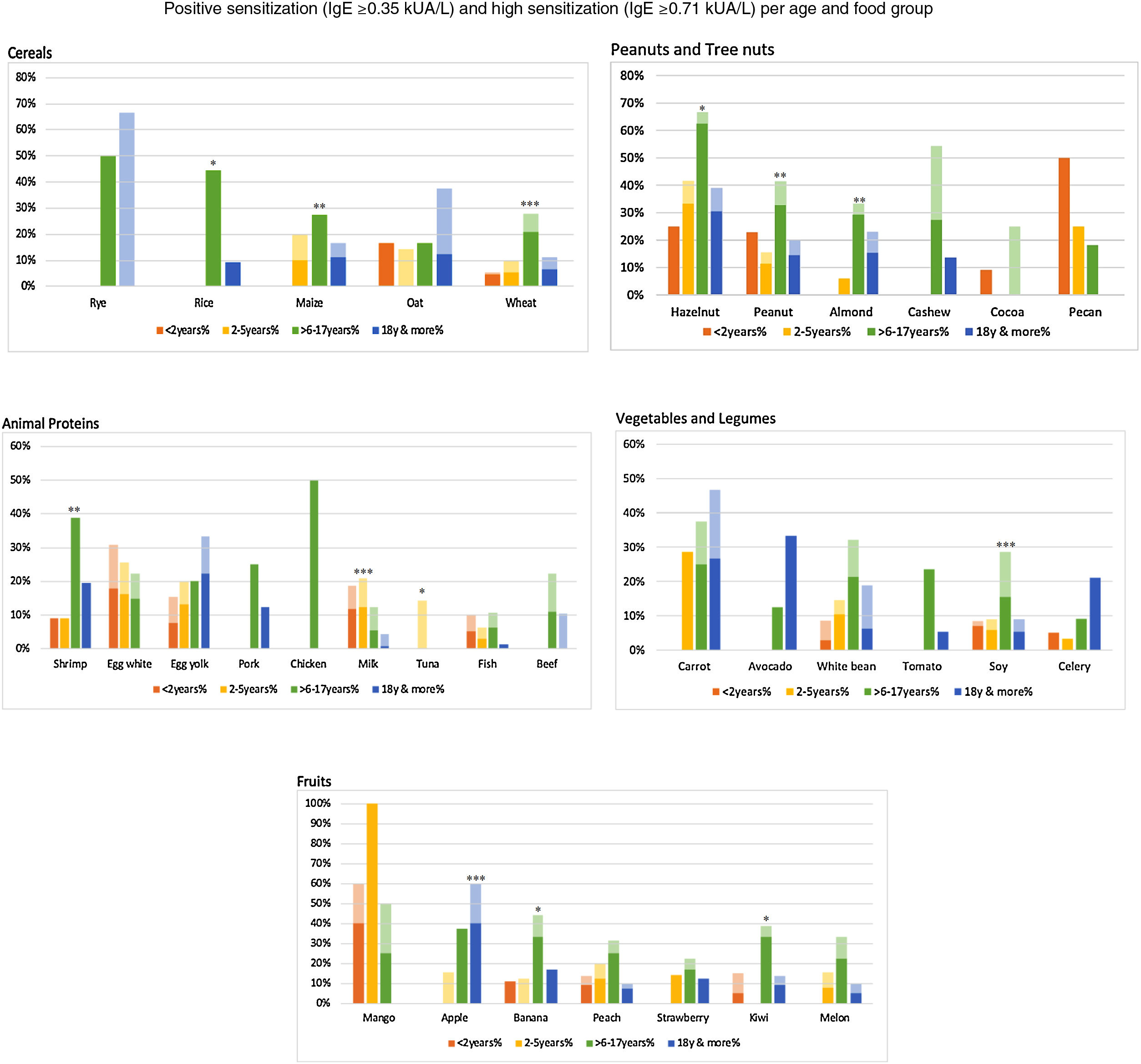
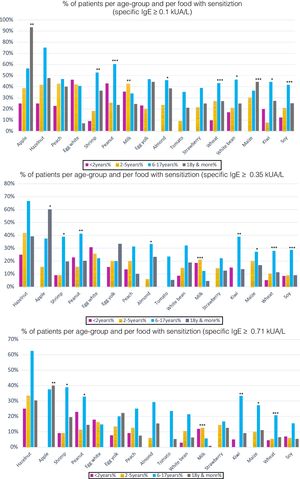
However, there were statistically significant differences between age groups at different cut-off points. Positivity was more frequent for cow's milk in children under two years; in older children (6–17 years), it was for peanut, almond, wheat, soy, maize and in adults, apple. For differences in sensitization and moderate-high positivity see Figs. 4–5.
