Common acute viral respiratory infections (colds) are the most frequent cause of exacerbations in infants with recurrent wheezing (RW). However, there is no quantitative information about the effect of colds on the lung function of infants with RW. This study was undertaken to determine the effect of common cold on forced expiratory parameters measured from raised lung volume in infants with RW.
MethodsSpirometric lung function (expiratory flows from raised lung volume) was randomly assessed in 28 infants with RW while they had a common cold and when asymptomatic.
ResultsIt was found that during colds there was a significant decrease in all forced expiratory parameters and this was much more evident for flows (FEF50%, FEF75% and FEF25–75%) which were definitively abnormal (less than −1.65 z-score) in the majority of infants. There was not association between family asthma, tobacco exposure, and other factors, with the extent of lung function decrease during colds. Tobacco during pregnancy but not a history of family asthma was significantly associated to lower expiratory flows; however, the association was significant only when infants were asymptomatic.
ConclusionThis study shows that common colds cause a marked reduction of lung function in infants with RW.
Most wheezing episodes and asthma exacerbations in children and adults are caused by viruses responsible for upper respiratory tract infection (URTI) or common colds.1–3 Of the several known viruses which cause the disease, the role of rhinoviruses is most prominent but several other common respiratory viruses are associated with respiratory illness and wheezing in infants and children.4–7
Experimental studies in adult asthmatics have demonstrated that rhinovirus, one of the most common causes of natural colds, can increase airway responsiveness, inflammation, and decrease lung function very early (in the first 2–4 days) after experimental inoculation,8 whereas other authors have not found significant changes in FEV1 in asthmatics after rhinovirus inhalation.9 Under clinical conditions, it has been demonstrated that airway inflammation occurred during natural cold caused by different viruses (influenza A, rhinovirus, adenovirus, respiratory syncytial virus, and coronavirus) is significantly greater in asthmatics than in healthy subjects.10
At present there is no information in infants with RW as to whether URTI caused by common respiratory viruses results in significant airway obstruction, regardless of the fact that some mechanisms involved in virus-induced airway obstruction may also be present in infancy.11,12 It has been found that viruses commonly causing URTI can induce an asthmatic clinical response in infants during the first year of life and later during childhood,13–15 but the effect of URTI on their lung function is unknown.
The objective of this study was to quantify the effect of URTI (common cold) on the spirometric lung function of infants with RW assessed with the raised volume rapid thoracic compression technique (RVRTC), a sensitive technique to assess bronchial obstruction in infants.16
Materials and methodsSubjects. Thirty infants with recurrent wheezing (three or more previous episodes of physician diagnosed wheezing) and without antecedents of hospitalisations; severe lower respiratory infection in the last 8 weeks; emergency room visits; or oral corticosteroids in the last 8 weeks, were randomly recruited from our paediatric respiratory outpatient clinic to measure their spirometric lung function. Lung function measurements were performed at random in two occasions for each patient (each one was its own control), either during the first days of an URTI or when they had been asymptomatic during 4 weeks and had normal physical examination at the measurement day. Infants with fever; respiratory distress; RSV bronchiolitis; pneumonia; chronic lung diseases; neonatal respiratory illness requiring oxygen or mechanical ventilation; or any other condition potentially affecting airway responsiveness, were not eligible for the study.
URTI (common cold) was defined by the presence of runny nose (watery nasal rhinorrea), sneezing, nasal obstruction, without fever, and eventually cough, plus the antecedent of family members with similar symptoms at home (parent, siblings) or other children with a cold if they attended day-care. No viral determinations were done because they were not available at the hospital. Infants were on treatment with inhaled salbutamol on-demand and regular budesonide delivered by valved holding chamber with mask; treatments were continued as prescribed by their doctors, excepting salbutamol which was withheld 12h prior to measurements. The study was approved by the Hospital Ethics Committee and full informed written and signed consent was obtained from all parents.
