INTRODUCTION
Investigation on hemopoietic precursor cells (HPC) is still a field of interest mainly devoted to haematology for transplantation purposes.
Several lines of evidence, nevertheless, stand for a critical role played by stem-cells in establishing allergic inflammation. Increased levels of circulating hemopoietic precursors committed to the myeloid lineage such as CD34+ granulocyte/macrophage, basophil/eosinophil and mast cell colony-forming cells have been reported in allergic rhinitis, asthma, nasal polyposis and atopic eczema (1-4). CD34+ progenitors have been also identified in cellular infiltrates at peripheral sites (5, 6) confirming the important contribution of systemic and local hemopoietic events to allergic inflammation (7, 8). The mentioned evidence has supported the hypothesis that tissue cellular allergic inflammation could be the result of a peripheral homing of bone marrow circulating precursors committed or, if lineage negative, "in situ" committed towards myeloid or lymphoid lineage by differentiation-growth factors supplied by local epithelium (9). This dynamic pathogenetic view was raised as an extension of the micro-environmental differentiation hypothesis of airway inflammation, originally proposed by Denburg and concerning only the myeloid lineage (10).
In the present work we evaluated the relation potential between the CD34+ cells raised peripheral traffic and the severity of the allergic inflammation and if such a measurement could constitute, together with the conventional study of circulant specific-IgE, a future useful laboratory tool for the assessment of the inflammation severity.
MATERIAL AND METHODS
Study populations
The whole amount of the peripheral CD34+ cells, containing more or less immature elements (Lin+, Lin), was assessed and compared in the peripheral blood of two groups of subjects. Patients demographic, clinical and laboratory parameters are resumed in table I. This research was planned as a no-profit, retrospective pilot study.
Blood samples were all obtained during the diagnostic routine procedures and subjects gave their oral consent for the data processing. The study has been notified to the S. S. Annunziata Hospital ethics committee.
Control group
Twenty healthy subjects (10 males and 10 females, mean age 24.5 years) negative to the skin prick test (SPT) allergen screening panel and negative for clinical history of allergy were selected as control group.
Group of pathological patients
Twenty-two patients (13 males and 9 females, mean age 28.9 years) independently whether under treatment or not, were enrolled. Eighteen had the extrinsic form (specific IgE +) of rhino-conjunctivitis (RC) and/or asthma (A), eczema (AD), urticaria (Ur) and food adverse reactions (FAR). Four patients had the intrinsic form (specific IgE-) of the same diseases. In all patients the test for CD34+ cells was performed at enrolment (T0) and in 12 subjects undergoing sublingual immunotherapy (SLIT) the measurement was repeated at different times of the therapy course (T1 = one year after; T2 = two years after). It must be underlined that in SLIT-treated patients T0 means only the time of the first test for CD34+ cells, and that only for a few subjects T0 means before the beginning of SLIT. Since allergic patients have often more than one organ or apparatus involved by inflammation, an arbitrary severity score (SS) able to take into account all relevant symptoms has been used. The SS was also referred to the minimal drug therapy required to control the inflammation. The disease severity has been thus assessed on a five-point scale: 0 = no symptoms (no drug therapy required); 1 = mild symptoms lasting less than two months a year (drug therapy on demand); 2 = mild symptoms lasting more than two months a year (intermittent therapy with last-generation oral antihistamines and topical steroids); 3 = moderate to severe persistent symptoms (continuous therapy with antihistamines and topical steroids); 4 = severe symptoms (emergency patients requiring systemic steroids).
Sublingual specific immunotherapy (SLIT)
SLIT was performed with commercial extracts whose major allergens content was known (ALK- Abellò, Milan and Anallergo, Florence, Italy) and administered according to the standard procedures as for respiratory allergies. This means a build-up phase with daily increasing dosages for around one month, followed by the administration of the maintenance dose 3 times a week for at least 3 years.
Skin tests and IgE measurements
Skin prick tests were performed with extracts of Grasses (Gr), Parietaria judaica (Pj), Olive (Ol), Mugwort (Mw), Cupressus arizonica (Cp), Plantago lanceolata (Pl), Alternaria alternata (Aa), Dermatophagoides pteronyssinus and farinae (Dp, Df) supplied by ALK-Abellò (Lainate, Milan, Italy) and Anallergo (Florence, Italy). Skin responses were expressed according to a scale: 0 = reaction elicited by negative control and 3 = wheal elicited by 1 mg/ml histamine solution. In patients assuming antihistamines or affected by severe symptoms, specific-IgE for the above mentioned allergens and/or total IgE have been studied in vitro by means of the routine CAP-FEIA System (Pharmacia, Uppsala, Sweden) in accordance with the manufacturer's instructions with concentrations of ≥ 0.35 kU/mL (CAP class 1) being classified as positive.
