CONCEPT
According to the classification proposed by the European Academy of Allergology and Clinical Immunology (1) egg allergy is an adverse reaction of an immunological pathogenic mechanism induced by egg ingestion.
The only currently well known pathogenic mechanism in egg allergy is that of immediate, type I, IgE-mediated hypersensitivity, although other mechanisms are possible.
The consumption of bird eggs, more specifically in our environment of hen eggs, constitutes an important source of proteins and is one of the basic foods in our diet from the first year of life.
Because of its high protein content, its introduction into the diet during the first year of life and its widespread consumption, egg is the most frequent cause of food hypersensitivity in young children in Spain (2).
EPIDEMIOLOGICAL DATA
Egg is the most frequent cause of food allergy in children (2-4). The allergy tends to develop before the child is 2 years old and in 55 % of cases it disappears during the first 6 years (5).
No studies have been published on the incidence of egg allergy and data on prevalence varies according to the type of study population.
Results on the prevalence of egg allergy in Europe are variable, ranging from 8 % (6) to 58 % among children allergic to cow's milk (7). No studies have been published on the prevalence of egg allergy in the general population.
In Spain, an observational study of 4,000 patients who consulted an allergist, found that egg allergy accounted for 16 % of food allergies in the general population and was the fourth most frequently implicated food (8). In the subgroup of children aged less than 5 years this frequency was 44 % and, together with milk, egg was the main cause of sensitization. Milk and egg were the allergens most frequently found to be involved in patients with atopic dermatitis and digestive symptoms. In children aged less than 15 years the frequency of egg allergy was 20 % and, together with milk and nut allergy, was the most common food allergy in this age group. Seventy-six percent of sensitizations to egg proteins developed before the child was 5 years old, 12 % between the ages of 5 and 10 years and another 12 % between the ages of 10 and 15 years. Thirty-seven percent of children allergic to egg had an associated inhalant-induced respiratory disease.
In another group of 355 Spanish children diagnosed with food allergy (2) the prevalence of allergy to egg proteins was 20.1 %, similar to that observed in the cited study (8). In 56.5 % of patients the allergy developed between the ages of 6 and 12 months and in 97 % during the first 2 years. Only 16 % of the children with egg allergy had associated food allergies (three or more).
FACTORS CONDITIONING AND FAVORING SENSITIZATION
The development of food allergy is determined by the interaction between genetic predisposition and environmental factors, especially exposure to food proteins.
Genetic predisposition
The importance of genetic predisposition in the development of allergic diseases is well known. A positive family history is found in 60-70 % of patients with atopic allergy. The risk of suffering from an atopic allergy in the future is increased not only by familial antecedents of atopy, but it also by the number of family members affected and is greater when both parents are allergic (9).
Currently, the presence of egg-specific IgE antibodies constitutes the earliest marker of atopy (10).
The nature of the antigen
In general, egg-allergic children react principally to the ingestion of egg white. Although egg yolk contains several proteins, egg white contains the greatest number of allergens. Up to 24 different antigenic protein fractions have been isolated, although the antigenicity of most of these is unknown. The main allergens are ovalbumin, ovomucoid, ovotransferrin and lysozyme. These proteins have been sequenced.
Ovalbumin (Gal d II) represents more than 50 % of egg white proteins. This 45 kDa protein contains 385 amino acids.
Ovomucoid (Gal d I) is a thermostable glycoprotein constituting 10 % of egg white proteins. It has a molecular weight of 28 kDa, contains 186 amino acids and is the most allergenic egg white protein (11). The use of commercial ovalbumin extracts contaminated with ovomucoid has led to overestimation of ovalbumin as the main allergen in egg white (12).
Ovotransferrin (Gal d III) represents 12 % of the total protein in egg white. It has a molecular weight of 77 kDa and is composed of 686 amino acids.
Lysozyme (Gal d IV) is a small protein, with a molecular weight of 14.3 kDa and 129 amino acids. Thirty-two percent of egg-allergic individuals are sensitized to lysozyme. Because of its bactericidal properties, this protein is used as an additive in numerous foods and drugs (13).
Other proteins have also been identified. Some of these proteins, such as ovomucin, ovoflavoprotein, avidin, ovoinhibitor, etc., are antigenic. Egg yolk proteins can also be allergenic (apovitellenis, phosvitins, livetins).