(a) Percentage of patients with specific IgE≥0.1kUA/L per age-group: 16 most frequent foods. Per food, statistically significant differences between the different age groups in sensitization (sIgE >0.1kUA/L) were found for the following foods: *p<0.05: almond, white bean, kiwi; **p<0.01: apple, shrimp, milk; ***p<0.001: peanut, wheat, maize, soy. (b) Percentage of patients with specific IgE 0.35kUA/L per age-group: 16 most frequent foods. Statistically significant differences for positivity (sIgE values >0.35kUA/L) were found for the following foods: *p<0.05: apple, shrimp, almond, maize; **p<0.01: peanut, kiwi; ***p<0.001: milk, wheat, soy. (c) Percentage of patients with specific IgE 0.71kUA/L per age-group: 16 most frequent foods. Statistically significant difference for moderate-high positivity (sIgE values >0.71kUA/L), there were statistically significant differences between age groups for: *p<0.05: shrimp, peanut, maize; **p<0.01: apple, kiwi; ***p<0.001: milk, wheat.
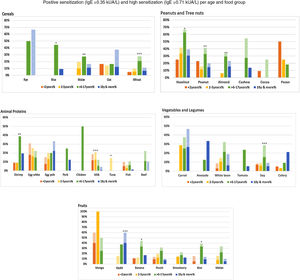
Specific IgE≥0.35kUA/L (light color) and sIgE≥0.71kUA/L (dark color) per food section and age-group. Food group positivity according to age groups. We depict IgE positivity in light color (specific IgE≥0.35kUA/L) and moderate-high IgE positivity in darker color (specific IgE≥0.71kUA/L). There is a statistically significant difference in the frequency of IgE positivity between age groups per food. *p<0.05: rice, banana, kiwi, hazelnut, tuna; **p<0.01: maize, peanut, almond, shrimp; ***p<0.001: wheat, apple, soy, milk.
Per food section, there were statistically significant differences. For cereals, we found more frequent positivity for rice, maize and wheat in older children. For fruits, banana and kiwi were more frequent in older children, and apple in adults. For vegetables and legumes, soy was more frequent in older children. In the peanut/tree nut category, hazelnut, peanut and almond were more frequent in older children; and in the animal protein category, cow's milk and tuna were more frequent in children between 2 and 5 years, shrimp in older children (Fig. 7).
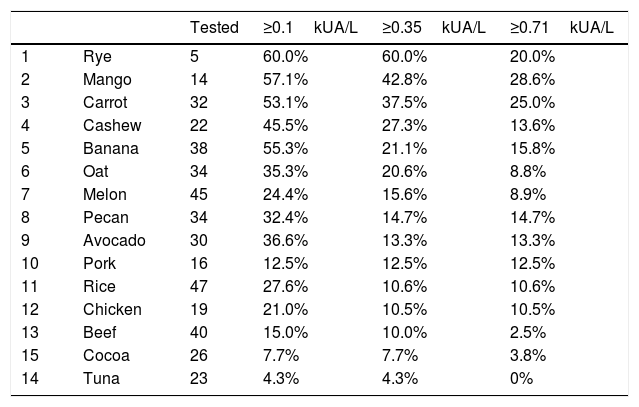
Foods with low sample size, fewer than 50 samples, also showed some results that should be highlighted and investigated further (Table 1). Rye, mango and carrot show a high percentage (>20%) of moderate-high positivity.
