Aging is associated with thymus involution leading to a reduction in naive T cells and to an accumulation of effector-memory cells. Apoptosis is a key mechanism to clear the immune system from activated and harmful cells. In asthma the stimulation of T cells by environmental antigens can decrease naive cells and sustain activated cells. The aim of this work was to evaluate the imbalance between CD45RA and CD29 cells during the aging process and their changes in elderly asthma and to evaluate how elderly and chronic diseases like asthma can affect susceptibility to apoptosis.
MethodsElderly and young adult healthy volunteers and elderly asthmatic patients were submitted to skin prick tests, immunoglobulin determination and flow cytometry analyses of CD3, CD4, CD8, CD45RA, CD29, and CD95.
ResultsSerum IgE was increased in allergic patients (p=0.0001). Asthmatics presented an increase in CD4 cells (p<0.05). CD45RA was significantly decreased in elderly individuals (p<0.05) and this decrease was higher in asthmatics (p<0.05). CD29 was increased in elderly healthy individuals compared to the control young group (p=0.0001). A negative correlation between CD29 and CD45RA (p<0.05) was observed. CD95 lymphocytes increased in elderly (p=0.0001) and a positive correlation between age and CD95 (p<0.05) was found. Asthmatic patients showed significant decreases in CD95 (p=0. 0001).
ConclusionsNaive cells are key cells in the defence against infections and their decrease in the elderly and in asthma is a bad prognosis factor. The reduction of apoptosis markers can promote the persistence of activated cells involved in chronic conditions.
Thymopoiesis provides the immune system with T cells with a naive phenotype (CD45RA, CD62L CD27, CD28) and although this procedure suffers a reduction during the growth process it can persist during adulthood.1,2 Antigen exposure determines the expression of new surface molecules and confers proliferative properties to cells transforming them into effector and memory cells. Repeated stimulation with identical antigenic proteins elicits responses of cross-reactivity increasing the survival of memory T lymphocytes (TL) and the expansion of these cell lines, while limiting the expansion of naive cell lines.3
The most profound age related changes described within T cell populations are the decrease in the number of naive cells and the accumulation of sensitised cells. Moreover, a further decrease of this lymphocyte population can be observed when the exposure to new pathogens induces the replacement of old memories for new ones.3 The decrease of naive T lymphocytes contributes to the impoverishment of lymphocyte repertoires with defective responses to new antigens.4,5 However, a substantial naive T-cell pool is maintained even in centenarian individuals and some data suggest that their relative and absolute values may remain virtually unchanged after 40years of life. Extrathymic differentiation of TL can become prevalent in the elderly.6 Immunohistological studies showed a reduction in the number of CD45RA naive lymphocytes in lymph nodes associated to aging.7
The CD29 is the β1 subunit of the integrin family from adhesion receptors that is expressed when a cell enters the G1 phase of the cell cycle. This molecule is often present in memory cells and tends to increase in the elderly.8,9
Apoptosis is a key mechanism in the elimination of activated cells that expand in excess during the course of a physiological immune response. The Fas death receptor (also named CD95 or APO-1) is an integral part of the physiological regulation of apoptosis. In aging, the process of apoptosis establishes the preferential elimination of memory senescent cells and its deregulation is associated to disease.10,11
Several environmental factors and health conditions have a recognised capacity for eliciting cell activation into memory and effectors populations and to trigger apoptosis with varying impact on successful aging.12
In allergic asthma there is a continuous stimulation of TL by several aeroallergens, which can lead to a decrease in naive T cells. A decrease in apoptosis can also help to sustain the airway inflammation that characterises the disease.
The aim of this work was to evaluate the imbalance between CD45 and CD29 cells during the aging process and to analyse the changes in these cell phenotypes in elderly asthma patients. A second goal was to compare susceptibility to apoptosis and how it affects aging and chronic respiratory diseases such as asthma.
MethodsPopulationA group of 58 elderly healthy volunteers older than 65years of age (mean age 79.2±7.1); a group of 33 healthy young adults (mean age 30.2+11.3years); and a group of 95 individuals with controlled asthma also older than 65years of age (mean age 72.4±5.1years) attending a chest disease outpatient department were studied. All the patients had a history of intermittent chest tightness, wheezing or shortness of breath for at least 30years prior to study participation consistent with the diagnosis of asthma according to the Global Initiative for Asthma.13 All the patients were using 250μg to 500μg of beclometasone dipropionate in mild to moderate asthma or 250μg of fluticasone propionate daily associated with long-acting ß2-agonists in severe asthma and short-acting ß2-agonists as needed. Other anti-asthmatic drugs were withdrawn at least four weeks prior to the study. All patients were examined by a physician and underwent spirometric testing using the same equipment (Vitalograph Compact) at least six hours after the last dose of any bronchodilator. Predicted values were measured according to Knudson et al.14 Subjects’ spirometric performances were assessed by means of a computerised program following the American Thoracic Society (ATS) 94 criteria. Approval for analysis was determined using the ATS 94 criteria, and accuracy was achieved if, within the same evaluation, three curves were acceptable and reproducible. Patients with severe asthma had FEV1 percentage values in spirometry lower than 60% of the predicted (Tables 1, 2).