In bird-egg syndrome, found in a group of patients sensitized to egg through bird proteins (feathers, excrement, and bird serum), sensitization was mainly to egg yolk. The allergen producing this cross-sensitization was alpha-livetin (14). This pattern of sensitization was infrequent in children (15). Digestive and respiratory symptoms after egg ingestion were more frequent in patients with bird-egg syndrome than in those with isolated egg protein allergy (12).
Cross-reaction takes place between egg white proteins and egg yolk proteins, as well as between different bird eggs (hen, turkey, duck and seagull) (16).
In egg-white allergic individuals, skin tests frequently reveal sensitivity to chicken meat with tolerance of ingestion. Clinically relevant cross-reaction between egg and chicken meat is less than 5 %.
An ovalbumin specific T cell epitope has been identified in patients allergic to egg (17). Activation of this cell leads to the production of type II cytokines. The identification of this epitope will lead to the future creation of peptide blockers.
Antigen transmission through breast milk
Sensitization to egg white, as with that to milk, develops early even in exclusively breast fed children. Sensitization is probably produced by the transfer of small doses of the antigen in breast milk. Infants sensitized to egg through this route can react to the first ingestion of egg (18).
CLINICAL ASPECTS
Hypersensitivity to egg can manifest in all forms of IgE-mediated reaction (19):
1.Cutaneous reactions:
Erythema.
Urticaria.
Angioedema.
2.Generalized reactions:
Anaphylaxis.
3.Gastrointestinal reactions:
Abdominal pain.
Nausea.
Vomiting.
Diarrhea.
4.Respiratory reactions:
Rhino-conjunctivitis.
Laryngeal edema.
Asthma.
The existence of factors modulating clinical response in immediate hypersensitivity reactions to foods is well-known (18). Some of these factors, such as the amount ingested, depend on the allergen while others, such as the specific IgE rate, the releasing capacity of the mediators, or the sensitivity of the target organs to the mediators released, depend on the individual. Thus, the clinical signs and symptoms of egg allergy can be varied.
Symptomatology usually develops after the first ingestion of egg white. Frequently, affected individuals have previously tolerated cooked egg yolk. Symptoms usually appear a few minutes after ingestion and almost always within an hour.
Clinical signs and symptoms
Immediate hypersensitivity reactions after egg ingestion usually manifest as acute dermatological or gastrointestinal symptoms (4).
Acute dermatological manifestations consist of erythema, urticaria and angioedema clearly associated with egg ingestion. The onset of symptoms may be rapid, developing a few minutes after ingestion of the causative allergen. Caffarelli et al (18) observed that 93 % of positive egg challenges elicited immediate symptoms, even though the child had not previously ingested egg. Symptoms developed within the first 20 minutes of egg ingestion and 53 % of the children presented cutaneous symptoms in the following order of frequency: pruritus, erythema, urticaria and angioedema. Like other foods, egg is rarely associated with chronic urticaria (20).
Up to 40 % of patients with atopic dermatitis are sensitized to some type of food (21). Lever et al (22) reported that the reduction in affected surface area and the symptom severity score was significantly greater in children who followed an egg exclusion diet than in controls.
Patients with oral allergy syndrome due to egg hypersensitivity have also been reported (23, 24).
Acute gastrointestinal symptoms normally develop between a few minutes and 2 hours after ingestion of the causative food and consist of nausea, abdominal pain, vomiting and/or diarrhea (4). Depending on the immunological mechanism involved, symptoms of gastrointestinal hypersensitivity, although markedly similar, vary in time of onset, severity and duration.
Isolated respiratory symptoms are infrequent and almost always are associated with cutaneous or digestive symptoms. In highly susceptible individuals, asthmatic reactions caused by the inhalation of the vapor or the smell of cooking egg have been observed. Respiratory symptoms after egg ingestion are more frequent in patients sensitized to bird proteins (bird-egg syndrome) (15, 25).
Food allergy is the most frequent cause of generalized anaphylaxis seen in hospital emergency departments and represents a third of all cases (26, 27). No specific data on egg hypersensitivity is available. Cases of anaphylaxis after the ingestion of small amounts of raw egg in individuals who previously tolerated cooked egg have been published (28). In cases of generalized anaphylaxis, in addition to cutaneous, respiratory and gastrointestinal symptoms, cardiovascular symptoms are also present, including hypotension, vascular collapse and cardiac dysrhythmia. Factors associated with severe reactions include concomitant asthma, a history of previous severe reactions and a delay in starting appropriate treatment. To date, we have found no reports of exercise-induced anaphylaxis after egg ingestion.