Flow cytometric enumeration of CD34+ cells: technical aspects
The Milan Protocol of Peripheral Blood CD34+ Cell Estimation, partially modified, was followed (11).
Peripheral or cord blood venous samples were collected in ethylendiaminetetraacetic acid (EDTA) tubes. Three aliquots of 50 μl of whole blood were incubated at 4 °C for 25 min. in the dark, one with 15 μl of phycoerythrin (PE)-conjugated murine monoclonal antibody (MAB) specific to CD34 molecule, one with PE-conjugated MABs specific to molecules unrelated to human leukocyte antigens, and one as unlabelled control. Monoclonal antibodies were purchased from Becton Dickinson (BD), Milan, Italy. PE anti-CD34 MAB was the Anti-HPCA2, Clone 8G12 (BD), suitable for routine use (12, 13). Then, 2 ml of red blood cell lysis buffer (BD) were added to each tube incubated afterwards at room temperature for 10 min, in the dark. After lysis cells were washed twice with 2 ml of cold phosphate buffered salt solution (PBS) (BD) at 1,200 rpm (300 g) for 7 min at 4 °C and then resuspended in 500 μl of PBS.
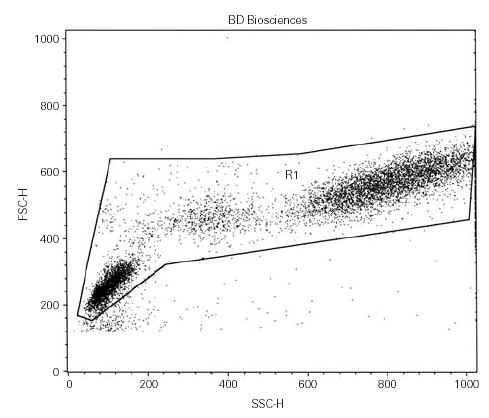
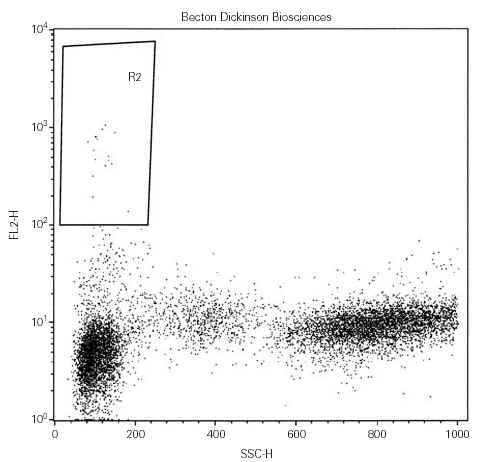
Cell were analyzed using a FACScan flow cytometer equipped with Lysis II software until June 2001, then using a FACSCalibur with the Cell Quest software. Three data parameters were acquired and stored in list mode files: linear forward scatter (FSC) (vertical axis), linear side-angle scatter (SSC) (horizontal axis) and log PE fluorescence, by gating whole viable cells (fig. 1). Since our aim was to enumerate both CD34bright and CD34dim cells, the setting of the fluorescence analysis region 2 (R2) was fixed with the lower limit at 102 avoiding any further compensation, when possible (fig. 2). At least 10,000 events were acquired for each measurement. Results were expressed as percentage of positive cells.
Figure 1. Cell analysis by flow cytometer.
Figure 2.
To test and standardize the method before running our research CD34+ cells were investigated in cord blood of 41 unselected newborns; in each sample the percent of eosinophils and total IgE levels have been evaluated.
Statistics
Data distribution was analysed by mean of the Shapiro-WilK W test for normal data and by the Skewness/Kurtosis test for Normality. Given the not- normal (asymmetrical) distribution emerged, data was displayed as range, median and interquartile range.
CD34 values and symptom scores relation was investigated with the non parametric test of Spearman.
Results
CD34+ cell values in healthy human beings ranged from 0.01 % to 0.09 %. The median was 0.03 % and the interquartile range 0.020.06 %. In the control group thus, all values were < 0.10 %.
