Immunotherapy has shown to be an effective treatment for the management of some IgE-mediated allergies. However, due to its long duration, a high number of patients withdraw from it before completion.
ObjectiveExplore if allowing patients to select the route of immunotherapy, educational sessions and strict follow-up could improve treatment compliance.
MethodsPatients consulting allergy service were divided into two groups; if they chose the route of administration of immunotherapy, they were selected for the active group; if their physician decided, they were selected for the control group. All patients had to attend the allergy service monthly for control. Before the first application of immunotherapy, all patients received an educative session about the benefits and risks of the treatment. Patients in the active group received an additional session about subcutaneous and sublingual routes and they chose the most appropriate according to their personal characteristics.
ResultsA total of 204 patients were in the active group and 103 were included in the control group. At six months, a total of 46 patients withdrew from immunotherapy during follow-up, 24 (11%) in the active group and 22 (21%) in the control group (p=0.02). In the active group we observed no statistically significant difference in adherence between those who preferred subcutaneous or sublingual immunotherapy; however in the control group, the drop out of sublingual immunotherapy was significantly higher than those who received subcutaneous (p=0.05).
ConclusionEducational sessions, strict follow-up and considering personal preferences of patients could improve adherence to allergen immunotherapy.
Allergen immunotherapy has proved to be an effective alternative for the control of symptoms and prevention of some allergic diseases. Multiple routes of administration of allergens have been proposed but currently the most popular are the subcutaneous and the sublingual routes. Although the subcutaneous route remains the most widely used, the sublingual one has increased in recent years. Apparently, this increase is due to the physician's preference for the lower frequency of systemic reactions compared with the subcutaneous route, and several articles have been published evaluating physicians’ reasons for their selection.1 However, the dropout rate of immunotherapy, especially from the sublingual route, is high, and little has been studied in relation to the preferences of patients regarding the route of administration, which perhaps could improve the rate of adherence.2
Previous studies have suggested that patient education and closer monitoring may improve the adherence rate,3,4 therefore in this study we evaluated these factors and we observed if patient preferences about the route of administration could improve adherence.
MethodsCharacteristics of the study population and selectionThis was a prospective pragmatic study. We included patients who attended the allergy service of the University of Antioquia (Medellín, Colombia) from June 2012 to July 2013 with a diagnosis of asthma, rhinitis or atopic dermatitis and required mites immunotherapy. Patients who had previously received immunotherapy were excluded. The disease diagnosis was established according to the GINA guidelines for asthma (http://www.ginasthma.org), ARIA for rhinitis5 and the criteria of Hanifin and Rajka for atopic dermatitis.6 Each patient, at the beginning of treatment, received an explanation about why immunotherapy was needed, and its benefits and risks. All patients signed an informed consent (Fig. 1). This explanation was given by an allergist physician.
Commonly in our allergy service the selection of the route of administration of immunotherapy is a medical judgement. However, since 2013 we have been doing additional educational sessions, in which we present to the patient the principal characteristics of each route, the application scheme and the method of administration, and we allow them to choice the route that they prefer. This protocol is optional, and the allergist is free to follow it, and it does not depend on patients characteristics. Therefore at the time of enrolment we found patients who made the selection of the route, and others who were being treated with the route that their treating allergist chose. Patients who selected the route of administration were included as “active group” and the other patients where included as “control group” (Fig. 1).
Sublingual vs. subcutaneous routeSubcutaneous immunotherapy was administered monthly (Alxoid, Inmunotek, Madrid Spain). Mite allergen extracts were administered in two refracted doses of 0.2 and 0.3ml (30min interval) during buildup, and 0.5ml in single doses (50 DPP) in subsequent monthly injections. Observation time in the allergy service was required after each injection, in order to treat a local or systemic reaction if necessary. Patients and/or patients’ parents were instructed to identify and report any delayed reaction. Patients with asthma had to wait one hour during observation time, whereas patients with rhinitis or dermatitis had to wait 30min.