Average percentage of predicted spirometric values in asthmatics.
| n | Fev1 (%) | Fev1% BD | VC% | Tiffeneau | |
| Severe Asthma | 19 | 51.8±10.0 | 10.2±3.7 | 68.1±10.2 | 70.1±9.7 |
| Mild Asthma | 76 | 87.6±17.8 | 12.4±6.2 | 94.5±15.5 | 83.5±13.0 |
Predicted values were measured according to Knudson et al.14
Fev1 - Forced expiratory volume in one second; Fev1BD - FEV1 after bronchodilatation;
VC - vital capacity.
All the studied individuals were able to perform their own daily tasks, carrying out regular physical activity. None of them had had any respiratory infections in the previous month to their inclusion in the study. No other clinically relevant diseases were reported.
The following were considered exclusion criteria: smoking, cancer, autoimmune disease, infection, diabetes, heart failure, renal failure, chronic hepatic disease, and recent exposure to environmental risk factors for pulmonary diseases.
Approval to this study was received from institutional review board of the Coimbra University Hospital, and all individuals were selected after informed consent.
MethodsSkin prick tests to 20 common aeroallergens were performed to all individuals involved in the study (ALKABELLO/Lancetter-tames Hollister Stier). Subjects were classified as allergic with one positive test associated with clinical symptoms. The remaining asthmatics were classified as non-allergic.
Immunoglobulin G (IgG), IgA and IgM were measured in total serum by nephelometry and total serum IgE was measured using a commercial kit (Coat-A-Count® Total IgE IRMA, DPC®, CA, USA) based on an Immuno-radiometric assay (IRMA) of solid phase. This kit uses monoclonal antibodies anti-IgE with I125-labelled in the liquid phase and polyclonal antibodies anti-IgE attached to the wall of the polystyrene tube.
Therefore, the IgE present in the sample was retained between these two antibodies. These tubes were measured using a gamma counter (Gamma-C-12, DPC®, CA, USA) for one minute. Total IgE concentration in the sample was directly proportional to the counts per minute. This concentration was determined by comparison with provided calibrators. The kit sensitivity was 0.5 IU/ml.
The study of peripheral blood (PB) lymphocytes was performed using flow cytometry assay. 100ul of PB was incubated with monoclonal antibodies for 10minutes at room temperature in the dark. To determine the main populations, a single tube with Lymphogram (Cytognos, Salamanca, Spain) containing monoclonal antibodies anti-CD4 stained with phycoerythrin cyanin 5 (PECy5), CD8 with fluorescein-isothiocyanate (FITC), CD3 with phycoerythrin (PE) was used. Moreover we evaluated CD45RA, CD29 and CD95 (Immunotech, Marseille, France) stained with FITC, CD8 (Dako, Denmark) with PE, CD3 and CD4 with PECy5 (Dako, Denmark), according to manufacturers’ specifications.
Flow Cytometry (FACS) data were collected on a FACS Calibur (BD Biosciences, San Jose, CA, USA) using the Cellquest® acquisition software and analysed by Paint-a-gate® software.
Statistical analysisStatistical analysis was performed using SPSS 12.0 software package. The Kolmogorov –Smirnov test was used to check if variables were normally distributed. For those with a normal distribution the parametric t test was used for two independent samples. Variables that were not distributed normally were evaluated using the Mann-Whitney non-parametric test. P-values less than 0.05 were considered significant. Statistical comparisons were made between elderly healthy volunteers and healthy young adults and between elderly asthmatics and healthy elderly individuals. Differences between allergic and non-allergic asthmatic patients were also analysed.
ResultsSixty-five asthmatic patients had positive tests to at least one allergen. The control population and the remaining asthmatics had negative skin prick tests.
Serum immunoglobulin (Ig) IgG, IgA and IgE were higher and IgM lower in allergic patients when compared to non-allergic patients (Table 3).