Foods with fewer than 50 samples. Percentage of patients with different cut-offs of specific IgE.
| Tested | ≥0.1kUA/L | ≥0.35kUA/L | ≥0.71kUA/L | ||
|---|---|---|---|---|---|
| 1 | Rye | 5 | 60.0% | 60.0% | 20.0% |
| 2 | Mango | 14 | 57.1% | 42.8% | 28.6% |
| 3 | Carrot | 32 | 53.1% | 37.5% | 25.0% |
| 4 | Cashew | 22 | 45.5% | 27.3% | 13.6% |
| 5 | Banana | 38 | 55.3% | 21.1% | 15.8% |
| 6 | Oat | 34 | 35.3% | 20.6% | 8.8% |
| 7 | Melon | 45 | 24.4% | 15.6% | 8.9% |
| 8 | Pecan | 34 | 32.4% | 14.7% | 14.7% |
| 9 | Avocado | 30 | 36.6% | 13.3% | 13.3% |
| 10 | Pork | 16 | 12.5% | 12.5% | 12.5% |
| 11 | Rice | 47 | 27.6% | 10.6% | 10.6% |
| 12 | Chicken | 19 | 21.0% | 10.5% | 10.5% |
| 13 | Beef | 40 | 15.0% | 10.0% | 2.5% |
| 15 | Cocoa | 26 | 7.7% | 7.7% | 3.8% |
| 14 | Tuna | 23 | 4.3% | 4.3% | 0% |
We report results for the first time of an initial screening on food allergen sensitization patterns in unselected, allergic patients in Mexico. The overall positivity (food-sIgE≥0.35kUA/L) in our population was 24%. The 15 most frequently positive foods were: hazelnut, apple, shrimp, peanut, egg white, egg yolk, peach, almond, tomato, white bean, milk, strawberry, kiwi, maize and wheat. When comparing the different age groups, milk (p<0.01) and egg (p<0.05) sensitization/positivity was more frequent among young children (≤5 years); while peanut, almond, wheat, soy, maize, shrimp and kiwi were more frequently positive among older children; and apple among adults. Moreover, other foods with fewer than 50 samples, like rye, mango and carrot, showed a high percentage of moderate-to-high positivity.
Concerning the well-known “eight major allergen group” generally accepted,2,3,8 in our population we only found six of the eight among the 15 most frequently positive foods. Soy only reached 16th place, and fish 18th. Instead, two new members among the eight most frequently positive food allergens in Mexico were apple and peach, both PR-10 (pathogenesis related proteins) related foods.
Notably, in our population we found a high percentage of sIgE positivity to foods not frequently tested, and not commonly included in panels commercially available, e.g. hazelnut, maize, beans, tomato, strawberry and kiwi. An under representation of the most commonly sensitizing allergens in commercial panels was noticed previously in EuroPrevall reports, where local food allergens with high sensitization frequency were not present in commercially-available testing panels either.20
In relation to the age distribution, we found more frequently positive results in younger children for milk and a trend for egg white; the 6–17-year old age-group was the one with statistically significant higher frequency of positive results, as compared to young children and adults.
Comparing our results with the existing literature, we found a high overall frequency of positivity: 24% of all tested patients. When interpreting this figure, it must be considered that our patients were pre-selected, as they presented a clinical suspicion of food allergy. In other Mexican studies the reported prevalence of food sensitization in the allergic population has been in the same range: between 24 and 65%, although sensitization in these studies was defined as a positive SPT or a positive clinical history only.12–18,23
In Latin America, there are reports of frequency of food sensitization from Brazil, Colombia, Costa Rica and Venezuela, based on the results of SPT or some only on self-reports. These studies have different aims, age groups and methodologies, making it difficult to compare them with our results. In general, in these publications the major allergens found have been: milk, egg, wheat and soy, which are only partly the same as we are reporting. However, milk and egg predominance in young children is universal.9,24–30 In the context of the frequency of food allergen sensitization in these Latin American studies, the prevalence in Brazil was reported between 42 and 79%,25,26 in Colombia, between 14.9 and 23%,24,27 in Costa Rica 60%,28 in Chile 8–18%29 and in Venezuela 80%.30 Surely, the pre-selection of the studied subjects varied per trial.
European studies conducted in non-selected populations found a prevalence of positive sIgE ≥0.35UI/mL to at least one food in 7.7–21.9%.7,20 In the United States a health record study found a 3.6% prevalence of food allergy in the population at large using only clinical data.31
As is widely reported, the highest prevalence is normally seen in children compared to adults.7 However this is not the case in our population, probably because our patients were preselected. We found no association between gender and the frequency of food sensitization. In some studies, mainly among children, male gender was associated with an increased risk.7,32–34
Food sensitization and ageIn children less than five years of age the most common food allergens are relatively similar worldwide, including milk, egg, peanut and seafood, with regional variation in the relative frequencies. In Australia and Asia, egg allergy is predominant over milk. In North America and the Middle East, milk is more frequent. In Europe, the pattern is more variable, but egg and milk tend to be the most common allergens in this age group. In Asia, seafood also appears consistently among the most common allergens.35 In our series, milk was more frequent in this young age group, but egg-white sensitization was still above 20% of the tested samples, and this continued even in the 6–17-year age groups. Interestingly, egg yolk shows a trend to be more frequent in older children and adults. No other foods in this age group showed a statistically significant difference as compared to the older age groups.