For sublingual immunotherapy with mites extract, buildup was done according to the manufacturer's instructions from each laboratory (Staloral Stallergenes, France or Oraltek, Inmunotek, Spain) but we included additional visits during the buildup. To the initial dose of SLIT, Inmunotek suggests that only the first administration required clinical observation for 30min. At day 14, we requested patients an additional visit to assess the presence of adverse effects and confirm the correct use of immunotherapy at home. This visit took less than 15min. For Staloral, different schemes have been proposed. We chose a rush scheme where buildup consisted of 11 days. In this scheme, the administration of day 1 and day 6 is under medical supervision (30min). We did the educational session during the first visit 30–60min before the first administration. SLIT patients during buildup had the option to call or attend the allergy centre in case of doubts about the method of administration or the dose of immunotherapy. An average of 1.2 (0–3) visits or calls per patient were received during the buildup phase. Maintenance doses of SLIT were administered at home. Each month patients attended the allergy centre to change bottle of immunotherapy and for a clinical evaluation (<15min).
Educational sessionWhen patients selected immunotherapy, the explanation about routes of administration was focused on the scheme to use (frequency of administration and monitoring time) and application techniques (drops and injections). Answers to patients’ questions about immunotherapy were strictly based on the recommendations of the third consensus of immunotherapy, ARIA guidelines and PRACTALL guidelines.5,7,8 Considering that the comparative evaluation of efficacy and safety between sublingual and subcutaneous route is open to discussion, we described a summary of the answers that we gave to patients for questions about some topics. For questions about effectiveness, we explained to the patients that both routes were effective and a high percentage of patients obtain a good control of symptoms and a significant reduction of drug treatment after a few months of continuous treatment (6–12 months), but the subcutaneous route could act faster than sublingual immunotherapy with mites.9 For the question about safety, we explained to patients that both routes were safe, but the subcutaneous route has more frequent systemic severe side effects, whereas the sublingual route is usually associated with only local effects.10,11 All doubts from patients were clarified and recorded. In the event that the answer to a question from a patient was not clearly defined in the guidelines above, the opinion of two additional allergists was requested to reduce the risk of subjectivity or biases that could incline the patient to choose one of the routes over the other. The reason for the preference was recorded and grouped according to whether they were related to the application scheme, the technique used, safety, or efficacy. The same patient could have two or more reasons for his or her selection.
AdhesionThis study was conducted for six months. Each month the patient had to attend the allergy service to receive the bottle with drops or the monthly injection. During the monthly assessment, adverse events and patients questions were recorded.
Statistical analysesAnalyses were performed with the IBM SPSS version 21 for Windows. The results and the general characteristics of the patients were expressed as percentages of frequency and in absolute numbers. Chi2 test or multivariate logistic regression were used to assess the difference between groups.
ResultsCharacteristics of patientsA total of 376 patients were included. 273 patients were able to choose the route of immunotherapy and 103 patients were included in the control group. Between patients who could choose the route, 204 (74.7%) chose it (active group) but 69 (25.3%) declined to take the decision and left the choice to the treating physician and were analysed separately. All patients were vaccinated at least with one mite or mites mixture (Der f 93%, Der p 97%, Blo t 42%). 52% of the population was female and the age range was 3–53 years, with 40% under 14 years. 53% of patients with immunotherapy to mites were sensitized to other sources (Table 1). We did not observe significant differences between the active group and the control group for age, patterns of sensitization, gender or frequency of allergic diseases (Table 1).
Patients’ characteristics.
| Characteristics | Active group n=204 | Control group n=103 | ||
|---|---|---|---|---|
| Route | Subcutaneous IT n=170 | Sublingual IT n=34 | Subcutaneous IT n=64 | Sublingual IT n=39 |
| Female | 88 (52%) | 17 (50%) | 35 (54%) | 21 (53%) |
| Age | 16 (3–49) | 12 (3–33) | 14 (3–53) | 14 (3–23) |
| Der f | 159 (93%) | 32 (94%) | 59 (92%) | 38 (97%) |
| Der p | 165 (97%) | 33 (97%) | 63 (98%) | 37 (94%) |
| Blo t | 72 (42%) | 14 (41%) | 28 (43%) | 16 (41%) |
| Dog dander | 81 (48%) | 16 (47%) | 33 (51%) | 18 (46%) |
| Cat dander | 18 (11%) | 5 (15%) | 9 (14%) | 5 (13%) |
| Rhinitis | 133 (78%) | 27 (79%) | 48 (75%) | 31 (79%) |
| Asthma | 57 (33%) | 11 (32%) | 24 (37%) | 12 (30%) |
| Dermatitis | 34 (20%) | 7 (20%) | 14 (21%) | 7 (17%) |
Patients characteristics according to allergy diseases and route of immunotherapy.