Lung Function Testing. Forced expiratory manoeuvres were obtained using the raised volume rapid thoracic compression technique (RVRTC) as previously described.17–19 Infants were measured 4 hrs postprandial and while sleeping under sedation with chloral hydrate (70–90mg/kg). Lung inflation pressure was set at 30cm H2O, and after delivering several sequential inflations rapid thoracic compression was from raised lung volume and maintained until residual volume was reached. Forced expiratory manoeuvres were repeated with increases of 10cm H2O in jacket pressure until there was no further increase in flows with subsequent increase in jacket pressure. The best curve was selected from three reproducible curves, as the single best shaped curve with the highest product of forced vital capacity (FVC) and FEF between 25% and 75% of FVC (FEF25–75%). The following parameters were recorded for all curves: FVC, FEV0.5, FEF50%, FEF75% and FEF25–75%.
Data analysis. Values for each pulmonary function parameter were converted to z-scores using the equations of Jones et al18 and the comparisons of spirometric values during URTI and when asymptomatic were done using parametric test for paired samples; p <0.05 was considered as statistically significant. For all computed lung function parameters a change of one or more z-score (increase or decrease) was considered as significant. Abnormal lung function at the time of measurements was defined as a z-score of ≤ −1.65 for a given spirometric parameter in both occasions (symptomatic or asymptomatic patients). Spearman correlation was used for continuous variables whereas chi-square tests and regression analysis were used to relate the change in lung function to history of a first-degree relative with asthma, gender, age, exposure to cigarette smoke during pregnancy or intra-domiciliary, order of measurement, time elapsed between measurements, age at onset of wheezing, and number of previous episodes of physician-diagnosed wheezing.
ResultsTechnically satisfactory spirometric measurements on both occasions (when patients were with URTI or asymptomatic) were obtained in 28 wheezy infants (18 males). In 10 patients lung function was measured first when they were asymptomatic and in 18 it was measured first when they were suffering from URTI. The mean delay between tests was 10.4 weeks (95%CI, 8.6–12.3). Anthropometric data, family history of asthma, and other characteristics of the studied infants are shown in Table 1. There was no significant difference in baseline oxygen saturation between measurements when infants were asymptomatic or during URTI [97.2% (95%CI, 96.9–97.5) and 97.6% (95%CI, 97.2–98.09)], respectively. History of family asthma was present in 15/28 infants whereas exposure to tobacco during pregnancy was positive in 9/28, Table 1.
Anthropometric and epidemiological characteristics of patients.
| N=28 | Asymptomatic | URTI |
| Age, wk | 66.53 (57.54–75.52) | 62.37 (54.04–70.70) |
| Length, cm | 76.88 (74.62–79.14) | 75.89 (73.68–78.10) |
| Weight, kg | 10.68 (10.08–11.28) | 10.40 (9.85–10.95) |
| Sex, M/F | 18/10 | |
| Weight newborn, g | 3489 (3272–3706) | |
| Height newborn, cm | 50.34 (49.34–50.66) | |
| Age at onset of wheezing, months | 2.68 (1.76–3.60) | |
| Number of previous wheezing episodes | 7.50 (5.82–9.18) | |
| Environmental tobacco smoke, yes/no | 17/11 | |
| Tobacco smoking during pregnancy, yes/no | 9/19 | |
| History of family asthma (first-degree relative), yes/no | 15/13 | |
Values are expressed as mean and 95% confidence interval.