In some patients contact with egg can cause urticaria, although ingestion is tolerated. These patients have recently been observed to have IgE antibodies that recognize egg white epitopes unstable to the action of digestive enzymes (29).
DIAGNOSIS
The clinical history can lead to suspicion of egg hypersensitivity and its possible mechanism. In IgE-mediated reactions, skin tests (prick-test) and serum IgE determination (RAST, Pharmacia CAP system, fluorometric and other methods) reveal the presence of specific IgE antibodies. However, it is the oral challenge that confirms clinical reaction.
Clinical history and physical examination
A clinical diagnosis should be based on a detailed history that includes the following: patient age at the first adverse reaction to egg, duration of consumption, amount ingested and form of cooking causing the reaction, symptomatology, time between ingestion and onset of symptoms, repeat reactions, the treatment required and the time to resolution, as well as the date of the last reaction. Familial and personal antecedents of atopy should also be noted, paying special attention to the presence or otherwise of atopic dermatitis.
The clinical history should be completed by a thorough physical examination, paying special attention to the presence of eczema or dryness.
Skin tests
The skin prick test is the technique of choice to demonstrate sensitivity to food. In general, it is highly reproducible and if quality extracts are used it is an excellent means of confirming IgE-mediated allergy. The test should be carried out using the appropriate technique (30); a positive result is a skin weal more than 3 mm greater than that observed with the negative control. Appropriate positive (histamine 10 mg/ml) and negative (saline glycerin solution) control substances should always be used.
The available commercial extracts tend to be glycerinated and are not well standardized. Some are labeled in P/V units and others in mg/ml. The concentrations used are also different. In some studies whole egg extracts have been used at concentrations of 1/10 or 1/20 W/V (31, 32). In others, commercial egg white and egg yolk extracts have been used at concentrations of 10 mg/ml (33). Some authors have used purified egg proteins, such as ovalbumin, ovomucoid, ovotransferrin or lysozyme, although these have been demonstrated to be incompletely purified and all of them can be contaminated with other proteins (12). In one study (34), in addition to egg white extract at concentrations of 10 mg/ml and egg yolk at concentrations of 1/20 W/V, purified ovalbumin and ovomucoid proteins were used in the skin prick test at concentrations of 10 mg/ml; the highest diagnostic yield was obtained with egg white followed by ovalbumin. Egg yolk is generally considered to be less allergenic than egg white, but it contains livetins and alfa-livetin has been identified as chicken serum albumin (35); egg yolk is unlikely to produce hypersensitivity reactions in children (25, 36).
When analyzing the diagnostic validity of the skin prick test in egg allergy compared with that of oral challenge, almost all studies have reported the sensitivity of the skin prick test to be high (73-100 %) (18, 32-34, 37) and with a high negative predictive value (86-91 %) (18, 33, 34, 38). Its specificity tends to be lower (53-71 %) (18, 32, 33) as is its positive predictive value (61 %) (18, 39). In study populations with a high prevalence of food allergy, high positive predictive values have been found (85-92 %) (33, 34). As is generally the case in the diagnosis of food allergy, in egg allergy a negative skin test excludes clinical reaction in most patients due to its high negative predictive value. If quality extracts are used at appropriate concentrations, the skin prick test is also a good predictor of egg allergy.
Intradermal allergy skin tests tend not to be used because of their lower specificity.
Although the skin of children under the age of 2 years does not react well (40), reactivity is good when the appropriate extracts are used at the right concentration (34).
In vitro tests
Sensitization can also be demonstrated by the presence of specific IgE antibodies in serum through the RAST or CAP techniques. Most studies report that RAST shows lower sensitivity and higher specificity than the skin prick test (37). A study comparing the skin prick test and RAST with oral challenge reported that RAST showed lower sensitivity than the skin prick test, but when the RAST score was 3 or more, the sensitivities were similar (39). CAP has higher sensitivity than RAST (41) and is a useful technique for correlating clinical reactivity with higher levels of sensitization (33, 42). CAP can be used to measure specific IgE antibody concentrations, thus identifying subtypes of patients with a high probability of positive challenge and eliminating the need to perform the challenge. In individuals with egg sensitivity and atopic dermatitis (33), the probability of a positive food challenge was greater than 95 % when egg white CAP values were 6 kU/L and was more than 90 % when egg white CAP values were equal to or higher than 2 kU/L. With values lower than 0.6 kU/L the probability of tolerance was greater than 90 %. In another study of children under the age of 2 years with egg allergy (34), egg white CAP values equal to or higher than 0.35 kU/L showed a positive predictive value of 94 %. The lower cut-off point found in this study could be related to the patients' age, a mean of 16 months compared with 5.2 years in the previous study. In the latter, moreover, all the children had atopic dermatitis, which could explain the higher IgE concentrations.