No significant differences were found when we stratified the active and control groups according to age: Under 10 years: 80 (39%) active vs. 40 (39%) control. Between 11 and 18:71 (35%) vs. 35 (34%). Over 18:53 (26%) vs. 28 (27%). However, we observed significant differences in the selected route according to age (p=0.02): sublingual route increased among children under 18, and in children under six years it was the most prescribed in the control group (active group: 24%, control group: 48%). There were no significant differences between the frequencies of routes according to the type of allergic disease among the 204 patients in the active group, 74% were under 18 years and their parents chose the route. The most frequently selected route was the subcutaneous one, in the active group (n=170, 83%) and in the control group (n=64, 62%).
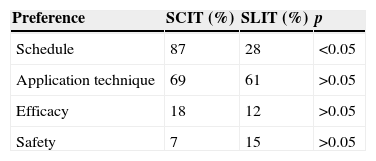
Reasons for route preferencePatients chose the route mainly for convenience in the scheme and the application technique, to them safety and efficacy were less important. Among the active group, patients took in consideration reasons such as scheme (87% vs. 28%, respectively), technique (69% vs. 61%), efficacy (18% vs. 12%) and safety (7% vs. 15%) in order to choose their route (Table 2).
We did not observe differences in the choice of the route between patients according to the distance and travel time to reach the allergy service, or between the sociodemographic characteristics. Among patients who were offered to select the route, we did not observe significant sociodemographic differences between those who chose the route and those who left the choice to the treating physician. Considering that in our service all patients with allergy immunotherapy, no matter the route, must attend monthly to an evaluation of control, we asked patients who chose the subcutaneous route if they preferred the sublingual route in the case that they had to come only every two or three months. 15% answered yes. The most frequent questions were related to the cost of immunotherapy (45%).
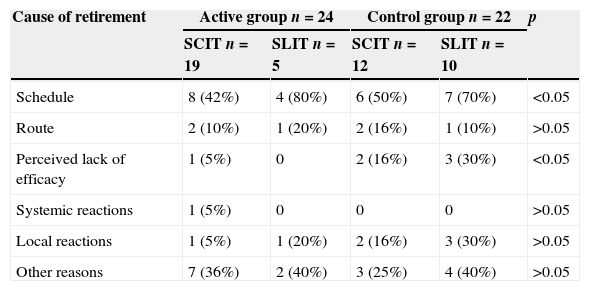
Adherence to immunotherapyAfter six months a total of 46 patients had withdrawn from follow-up, 24 (11%) in the active group and 22 (21%) in the control group (p=0.02). In the active group, we observed no statistically significant difference in adherence between those who preferred subcutaneous or sublingual immunotherapy (drop-out: SCIT 19 (11%) vs. SLIT 5 (14%)), however in the control group, the drop-out of sublingual immunotherapy was significantly higher than those who received subcutaneous (drop-out: SCIT 12 (18%), vs. SLIT 10 (25%) p=0.05). In both groups, the main reason for discontinuation was the difficulty in complying with the scheme (active group 12 (50%) vs. control group 13 (59%)). Other reasons for dropout were the opinion of lack of efficacy by the patient, which was significantly higher in the control group (active group one (4%) vs. control group five (22%) p<0.01), systemic adverse reactions (active group one (4%) vs. none control group) and local reactions (active group two (8%) vs. control group five (22%)). The same patient could have several reasons for leaving immunotherapy (Table 3).
