Hypersensitivity reactions to hymenoptera venom are infrequent in paediatric patients. A study was made to determine the incidence of this pathology in children, based on an epidemiological survey targeted to all members of the SEICAP (Sociedad Española de Inmunología Clínica y Alergia Pediátrica/Spanish Society of Paediatric Clinical Immunology and Allergy), and designed to collect the data on patients under 17 years of age diagnosed with hymenoptera venom allergy. Results: The data corresponding to 175 patients (135 males) were collected. The mean age was 9.9±3.6 years. Seventeen percent (32 patients) were the offspring of beekeepers, and 68.9% had experienced previous stings. The causal insect was Apis melifera, implicated in 55 cases, followed by Polistes dominulus (33 cases). In 151 patients (83.9%) the condition consisted of a local reaction. The most frequent systemic response was urticaria and angio-oedema. Fourteen patients suffered anaphylactic shock. The diagnosis was based on skin test (intradermal and prick) and/or specific IgE testing. Three treatment categories were established: (a) prevention and educational measures; (b) symptomatic treatment with oral antihistamines as well as self-injectable adrenalin; and (c) immunotherapy. In this context, 135 patients underwent immunotherapy with a mean duration of 3.5±1.7 years (range 2–5 years) – with excellent tolerance. The starting regimen was predominantly conventional (92 patients). Conclusions: The results of this survey show hypersensitivity reactions to hymenoptera venom to be infrequent in paediatrics, though with a strong impact upon patient quality of life.
The results of the Alergológica 2005 epidemiological study on allergic diseases in Spain have recently been published.1 In the chapter corresponding to hypersensitivity reactions to hymenoptera venom, paediatric patients are reported to be barely affected in comparison with the adult population. However, as has also been mentioned by other authors,2 it is not easy to establish the true prevalence of this problem in paediatric subjects. On the other hand, it has been postulated that such reactions are underdiagnosed, particularly considering that children show greater exposure levels than adults (except in the case of beekeepers), especially in urban settings, and their self-protection capacity is much more limited.
The SEICAP (Sociedad Española de Inmunología Clínica y Alergia Pediátrica/Spanish Society of Paediatric Clinical Immunology and Allergy), through its Immunotherapy Work Group, conducted a survey among all its members with the purpose of determining the situation of allergy to hymenoptera venom in Spanish children. Specifically, the aim was to determine the number of affected patients and the diagnostic and management approaches used, as seen from the specialised care setting.
Material and methodsIn 2006, a survey was conducted among all members of the SEICAP, designed to collect the data on all patients under 17 years of age diagnosed with hymenoptera venom allergy (HVA) by a specialist.
Specifically, the following information was collected:
Clinical history: patient relationship to beekeeping, personal and family history of atopic disorders, family antecedents of reactions to hymenoptera stings, and previous stings experienced by the patient.
The type of reaction experienced as a result of the sting leading to allergological study of the patient was registered, along with the time elapsed from sting to reaction.
Diagnostic tests used: specific IgE testing and skin tests.
Repeat stinging (spontaneous or induced) was also recorded.
Treatment provided: educational and preventive measures, symptomatic treatment and/or immunotherapy.
The characteristics of immunotherapy and tolerance of the latter were registered.
ResultsData were received on a total of 175 patients, of which 135 corresponded to children who were receiving or had received specific immunotherapy.
The number of patients studied in each region in the country is shown in Figure 1: most of the subjects were from Andalusia, followed by Navarra, the Canary Islands and Catalonia.
The mean patient age was 9.9±3.6 years (range 2–17 years). In the immunotherapy group these data were 10.3±3.7 years (2–17 years), versus 8.7±2.8 years (4–14 years) in the non-immunotherapy group.
Most of the patients (n=135; 75%) were males, 80 had a history of atopy (44.4%), 32 were the offspring of beekeepers (17%), and 68.9% had experienced stings prior to the episode leading to specialist consultation.
The most frequently implicated insect was Apis melifera (55 patients), followed by Polistes dominulus (33 patients). In 32 patients the causal insect could not be identified, and in 39 patients several insects were involved.
Type of reaction and clinical characteristics of the stingA total of 151 patients suffered local reactions (83.9%). As regards systemic reactions at the time of the sting, the most common manifestations were urticaria and angio-oedema. Fourteen patients suffered anaphylactic shock.
The time elapsed from sting to onset of the reaction was under 30min in 154 cases; 30–60min in three cases; and over one hour in 11 cases. The time from sting to onset of the reaction was not known in 12 cases.
DiagnosisThe diagnosis was based on skin test (intradermal and prick) and/or specific IgE testing.
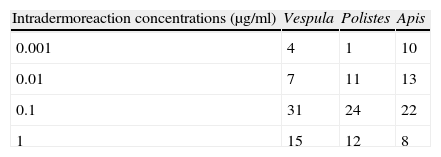
The results of the skin tests (Table 1) show that at the lowest concentrations (0.001 and 0.01μg/ml), the number of positive readings was very low. Most positive readings corresponded to the concentration of 0.1μg/ml.
Table 2 in turn presents the specific IgE values obtained, showing predominance of the intermediate IgE classes (2 and 3).
A total of 18 patients (17 belonging to the immunotherapy group) suffered repeat bites (accidental in 12 cases), without any associated systemic manifestations.
TreatmentThree treatment categories were established:
- 1.
Educational and preventive measures.
- 2.
Symptomatic treatment with oral antihistamines as well as self-injectable adrenalin.
- 3.
Immunotherapy.
In the group of patients without immunotherapy, 19 children only received educational and preventive measures, while 26 received such measures together with symptomatic treatment in the form of oral antihistamines and self-injectable adrenalin.