The basophil histamine release test is not used in daily clinical practice and tends to be used only in research. On the other hand, tryptase, a mast cell mediator, increases in serum following oral challenge, showing high specificity but low sensitivity, that which its titration is not usual practice.
Provocation tests
Controlled oral food challenges are used to confirm clinical reactions to food ingestion. Only approximately 50 % of suspected allergies are confirmed by double-blind food challenge (18, 37-39).
As in any provocation test, certain requirements should be fulfilled when performing oral food challenge: the patient should not be receiving medication that could inhibit skin tests and should be asymptomatic. The challenge should be performed by personnel experienced in treating allergic reactions and resuscitation equipment should be readily available (43).
Oral challenge can be open, in which both the patient and the physician conducting the challenge know which substance is administered, simple-blind, in which only the patient does not know which food is administered and double-blind, in which neither the patient nor the doctor know which food is administered. The double-blind placebo-controlled food challenge (44) is considered the gold standard for diagnosing adverse reactions to food (45). It is used in research investigating the effect of food in chronic conditions such as atopic dermatitis and bronchial asthma and when the involvement of multiple food substances is suspected. It is also used when a large subjective component may be involved. In blinded challenges, the substance is administered freeze-dried in capsules or, depending on the child's age, disguised in juices, milkshakes or infant formulas (31). In the placebo-controlled challenge, two challenges can be performed daily, one active and another with a placebo (46) or the placebo and the food extract can be administered on different days.
Open challenge can be used in the diagnosis of egg allergy in children aged less than 2 years because manifestations are easily observed and at this age the subjective component is limited. The challenge is performed gradually in fasting patients, beginning with the administration of an amount of the substance smaller than that which provoked symptoms and progressively doubling the amount until it equals half an egg white as a single dose or until symptoms appear (34). In immediate reactions, the substance can be administered at 15-90 minute intervals (46). If the reaction occurs later, this interval is greater. The patient should be followed up for 2 hours after the challenge in case immediate reactions develop. The challenge is positive when objective symptoms (cutaneous, gastrointestinal or respiratory) appear within 2 hours. Subjective symptoms, such as abdominal pain, nausea or pruritus are not used in the diagnosis. When subjective symptoms are reported, the double-blind challenge should be carried out.
Oral challenge has demonstrated cooked egg to be less allergenic than raw egg (11). In some studies, whole egg has been used in the oral challenge (32). In others, open challenge has been carried out, starting with cooked egg white and if results were negative, raw egg white was used (34). The amount of egg and the intervals at which it is administered can be modified according to the initial symptomatology presented.
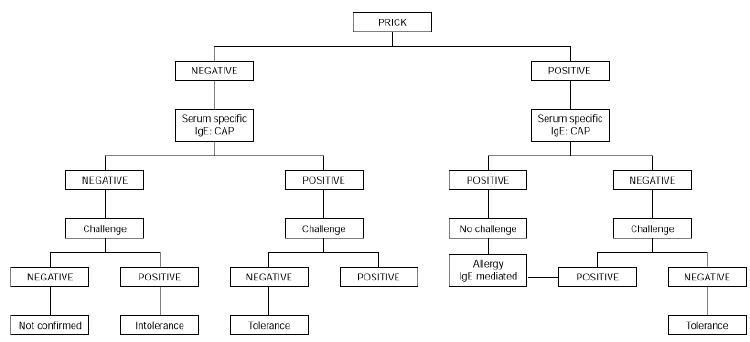
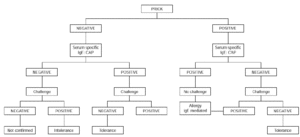
Oral challenge is contraindicated when severe symptoms of anaphylaxis and/ or glottis edema are present. It should not be carried out in children aged less than 2 years with immediate cutaneous, digestive and/or respiratory symptoms occurring within 2 hours of egg ingestion and with positive skin tests to egg white and an egg white CAP equal or greater than 0.35 KU/L. However, it is indicated if the skin prick test and the CAP are both negative or if the results are conflicting (34) (fig. 1).