Serum Igs values in asthmatics.
| IgG | IgA | IgM | IgE | |
| Allergic asthma | 11.6±2.7 | 3.2±2.1 | 1.2±1.4 | 362±310/a |
| Non allergic asthma | 10.8±2.3/ns | 3.1±2.2/ns | 1.3±0.7/ns | 92±193 |
IgG, IgM and IgA are expressed in mg/l; IgE is expressed in IU
The percentual values of CD3,CD4 and CD8 blood lymphocytes were within normal ranges. CD3 lymphocyte percentage in asthmatic patients was similar to the control groups and CD3+CD8+ lymphocytes were slightly decreased without statistical significance in asthmatic patients compared to control group of the same age. Only CD3+CD4+ cells were significantly increased in asthmatic patients (Table 4).
Percentual values of lymphocytes populations in asthmatics and control groups.
| Control Young | Control Elderly | Asthma | |
| CD3 | 75.0±3.1 | 73.6±9.9/ns | 73.4±7.6/ns |
| CD3+CD4+ | 43.2±5.1 | 41.8±10.4/ns | 45.4±9.6/a |
| CD3+CD8+ | 22.2±4.3 | 25.0±9.5/ns | 22.9±8.0/ns |
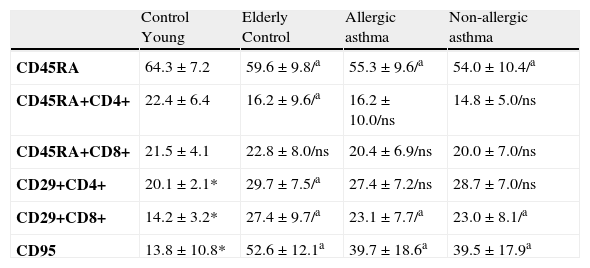
The mean percentage of naive CD45RA and CD4+ CD45RA + decreased significantly in the elderly individuals studied. The mean percentage of naive CD45RA was also significantly deceased in asthmatic patients when compared to the control group of the same age. CD4+ CD45RA + and CD8+CD45RA + cells were slightly reduced in asthmatics (Table 5).
Percentual values of lymphocytes populations in non-allergic asthmatics, allergic asthmatics and control groups.
| Control Young | Elderly Control | Allergic asthma | Non-allergic asthma | |
| CD45RA | 64.3±7.2 | 59.6±9.8/a | 55.3±9.6/a | 54.0±10.4/a |
| CD45RA+CD4+ | 22.4±6.4 | 16.2±9.6/a | 16.2±10.0/ns | 14.8±5.0/ns |
| CD45RA+CD8+ | 21.5±4.1 | 22.8±8.0/ns | 20.4±6.9/ns | 20.0±7.0/ns |
| CD29+CD4+ | 20.1±2.1* | 29.7±7.5/a | 27.4±7.2/ns | 28.7±7.0/ns |
| CD29+CD8+ | 14.2±3.2* | 27.4±9.7/a | 23.1±7.7/a | 23.0±8.1/a |
| CD95 | 13.8±10.8* | 52.6±12.1a | 39.7±18.6a | 39.5±17.9a |
a Statistical significance p=0.0001. ns = no significance.
* Only done in 20 individuals (mean age 37.8±12.2 years).
The mean percentage of CD4+CD29+ and CD8+CD29+ lymphocytes in the elderly was significantly increased both in asthmatics and healthy individuals. The CD8+CD29+ percentage in the asthmatic patient group was above the percentage found in the young control group but was significantly decreased when compared to the elderly control group (Table 5).
The mean percentage of CD95 lymphocytes in elderly health individuals was significantly increased when compared to the young control group. Conversely, the CD95 lymphocytes in the asthmatic patients group was significantly decreased when compared to the elderly control group although the values found were still higher than those of the younger healthy individuals (Table 5).
Discussion and conclusionsAlthough the skin reactivity and IgE values tend to decrease with age, elderly asthmatics have higher sensitisation to environmental allergens than the general population of the same age group. The asthmatics studied had intense cutaneous reactivity to aeroallergens, a Th2 immune response conserved and high levels of total serum IgE when compared to the control group (p=. 0001)15–17 The asthmatics also presented a significant increase of CD3+CD4+ lymphocytes compared to the control group (p<0.05), which can be associated to preserved humoral response despite the long disease evolution of the patients studied.