In the group of patients administered immunotherapy, two children received only immunotherapy, while 19 additionally received educational and preventive measures, and 114 received all three of the above-mentioned treatment categories.
In reference to immunotherapy, 135 patients followed treatment with a mean duration of 3.5±1.7 years (range 2–5 years). The immunotherapeutic regimen used was predominantly conventional (92 patients), followed by a rush protocol (25 patients) and, less frequently, a cluster protocol (16 patients). An ultra-rush immunotherapy protocol was employed in only two cases.
With regard to the type of immunotherapy used, a Vespula extract was administered in 39 cases, a Polistes extract in 45, and an Apis extract in 45. Two extracts were used in two patients: Vespula and Polistes.
The tolerance of immunotherapy was excellent: 35 patients experienced local reactions – 7 treated with Vespula (17.9%), 14 with Polistes (31.1%), and 14 with Apis (28.6%). Systemic reactions in turn were documented in 6 patients: two with Apis extract which proved mild, while three developed Müller type 3 reactions3 (two with Vespula and one with Apis extract), and one patient developed a type 3 reaction with Polistes extract. As regards the regimen employed, two reactions occurred with conventional immunotherapy, one with a rush regimen, and another with a cluster regimen.
DiscussionEvaluation of the data obtained on the 175 patients relating to the diagnosis and treatment of hymenoptera venom allergy in children showed the distribution of patients to vary with respect to the information published by the Alergológica 2005 study,1 where most cases were seen to come from Andalusia, followed by Galicia and Castilla. However, in our series most cases were from Andalusia, the Canary Islands, Navarra and Catalonia. Clearly, the voluntary nature of collaboration and the non-utilisation of sampling techniques explain this situation.
Coinciding with the literature, the percentage of affected boys (75%) was far greater than the percentage of girls (23.3%),1,4 though in reference to beekeeping or agriculture our findings differ, since only 17.8% of the children were the offspring of beekeepers. This indicates that insect stings in our paediatric patients occur in the context of outdoor playing activities, since among adults the highest percentages (70.1%) correspond to the rural setting, and in 52.8% of the cases occupational or leisure activities entail a high risk of insect stings.1,5
In turn, previous stings were reported in 68.9% of the patients – this percentage being similar to the values reported in the literature for the general population.1,5,6
In turn, 44.4% of the children had antecedents of atopy – this figure being higher than that reported in the literature.1,4
With respect to the type of insect involved, Apis melifera was implicated in 69 cases, followed by Polistes dominulus (42 patients). Vespula was not often involved – this possibly being explained by the fact that the activities of the children are related to their playing areas. Among adults, the causal insect is related to professional or occupational factors: Apis melifera in beekeepers or farm workers, etc.1,5,7
In reference to the type of clinical manifestations recorded, we found that the patients not administered immunotherapy logically experienced milder reactions (generally grade 1), with exclusively non-specific symptoms in a large number of cases. The systemic manifestations documented in the literature for paediatric patients6 are generally urticaria and anaphylaxis in 0.4–0.8% of cases. In our series, the systemic manifestations were of grade 2–3 (urticaria and angio-oedema).
In addition to the clinical history, explorations, skin tests and/or specific IgE determinations were made to confirm the diagnosis, in compliance with usual practice in all studies of hymenoptera allergy. In relation to skin testing, mention should be made of the fact that scant positivity was recorded for concentrations under 0.1μg/dl. In children, the relationship between the concentration at which testing proves positive and the severity of the reaction has not been established,8,9 although it does seem that the lower concentration is of little use in establishing the diagnosis. Repeat sting testing was not carried out in any of our patients for diagnostic conformation or for follow-up of the response to immunotherapy, in line with the little use made of this technique in the paediatric population.
All patients subjected to immunotherapy are advised and instructed on the use of self-injectable intramuscular epinephrine,8,10 as well as on the avoidance of stings, in abidance with the international guidelines for patient control and follow-up.
Immunotherapy has been indicated in patients with systemic manifestations after stings,8,10 although it has also been used in subjects experiencing important local reactions, with positivity in the in vivo and in vitro tests, as these are patients at risk due to exposure in leisure areas. Nevertheless, the indication of immunotherapy in these patients should be assessed on the basis of the consulted literature sources.10,11
In most cases the type of immunotherapy regimen was conventional, although in 43 patients other regimens were used, in view of the good tolerance presently demonstrated by the rush regimens.12–15 Tolerance in our series was good, and only 35 patients developed local reactions which required no change in regimen. Regarding the systemic reactions, our recorded tolerability was better than that indicated in the literature 16–18 and that described with other types of extracts. In this sense, improved tolerance of these extracts can be assumed in children, with good acceptance and only few dropouts.
ConclusionThe results of this survey show hypersensitivity reactions to hymenoptera venom to be infrequent in paediatrics, and involve conduction of a complete allergological diagnosis. Correct patient education is indicated both to avoid further stings and to treat any accidental exposures. The use of immunotherapy in these children is established according to the indications related to this pathology in paediatric patients.
The authors thank the contribution of the following investigators: F. de la Torre-Morín. J.M. Lucas-Moreno, J.C. Cerda-Mir, A. Martorell-Aragones, S. Nevot-Falco, R. Guspi, J.M. García-Martínez, M.F. Martín-Muñoz, M.C. García-Ara, M.M. Bosque-García, M.T. Marco-Valls, H. Larramona-Carrera, A. Malet-Casajuana, P. Amat, M. Roig-Riu, L. Moral-Gil, A. Rodríguez-Caamaño, A. Molina, J. Torres, L. Zapatero-Remon, E. Alonso-Lebrero, M.I. Martínez-Molero, J.E. Sancha-Pacheco, A.M. Paya-López, A. Armentia-Medina, A. Bilbao-Aburto.