Figure 1.--Diagnostic algorithm for egg allergy: immediate symptoms.
Exclusion diet
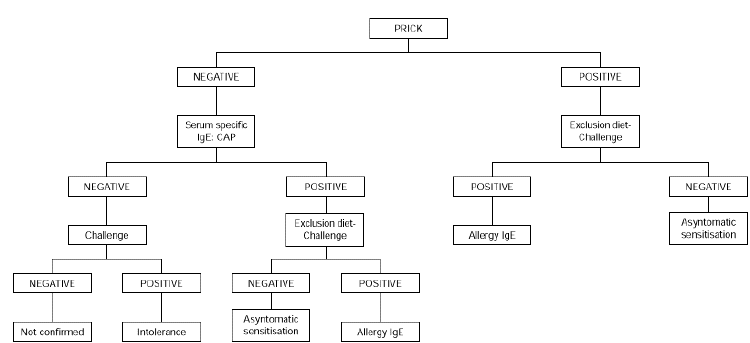
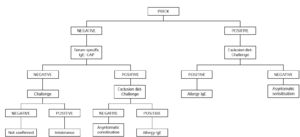
Exclusion diets should be used in patients with chronic symptoms such as atopic dermatitis, delayed gastrointestinal symptoms or asthma in an attempt to determine the role of egg sensitization in producing symptomatology. Egg is excluded from the diet for 2 weeks. If no improvement is seen during this time, eggs are unlikely to be causing symptomatology. If there is improvement, open challenge is carried out. If the result is negative, allergy is ruled out. A positive result should be confirmed by double-blind challenge (fig. 2).
Figure 2.--Diagnostic algorithm for egg allergy: atopic dermatitis and/or delayed gastrointestinal symptoms.
FOLLOW-UP, EVOLUTION AND PROGNOSIS
In the natural history of food allergy the clinical sensitization period is followed by another period of asymptomatic sensitization until complete tolerance is achieved and specific IgE antibodies disappear (47). The clinical sensitization period depends on the food involved, among other factors.
Not all egg-allergic individuals achieve tolerance. In some, the allergy persists for years and the longer symptomatic sensitization lasts, the lower the probability that future tolerance will be acquired (48, 49).
Studies of the natural history of egg allergy have reported that only 24 % of patients lost clinical hypersensitivity after following an exclusion diet for 1-2 years (49), between 32 % and 44 % lost clinical hypersensitivity after three years (49-51) and after a mean follow up of 4 years, 55 % of patients (with a mean age of 6 years) achieved tolerance. Clinical hypersensitivity that persisted at the age of 9 years was an index of poor prognosis (48).
Sensitization to egg before the introduction of this food into the diet has been demonstrated in lactating infants (52). The sensitization could have occurred during pregnancy but most probably took place after birth through exposure to egg proteins in the mother's milk. Breast-fed children sensitized to egg through this route can react on first exposure to egg (53). In a series of 21 patients aged between 5 months and 3 years (all of them were initially exclusively breast fed) with serum egg-specific IgE antibodies and/or positive skin tests to egg, which they had never previously ingested, double-blind foods challenges vs. placebo were positive in 13 (61 %) (18). Moreover, prognosis seems to be worse in these children, as indicated by a published study on long-term evolution, in which only 24 % of patients developed tolerance by the age of 14 years (54).
A progressive reduction in symptom severity and final tolerance can be expected with age even in anaphylactic reactions (55). Currently no clinical or serological parameters have sufficiently high sensitivity and specificity to determine the point at which tolerance begins but the following data may serve as a guide:
1. Multisystemic clinical reactions with angioedema and respiratory symptoms indicate a poor prognosis for future tolerance (50).
2. Although skin tests can remain positive in 50 % of individuals who achieve tolerance (48), a negative result is a good indicator of tolerance (50).
3. Initial serum specific IgE levels, especially to ovomucoid, may have prognostic value because individuals who develop tolerance have significantly lower serum IgE levels at the onset of symptomatology than children with persistent clinical hypersensitivity (12, 33, 51).