Causes of withdrawal from immunotherapy.
| Cause of retirement | Active group n=24 | Control group n=22 | p | ||
|---|---|---|---|---|---|
| SCIT n=19 | SLIT n=5 | SCIT n=12 | SLIT n=10 | ||
| Schedule | 8 (42%) | 4 (80%) | 6 (50%) | 7 (70%) | <0.05 |
| Route | 2 (10%) | 1 (20%) | 2 (16%) | 1 (10%) | >0.05 |
| Perceived lack of efficacy | 1 (5%) | 0 | 2 (16%) | 3 (30%) | <0.05 |
| Systemic reactions | 1 (5%) | 0 | 0 | 0 | >0.05 |
| Local reactions | 1 (5%) | 1 (20%) | 2 (16%) | 3 (30%) | >0.05 |
| Other reasons | 7 (36%) | 2 (40%) | 3 (25%) | 4 (40%) | >0.05 |
Patients could select one or more reasons to withdraw immunotherapy.
The efficacy and safety of immunotherapy have been widely demonstrated in asthma, rhinitis and may be beneficial for selected patients suffering from atopic dermatitis, although the frequency of success appears to depend on the severity of symptoms, the extract used, and patient adherence to therapy. This last point is especially critical in long-term treatments where drop-out rates are usually quite high.
Our hypothesis in this study was that if patients consider their personal characteristics after an educational session about immunotherapy, they will have the freedom to select the route, and this could improve the adherence to the treatment. We note that in our population most patients preferred the subcutaneous route, in contrast to the control group where the preference of physicians to the sublingual route was higher in children under six years. Some studies have shown that the physician selects the route of application considering the safety of the treatment, which may explain the preference for this route in the control group in our study. In the active group, patients’ preference were strongly linked to the scheme and an important factor that could influence the selection of the route was that patients with SLIT had to attend one or two additional visits to the health centre during the buildup phase, while patients with SCIT only had to attend one. However, the majority of patients who preferred subcutaneous immunotherapy explained that this was more comfortable for them, since they recognised a high probability of forgetting the drops if they should be administered daily at home. We observed that between patients or parents who preferred the sublingual route, the fear of needles was the main factor, especially in children.
Recently, Savi et al.,4 presented some strategies to improve adherence to sublingual immunotherapy based in a pragmatic study. They concluded that it was necessary to explain with more detail to the patient questions about immunotherapy and make more frequent monitoring. In agreement with these recommendations, we observed that patients who chose the route of immunotherapy (subcutaneous or sublingual) had a lower dropout rate than in the control group (physician selection), indicating that additional education and choosing the most comfortable route for the patient in consideration to their personal characteristics can substantially improve treatment adherence. 15% of patients who selected the subcutaneous route said that they would have preferred the sublingual route if this would have allowed them to space out the visits to the allergy service to every two or three months. There are some data that demonstrate a good compliance for SLIT even if patients are not monitored monthly .12,13 However, the dropout rate during the six months of follow up in our study was less than in other studies with more spaced visits,4,14 suggesting that monthly monitoring, independently of the route chosen, improves adherence.
Some studies have observed that the more frequent causes of dropout in immunotherapy are the side effects and the lack of clinical improvement.14,15 Interestingly, we observed that these factors were less decisive during the selection of the route by the patient, perhaps for patient confidence in treatment recommendations from their allergist.
Our study has the advantages and the limitations inherent to all pragmatic studies16; during the educational session, some answers to patient questions might be biased by physician preference, but we try to reduce this bias basing our responses according to international guidelines. When the answer was not explicit in the guidelines and depended on the physician's opinion, the answer was provided by a staff of at least three allergists. An important point that was not assessed in our study was how many days per month patients had to discontinue sublingual immunotherapy. However, we could not objectively evaluate this point without altering the observational and pragmatic design of the study.
In conclusion, in our population there appear to be significant differences between patient preferences regarding the route of administration of immunotherapy and physician preference. A better education and monthly monitoring, independent of the route selected, can improve patient adherence to therapy.
FundingNo support from any institution was required.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe author has no conflict of interest to declare.