In cord blood CD34+ cell values ranged from 0.11 to 3.24 %. The median was 0.38 % and the interquartile range 0.270.46 %. No significant correlations were found among CD34+ cell number, eosinophil number and total-IgE amounts.
In the patients' group CD34+ values ranged from 0.03 % to 0.79 %. The median was 0.25 % and the interquartile range 0.130.33 %. The relation between CD34+ values (as first measurement at T0) and severity scores was highly significant (number of observation = 21; Spearman's rho = 0.954. Test of Ho: CD34 and score independent: Pr < t = 0.000).
Data concerning the CD34+ traffic reduction during the therapy course (repeated measurements at twelve-month intervals in the same patient) is summarised in figure 3 and descriptively commented.
Figure 3. CD34 peripheral cells at different SLIT time-course.
Discussion
Several studies have established that most of the ability to reconstitute multilineage hematopoiesis resides in the CD34+ cell population (14). CD34 antigen in particular is believed to be up-regulated by activation-proliferation signals and thus to represent a marker of activated stem cells when CD34- compartment appears to contain quiescent HPC (15, 16).
Flow cytometric study of HPC is widely employed for stem cell grafts or for immunophenotyping leukemias and lymphomas. Thus far several protocols with increasing degrees of complexity have been developed for CD34+ cell enumeration and recently compared and well reviewed (17). Among the many based on multiparametric and multicolor analysis criteria capable of providing all the detailed information essential to the onco-hematology and transplantation requirements, we have chosen the simplest one fulfilling our needs, i.e. the study of the whole peripheral cells expressing on the surface high and low density of the investigated marker. Such an approach, in fact, could furnish information about the total amount of circulating activated stem and progenitor cells, independently from the lineage-commitment, the differentiation status and the site of mobilization. Bone marrow is the major but not the unique source of circulating precursors since developmental events have been reported at peripheral sites not only for cells of the myeloid lineage but also for the lymphoid lineage (18-22). It is therefore possible that part of the circulating immature cells originate from peripheral re-mobilization mechanisms.
The results of our study support the methodological choice inasmuch CD34+ values, even following an asymmetrical distribution, highly relate with score severity.
Of interest is the slightly increased traffic in patients suffering from intermittent or rare symptoms (SS = 1). Such a situation is frequent when allergy develops (disease onset) or in subjects close to the clinical recovery as the consequence of a well performed and effective specific immunotherapy. Acute inflammation is clinically evident through severity of signs and symptoms, but allergists have a great need for non-invasive tests that could be routinely used to assess low and minimal persistent inflammation (MPI).
The latter is a fundamental concept, proposed and investigated by the Genoa School of allergy (23, 24), closely linked to the concept of mucosal non-specific hyperreactivity. They have demonstrated that a persistent inflammation is constantly detectable in patients without symptoms. On the other hand, MPI has been related with the perennial exposure to allergens.
MPI and hyperreactivity are nonetheless clinical events also present in patients with, e. g., olea allergic rhinitis in November when pollen grains are undetectable in the air.
The "minimally increased CD34+ cell traffic" identified in quite-asymptomatic subjects, raises the question of the potential causative relation linking both phenomena.
Actually, non-specific hyperreactivity could be also observed in the intrinsic form of allergy in which allergen-specific IgE are, by definition, lacking. The increased CD34+ traffic in all four subjects suffering from intrinsic rhino-conjunctivitis, asthma and atopic dermatitis (patients 19-22), strongly suggests that cellular inflammation in intrinsic and extrinsic forms of allergy could have a common origin, being the result of a peripheral homing of more or less immature circulating precursor cells (9, 10). This could explain the reason why patients with atopic dermatitis and without asthma are positive to non-specific provocative tests and show an increased permeability of the gut mucosa (25-27).
Because the method used by us showed a highly significant relation between CD34+ traffic and symptom score, it should be regarded as a future candidate for both the monitoring of the disease activity and the effectiveness of the therapy.
The number of CD34+ circulating cells appears to be highly sensitive to drug therapy. We have in fact observed a dramatic fall of the values just one day after the beginning of a steroid-based local treatment. This may be due to the production at inflammation sites of factors (stem cell factor) capable of acting at distance on the bone marrow HPC mobilisation.