4. If, throughout evolution, specific IgE concentrations are higher than a RAST score of 2, food challenge is almost certain to be positive (48). In egg-allergic patients with atopic dermatitis, Sampson et al (33) recommend waiting for a reduction in serum specific IgE concentrations to 2 KU/l before repeating oral challenge. In patients without atopic dermatitis specific IgE values above 1.20 KU/l indicate a high probability of positive challenge (56).
In view of the current data, we recommend yearly monitoring of skin tests and egg-specific IgE antibodies each year. Oral challenge should be carried out to verify whether tolerance has been acquired when the results of skin tests are negative, when specific IgE levels are less than 2 KU/l in patients with atopic dermatitis and when these levels are less than 1.20 KU/l in patients without atopic dermatitis.
Although there is not an absolute contraindication to oral challenge, when symptoms of anaphylaxis are severe, the interval since the first episode should be more carefully evaluated, taking into account skin tests and variations in specific IgE concentrations.
The development of egg-specific IgE antibodies in children under the age of 1 year is a predictive risk index for atopic disease. Several studies suggest that hypersensitivity to egg might currently be the principal and earliest serological marker of risk for subsequent sensitization to inhaled allergens and for the development of allergic respiratory disease (10, 57, 58). At this age, a combination of a positive family history (history of atopic disease in at least one first generation family member) and concentrations of egg-white specific IgE antibodies greater than 2 KU/l, constitute a marker of future sensitization to inhaled allergens. This marker has a specificity of 99 % and a positive predictive value of 78 % (59). If sensitization persists for more than 1 year, there is a high risk for asthma (67 %) and rhinitis (50 %) at the age of 5 years (60).
TREATMENT
Etiological treatment: strict exclusion diet
Once a diagnosis of hypersensitivity to egg proteins has been established, a strict exclusion diet, the treatment of choice, should be started (61, 62). Exclusion diets should be adequately supervised to eliminate egg derivatives and possible contamination with these proteins. Special attention should be paid to certain proteins used as food additives that can provoke symptoms when unnoticed. Egg lysozyme is used in some drugs and in numerous foods as a bactericide to prevent the development of anaerobic bacteria such as Clostridium tyrobutyricum (13).
Clinical cross reactivity does not usually occur between egg and chicken meat (63) and consequently in most patients this source of protein need not be avoided. In feather-allergic individuals egg tolerance should be tested. Equally, egg-allergic individuals egg should take precautions when exposed to aviaries (15, 25, 64, 65).
Egg-containing foods
Table I lists the main foods containing egg. Careful reading of product labels is essential.
Lysozyme and other egg proteins can also be present in some medications (mainly suppositories, nose drops and some anesthetic preparations). For this reason, the list of components and excipients should always be read.
Symptomatic pharmacotherapy
If accidental egg ingestion is followed by anaphylactic reaction, parenteral adrenalin should be administered and repeat doses are often required. Intramuscular administration is recommended because absorption is more rapid (66). Treatment should be completed by antihistamine and corticosteroid administration. When the reaction is localized in the upper airways (laryngeal or oropharyngeal angioedema) inhaled adrenaline can be used. With asthma or spasmodic cough, a beta-adrenergic inhalant can be used. Children presenting an acute anaphylactic reaction should remain under observation for 24 hours (61).
Children with a history of anaphylactic reactions should keep a dose of adrenaline both at home and at school (67).
In cutaneous manifestations (urticaria and/or angioedema) the administration of an oral antihistamine may be sufficient.
Preventive pharmacotherapy
Preventive drug treatment has also been used (disodium chromoglycate, ketotifen and cetirizine) but the results are irregular and offer no improvement on avoidance of egg ingestion (61, 68, 69).
Immunotherapy
Experimental immunotherapy has been tried with other foods (70-73) but the results have not been encouraging. No experience exists of parenteral immunotherapy with egg and we have found only one study of oral hyposensitization that reported satisfactory results (74).
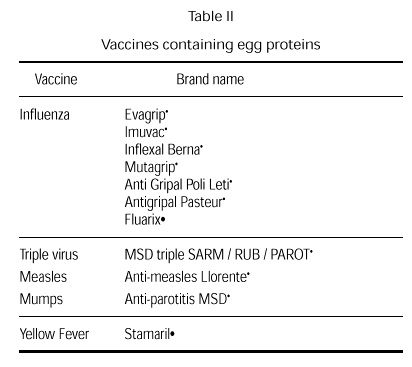
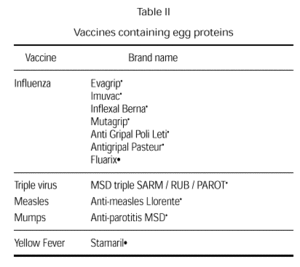
Vaccines that may contain egg proteins. What should be done?