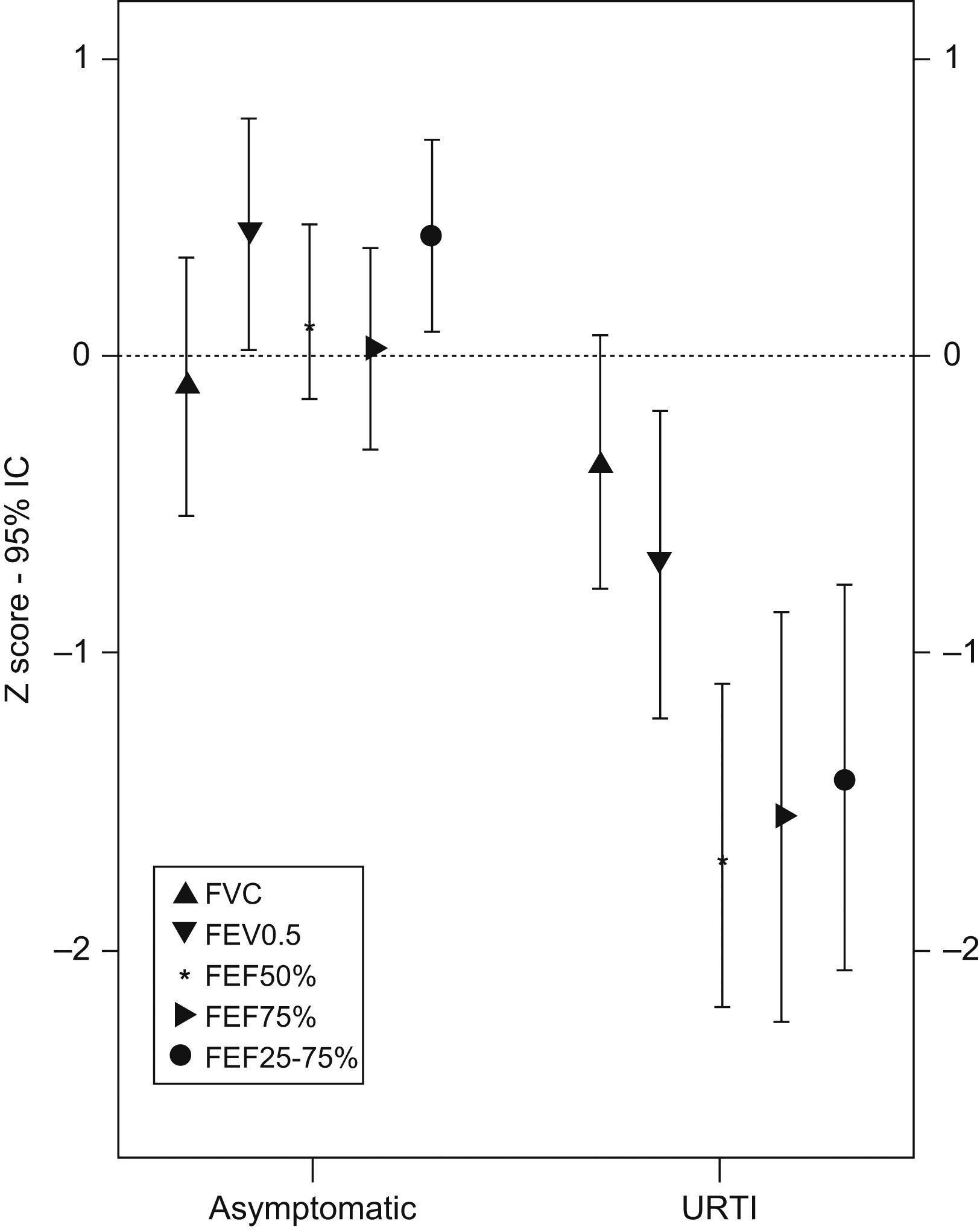
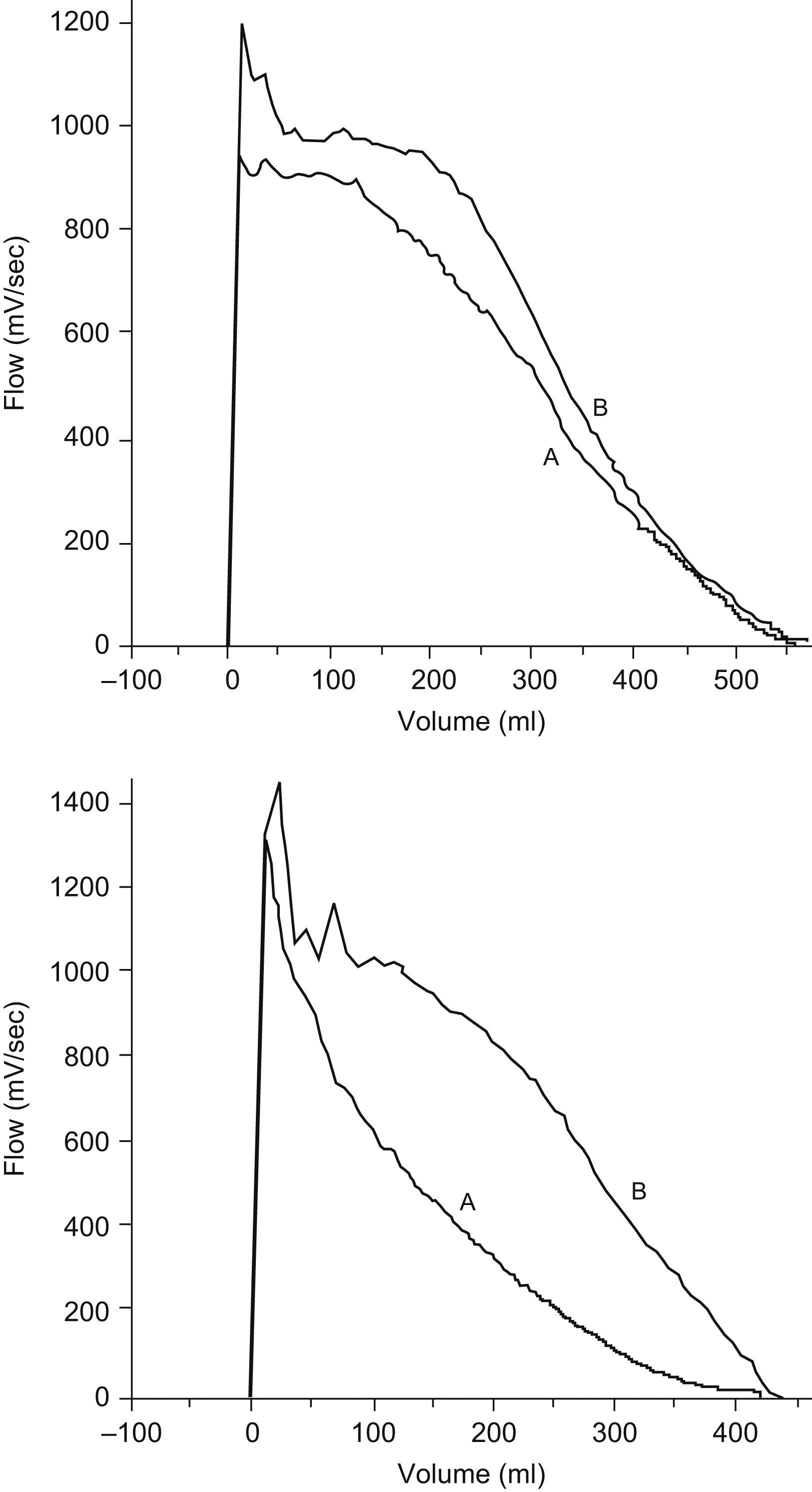
Mean values and 95% CI for lung function measurements in z-score are shown in Table 2. URTI caused a marked and significant decrease of lung function as compared with measurements performed when infants were asymptomatic. The percentage of infants with a lung function decrease of ≥1 z-score during URTI was as follows: 14.3% for FVC, 82.1% for FEF50%, 60.7% for FEF75%, 71.4% for FEF25−75%, and 53.6% for FEV0.5. When the abnormality criterion for any value of lung function was set at a z-score equal or less than −1.65, it was found that during URTI 50% of infants (14/28) had one or more abnormal forced flow parameters. During URTI, the most compromised parameters were FEF50% and FEF25–75%, and forced expiratory flows were more affected than volumes (Table 2 and Figure 1). Two examples of flow-volume manoeuvres from two patient when they were suffering URTI or asymptomatic are shown in Figure 2. It was found that almost all infants had normal lung function (all the spirometric parameters assessed) when they were asymptomatic excepting two of them who remained under the −1.65 z-score for one isolated parameter.