Globally, in older children (>5 years), peanuts, tree nuts, seafood, egg and milk tend to be the most common foods. In many European countries, fruits such as apple and kiwi are common allergens, this is also seen in Central and South American countries.36 We recorded very similar trends in our series: peanut, almond, shrimp and kiwi were the most frequent foods with sIgE positivity in that age group, but also other foods such as wheat, soy and maize were of importance.
In adults, in the EuroPrevall analysis, the foods with the highest prevalence in 20–44-year-old subjects were hazelnut, peach and shrimp. In our series, apple, closely related to peach but more frequently ingested, was the most frequent food in that age group; unexpectedly we found that egg yolk also tends to be more frequent.20
Regional foods and food sensitization profilesOther studies conducted in Mexico, mainly by SPT and self-reports, found a high frequency of sensitization for the most common food allergens worldwide (milk, egg, fish, shrimp, soy, wheat), but also for local staple foods like beans, maize, fruits (apple, peach) and vegetables (tomato).11–18,23,37
Studies in other Latin American countries report a high frequency of sensitization to fish, maize, beef and regional fruits and vegetables.9,24–30 These results show some similarities with ours, highlighting the presence of sensitization to staple foods.
The differences we found in frequency of sensitization rates for some foods among our Mexican population and world literature could probably be explained by the level of consumption of certain foods. Soy and fish sensitization were low, and maize, beans, hazelnut, tomato, strawberry and kiwi sensitization were frequency higher than internationally reported. Although soymilk is quite frequently consumed by infants in Mexico, its consumption in older children and adults is low. Similarly, fish is not one of the major protein sources, as red meat, pork and chicken can rather be considered staple foods. On the other hand, maize and beans are major staple foods, which could explain their rather frequent sensitization. As for hazelnut, the explanation can be dual: a true sensitization due to a recent increase in its consumption among children in the form of commercial hazelnut-chocolate spread. Moreover, a similar situation as in north-mid Europe38 might be the explanation for hazelnut sensitization in Mexico: e.g. a pollen-food syndrome, with the difference that the second most frequent tree allergy in Mexico is not birch pollen,39 but its highly cross-reactive oak tree pollen.40 Thus, hazelnut sensitization might be driven by oak tree pollen allergy in Mexico. Something similar could happen with shrimp sensitization, a food that is not often consumed but was frequently positive even in children. This could possibly be due to cross reactivity with tropomyosin, the group 10 protein of house dust mite.
One of the strengths of our study is its sample size and the quality analysis of the serum samples with ImmunoCAP. There is only one study in our country involving more patients, but diagnosis was based on self-reports11; there has been no other study using ImmunoCAP data. Moreover, we did not exclude any food- nor age-groups, which allows us to deliver a broad description of the sensitization patterns. On the other hand, the limitation of our study is its retrospective design, the fact that patients were referred and tested for a heterogeneous number of extracts, with most of them having their test directed to a few allergens, selected by treating physicians and the indications for testing are not known, e.g. eczema, nasal/ocular immediate allergic reactions. Just a few subjects were tested with the complete panel. Therefore, data must be confirmed in a large prospective trial with strict inclusion criteria.
For future studies, other foods that were frequently positive, but which were not included in commercial panels should be investigated further. This holds especially true for those foods often consumed in Mexico. In this sense we can mention maize, beans, tomato, celery, fruits such as strawberry, kiwi, peach, apple and hazelnut. Other fruits, vegetables and cereals with few samples in this study but high levels of sIgE could also be considered.
Funding sourceNone declared.
Conflicts of interestNone declared.
This work is part of a Master's thesis of the Master's Program in Clinical Research, Center for Clinical Research and Management Education, Division of Health Care Sciences, Dresden International University, Dresden, Germany.
We thank the laboratory staff who performed the tests and helped to collect the data.