Vaccines have always been controversial since they may contain small amounts of egg due to the way they are produced. Current vaccines that could potentially include egg products are the rubella, measles and mumps vaccine, the triple virus vaccine (measles, rubella and mumps), and the influenza and the yellow fever vaccines (table II). Small amounts of egg also used to be a possibility in the anti-typhus and antirabies vaccines (75). Anaphylactic reactions to vaccine components are rare. At times, it is even difficult to determine whether a reaction has been caused by the vaccine antigens or by any of the vaccine components (neomycin, sorbitol and, fundamentally gelatin) or whether the reaction is concomitant (76-83).
Triple virus vaccine
The triple virus vaccine (measles, rubella, mumps), obtained by chicken embryo culture in fibroblastic tissue, does not contain significant amounts of egg proteins (84, 85). Egg-allergic children, even those who are highly sensitized, are at very low risk for presenting anaphylactic reactions to these vaccines (86-89), although such reactions have been described (90, 91). Skin tests with diluted vaccine preparations do not appear to be predictive of possible allergic reactions after vaccine administration (85, 92-94). In 1997 the Committee of Infectious Diseases of the American Academy of Pediatrics proposed routine administration of the vaccine without prior skin tests (95), recommending that vaccinated patients should be observed for 90 minutes after vaccination by a team experienced in treating anaphylaxis (84).
In a study of 140 children with hypersensitivity to egg (85), 97.5 % of the children tolerated the triple virus vaccine (with 95 % reliability), showing no significant reactions, while tests were not predictive of adverse reactions. Another series of 410 children with hypersensitivity to egg showed no adverse reactions requiring treatment (92). Another study of 26 children with a history of anaphylactic reactions after egg ingestion reported no adverse reactions after vaccine administration (86).
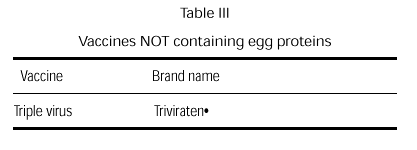
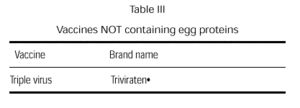
A triple virus vaccine cultivated in human diploid cells is currently available (table III). Although the possibility of an anaphylactic reaction is remote, we believe that this egg-free vaccine should be used in children with egg allergy.
Influenza vaccine
The influenza vaccine is prepared in chicken embryo and has been reported to contain small quantities of egg proteins (1-7 mg/ml). The Committee of Infections Diseases of the American Academy of Pediatrics has recently recommended that patients with anaphylactic reactions or very severe reactions after egg ingestion should not be administered this vaccine without prior skin testing with a diluted preparation of the vaccine (95). A positive result contraindicates vaccine administration. If the clinical situation indicates vaccination and if the results of tests are negative, the vaccine can be administered under medical supervision. In general, these children should not undergo influenza vaccination because of the risk of reaction and because yearly vaccination might be required.
Yellow fever vaccine
The yellow fever vaccine is also prepared in chicken embryo. A medical history of egg allergy and adverse reactions to previous yellow fever vaccines or other vaccines should be carried out in all patients before vaccination against yellow fever.
Skin tests should be only carried out before yellow fever vaccination in patients with a history of systemic anaphylaxis after egg ingestion (84, 96, 97). If immunization is required, the vaccine should be carefully administered at a medical centre in multiple gradual doses and by personnel with experience in treating anaphylaxis (84, 92, 95).
PREVENTION
Preventive measures are designed either to prevent sensitization and the development of allergic diseases or to prevent the manifestation of the disease in sensitized individuals.
Restriction diets in pregnant women to prevent sensitization before birth are not recommended nor do they guarantee effectiveness (67, 98). Moreover, unless perfectly controlled by a nutritionist, these diets can be dangerous due to the risk of fetal and maternal malnutrition.
Maternal avoidance of egg during lactation does not seem to be justified except in cases of high risk and when the family is highly motivated (67, 99-101).
In children at high risk, a skin test before the first egg ingestion can be predictive and prevent an adverse reaction to egg (18).
Independently of possible food hypersensitivity in children, a series of general environmental preventive measures should be implemented against possible allergens (102).