A crucial problem of the immune system in the elderly is the reduced proliferative cell capacity and the delay of clonal expansion in response to antigenic exposure as a result of stem cell decrease. The production of specific antibodies in response to vaccines and to environmental antigens is reduced in elderly but the production of autoantibodies is increased and the total serum immunoglobulin levels remain unchanged.18 A selective decline in IL2 and IFN γ is observed while IL4 and IL6 production is kept within normal values. This adjustment in the cytokines balance is accomplished by a normal or increased humoral response and a decrease in cellular response.19 The CD45 family (common leukocyte antigen) consists of multiple isoforms, which differ only in length and glycosylation of their extracellular domains. The different distribution of these isoforms in leukocytes and in particular on T cells plays a crucial role in cell activation and pathways signalling mediated by this receptor. The expression of different isoforms of CD45R is thus regulated during the maturation and activation of T cells. T cells with a naive phenotype express CD45RA differentiation markers and diminish throughout life, which is associated with a gradual onset of memory phenotypes. When asthmatics are exposed to allergen stimulation only CD45R0 cells can express activation markers.20,21
This study showed a significant reduction in the expression of CD45RA in elderly health individuals when compared with a young control group (p<0.05). This finding confirms the reported decrease of naive cells during the aging process. It was also decided to evaluate if the asthma condition posed an additional risk for this reduction. In fact the reported reduction was even more evident in elderly asthmatics (p<0.05). The expression of CD45RA on CD4 and CD8 was also reduced in asthma. The CD4+CD45RA+ subtype reached 16% while the values of CD8+CD45RA+ exceeded 20%.
The CD45RA can be expressed in cells previously activated that entered in a resting state. This decreased response to stimulation and lower functional activity, is probably linked to a lack of IL2 production.22,23 In asthmatics the existence of a continued exposure to allergenic sources or other airborne hazardous substances is accepted, resulting in a continuous stimulation of the immune system, mainly of the Th2 cells. This stimulation will sustain the setting up of memory phenotypes particularly in chronic diseases. Even in non-allergic asthmatics the bronchi inflammatory response is connected with exposure to inhalatory triggers. This phenomenon was also described in individuals with chronic infections where the offending agent was present as a persistent stimulus for the immune response. In this age group a marked reduction in the ability of identifying new allergens and promoting an effective immune response is observed. This reduction in acquisition of new skills is connected to the preservation of memory phenotypes acquired throughout life, which are specific to an extensive repertoire of antigens. It is therefore assured a prompt and effective response to the recall antigens, while there is a restrict response to new challenges.24,25 Naive cells are essential in the defence against acute infections and as so their decrease in elderly asthma, particularly in CD8 phenotypes, should be regarded as a bad prognosis factor.
The CD29 receptor is a homing molecule expressed in activated cells that potentiates T cell adhesion to endothelium and is involved in cellular response to specific antigenic challenge. The CD29 is preferentially expressed in CD4+CD45R0+ memory cells. In asthma this molecule tends to increase following allergen stimulation, and is especially associated with memory phenotypes.26 CD29 lymphocytes were significantly increased both in elderly healthy individuals and elderly asthmatics. The mechanism leading to increased expression in aging is probably dominant and therefore overlaps the arise expected from asthma.3
If we analyse the elderly population, a significant negative correlation between CD29 and CD45RA is observed meaning that the presence of a higher number of cells marked by CD45RA is associated to a smaller number of positive cells for CD29, both in asthmatics (PC=-0.25, p<0.05) and in group control (PC=-0.45, p<0.05). This observation strengthens the fact that the decrease of naive cells tends to be inversely related to the expression of CD29, a marker of activation and homing present in memory cells also in elderly.
Apoptosis is a natural defence mechanism against disabled or dead cells that are harmful to the physical integrity of the individual. Susceptibility to apoptosis measured by CD95 expression is reflected by an increase of this receptor in the elderly and suggests that apoptosis is important in the aging processes.26,27 The studied elderly healthy individuals had a significantly increased value of CD95 compared to the control group of young adults (p=0.0001) and compared to asthmatic patients (p=0.0001). A positive correlation between age and CD95 was found (PC=0.446, p<0.05). The resistance of T lymphocytes to apoptosis, namely the reduction of expression of receptors that can initiate this process of cell death, could act by promoting the maintenance of activated and memory cells and their intervention in the inflammatory process characteristic of bronchial asthma.28–31 In asthma there is a higher probability of decreased expression of apoptosis markers by blood cells overcoming, in some cases, the growing trend associated with the aging process. Recent studies have shown an increased incidence of cancer, particularly lung cancer, in elderly asthmatics.32,33 The observed reduction of markers expected to initiate processes of apoptosis can also promote the persistence of abnormal or damaged cells and consequent neoplasic condition.
The better knowledge of the immunoinflammatory pathway of elderly asthmatics can provide important information to improve the outcome of these patients.
Conflict of interestThe authors have no conflict of interest to declare.