But the HPC traffic appears to be also affected by the action of sublingual specific immunotherapy (SLIT). All SLIT-treated patients in which serial measurements were performed, underwent a progressive reduction of the peripheral CD34+ values, also when pharmacological therapy was discontinued and also when the allergen-specific IgE appeared quantitatively unmodified by SLIT.
Patient n.º 4, for example, before starting SLIT experienced severe seasonal allergic asthma episodes leading to repeated recoveries in an emergency unit. On the 3rd and 4th year of specific immunotherapy this subject was free from any symptom (SS = 0) and the CD34+ value was within the normal range whereas levels of IgE specifc to olea and grasses remained very high. We have discussed elsewhere the potential qualitative modifications induced by specific immunotherapy on IgE leading to a lack of pathogenicity (28). In this report we show that the measurement of CD34+ cells seems to be a reliable indicator of the running down of the disease activity.
Monitoring CD34+ cell traffic appears furthermore useful in supporting the clinical decision to continue/discontinue specific immunotherapy in asymptomatic, clinically recovered subjects. Commonly recommended inflammation markers such as exaled nitric oxide (NO), serum eosinophil cationic protein (ECP) and eosinophil count in induced sputum are still controversial in their relationship with current symptoms and physiological measurements (29-32).
CD34+ cells measurement, likewise, could give reliable information when drugs can be discontinued during specific immunotherapy. The currently accepted global therapeutic strategy for allergic diseases is now targeted to inflammatory phenomena rather than to symptoms, thus so direct information over the amounts of circulating HPC are essential to verify the effectiveness of combined therapies.
Very recently we have proposed that last-generation antihistamine drugs could facilitate the SLIT mechanism of "physical immuno-deviation" (33, 34) by interfering with homing processes.
HPC mobilisation from bone marrow matrix, re-circulation and homing at peripheral sites are phenomena in which cell adhesion molecules (CAMs) play a pivotal role, particularly for those expressed on HPC (homing receptors) and on endothelial cells (addressins).
Among the many molecules involved in cellular allergic inflammation and in HPC traffic, that are integrins, Ig-gene superfamily products, cadherins and selectins, the former are a key family of cell-surface receptors mainly involved, together with their reciprocal counter ligands expressed on endothelial cells, in the mentioned events. Such systems have been therefore extensively investigated as target potential of manipulation.
Attention has been focused on aL integrin (LFA-1, CD11a) that is the cell homing receptor to the endothelial ICAM-1 (CD54, a member of the Ig-gene superfamily) and on α4 integrin (CD49d, with β1 integrin constitute the very late activation antigen VLA-4) that is the cell homing receptor to the endothelial VCAM-1 (CD106) (35-39).
Circulating inflammatory cells home tissues at the peripheral places where they are captured by addressins (mainly VCAM-1 and ICAM-1) expressed on the surface of vascular endothelia.
Last generation anti-H1 drugs like cetirizine, mizolastine, loratadine and particularly the newly developed desloratadine have been proved to be capable of inhibiting the expression of vascular adhesion molecules (40-43). As the result of such an inhibition (vascular CAMs down-regulation) inflammatory cells could not be recruited at periphery and continuously re-circulate in the blood stream. The contemporary oral administration of allergen extract during SLIT, being the stimulation concentrated and constant, could get over drug inhibitory effects and induce an over- expression of addressins (vascular CAMs up- regulation) on the vessel endothelium restricted to the oral district. This may lead to an obliged and selective cell homing toward the oral mucosa (physical sequestration), where cells are subsequently negative selected.
The proposed mechanism, actually under experimental trial, recognises its theoretical basis in that VCAM-1 expression could be induced by a variety of polyclonal stimuli such as phorbol-myristate acetate (PMA) (38), bacterial lipopolysaccharide and cytokines as tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL1β) (44-48).
Because TNF alpha and IL1 beta belong to the mannose-binding lectin (MBL) family (C Type) (49-51) as the dust mite major allergen Der p2 does (34, 52, 53) an analogue effect on the vascular CAMs expression can be expected.
In conclusion, data from our pilot study suggests that the investigation of the CD34dim/bright cell traffic with the described method is highly promising for monitoring cellular inflammation in allergic diseases. The potential of the test, as well as its sensitivity and specificity, should be exploited through a multicentre study currently in the planning phase.
AKNOWLEDGMENTS
The authors thank Prof. Judah Denburg for helpful discussion and advice during his stay in Taranto in 1999 and Dr. Luisa Zanolla, Central Hospital of Verona, Cardiologic Department, for statistical assistance.