Lung function in infants with RW during URTI and when asymptomatic.
| Asymptomatic | URTI | P value | |
| FVC | −0.04 (−0.47–0.39) | −0.31 (−0.73–0.11) | 0.03 |
| FEV0.5% | 0.48 (0.08–0.88) | −0.61 (−1.14 to −0.08) | 0.00 |
| FEF50% | 0.19 (−0.10–0.48) | −1.54 (−2.10 to −0.98) | 0.00 |
| FEF75% | 0.10 (−0.26–0.46) | −1.44 (−2.12 to −0.76) | 0.00 |
| FEF25–75% | 0.47 (0.14–0.80) | −1.30 (−1.96 to −0.64) | 0.00 |
z- score (mean and 95% confidence interval).
There was no association between the extent of the lung function decrease during URTI after adjusting for sex; age; perinatal tobacco exposure; history of family asthma; age of onset of recurrent wheezing; order of measurements; and number of previous wheezing episodes. However, there was a significant association between expiratory flows measured when infants were asymptomatic and tobacco during pregnancy. No significant association was found for lung function during URTI.
There were not unwanted side effects derived from sedation or measurement procedure in any of the studied infants.
DiscussionThis study demonstrates that URTI causes a notorious and significant decrease in spirometric parameters of infants with RW, and such an effect is predominantly on forced expiratory flows. An abnormal lung function during URTI occurred in 50% of patients but all of them returned to normal lung function when asymptomatic.
Information about the quantitative effect of URTI on the lung function of normal or asthmatic children is scarce.20–22 The latter is rather surprising since common viral infections account for 80–85% of asthma exacerbations in children1 and are strong predictors for repeated asthma-like symptoms episodes in infancy and school age.1,2,5–7,14, 23–25 Similarly, the effect of respiratory viral infections -the main cause of wheezing exacerbation in infancy- on lung function in infants who are or not at risk of asthma had not been reported.
This study suggests that a positive history of family asthma and tobacco exposure during pregnancy would not be a risk factor for a higher degree of airway narrowing during URTI in infants with RW; however, both factors have been found to be related with higher airway responsiveness in healthy infants.26,27. In the same direction, we also found a clear association between tobacco exposure during pregnancy and lower expiratory flows, but only when infants were asymptomatic. A possible explanation for this finding may be that in our infants the marked reduction of forced expiratory flows during URTI may have obscured a potential effect of family asthma, perinatal tobacco and larger number of previous episodes of wheezing on the degree of decrease of lung function. Another explanation is that common respiratory viruses can directly induce important mucosal oedema by increasing vascular permeability, particularly during the initial stage of a URTI;28 in infants with RW it may be that the degree of such a response is unrelated to familiar predisposition or environmental exposures. Recently, it has been found that both, decreased lung function and enhanced bronchial responsiveness, were associated to atopic sensitisation of the infant rather than to family history of asthma or allergies.29
We did not evaluate the response to an inhaled bronchodilator in our infants; therefore it is unclear whether the lower baseline airway function during URTI reflects increased baseline airway tone, intraluminal airways obstruction due to mucosal oedema, or both. However, the significant increase of lung function achieving normal values when infants were asymptomatic, suggests that in this group of infants a pre-existing fixed smaller-sized airway calibre, as a determinant for the observed lung function decrease, could be ruled out.
Our results are limited because we only included infants with recurrent wheezing, we did not get viral determinations, and there was not a control group of healthy infants. However, the group was balanced regarding the antecedents of family asthma and tobacco exposure. Other studies which assessed the effect of viral respiratory infections on pulmonary function in atopic and non-atopic patients, have not included a control group.30,31 Considering its limitations, this study provides, for the first time, information about the effect of URTI or common cold on the spirometric lung function of infants with RW.
In conclusion, this study shows a remarkable decrease in expiratory forced flows from raised lung volume during URTI in infants with RW, and the degree of this decrease was unrelated with family asthma, tobacco exposure and the number of previous episodes of wheezing. Furthermore, infants recovered a normal lung function when they were asymptomatic, although it was slightly decreased in the group exposed to tobacco during pregnancy.
Conflict of interestThe authors have no conflict of interest to declare.