We aimed to establish the characteristics of anaphylaxis in childhood.
MethodsForty-four patients who had experienced anaphylaxis in a period of 10 years (from 1999 to 2009), were included in the study. Parameters analysed were age, gender, concomitant allergic disease, trigger, setting, clinical symptoms, treatment, prognosis and prophylaxis.
ResultsThe total numbers of anaphylaxis cases were 44 in a ten-year period. The ages of patients ranged from 3 to 14 years (11.50±3.87 years) and the majority were male. 33 of the patients (75%) had a concomitant allergic disease. The trigger was determined in 93.2% of the cases, being most frequent: food (27.3%), and SIT (25%), followed by bee sting, medications and others. Respiratory (95.5%), dermatological (90.9%), cardiovascular (20.5%), neuropsychiatric (25%), and gastrointestinal (11.4%) symptoms were seen most frequently. For anaphylaxis triggered by food, the duration of anaphylactic episode was significantly longer (p<0.05). No biphasic reaction was observed during these attacks. Of our patients, only one developed respiratory failure and cardiac arrest due to SIT, and intensive care support was required.
DiscussionAs a trigger for anaphylaxis, the frequency of SIT is so high that it cannot be described by the study group including patients who were followed up in an outpatient allergy clinic.
Anaphylaxis is an important paediatric emergency situation which is seen more and more frequently that should be well recognised by the paediatricians. Anaphylaxis was defined as a life threatening systemic hyperreactivity situation with a sudden onset.1,2
Studies regarding the epidemiological characteristics, clinical features and management of anaphylaxis usually deal with adults or general populations.3,4 However, anaphylaxis is predominantly a childhood disease and most of the new cases occur among children and adolescents.5 Anaphylaxis in the childhood has many differences in aetiology, risk factors, and clinical outcomes from adults, so studies to determine general properties of anaphylaxis in childhood are needed.
The purpose of this study was to describe the demographic characteristics of the patients, aetiology, clinical features and management of anaphylaxis.
MethodsA retrospective study was performed including all patients referred to the outpatient clinic of the Paediatric Allergy Department from 1 January 1999 to 31 December 2009. We selected the patients who admitted to be investigated for anaphylaxis or who have experienced anaphylaxis in our hospital. The patients should also fulfil the clinical criteria of anaphylaxis.2 The study was approved by the local ethics committee.
Data were collected using a standard questionnaire: (1) demographic data; (2) atopic status of the child and the family; (3) the symptoms of anaphylactic event, (4) the course of anaphylaxis—that is time lapse between contact with the trigger and onset of symptoms, the first symptom of the reaction, total duration of symptoms, and being biphasic or not; (5) the setting; (6) the treatment; (7) recurrence; and (8) prophylaxis of anaphylaxis.
StatisticsAll analyses were performed using SPSS software version 10.0 (SPSS Inc., Chicago, IL, USA). A descriptive analysis was used for demographics of the study group. Non-parametric variables were analysed with the Pearson chi-square test or the Fisher's exact test when needed. The Wilcoxon–Mann–Whitney U test and Kruskal–Wallis one-way ANOVA were used to compare age, time lapse between contact with the trigger and onset of symptoms, time lapse between the onset of symptoms and administration of medications, and total duration of symptoms between two or more groups.
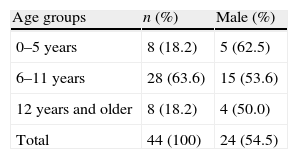
ResultsThe study group consisted of 44 cases who were aged between 3 and 14 years (11.50±3.87 years), 24 boys (54.5%) and 20 girls (45.5%). Distribution of age groups is given in Table 1. There was no difference for sexes between in age groups (p>0.05).
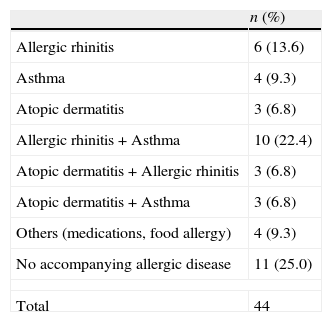
Thirty-three children (75%) had a personal history of allergic diseases. Of them, 18 (40.9%) had asthma, 22 (50%) had allergic rhinitis, 10 (22.7%) had atopic dermatitis, 3 (6.8%) had drug allergy, and 2 (4.5%) food allergy. There was a familial history of atopy in 27 (61.4%) children. Some cases had more than one allergic disease (Table 2).
Distribution of atopic diseases.
| n (%) | |
| Allergic rhinitis | 6 (13.6) |
| Asthma | 4 (9.3) |
| Atopic dermatitis | 3 (6.8) |
| Allergic rhinitis+Asthma | 10 (22.4) |
| Atopic dermatitis+Allergic rhinitis | 3 (6.8) |
| Atopic dermatitis+Asthma | 3 (6.8) |
| Others (medications, food allergy) | 4 (9.3) |
| No accompanying allergic disease | 11 (25.0) |
| Total | 44 |
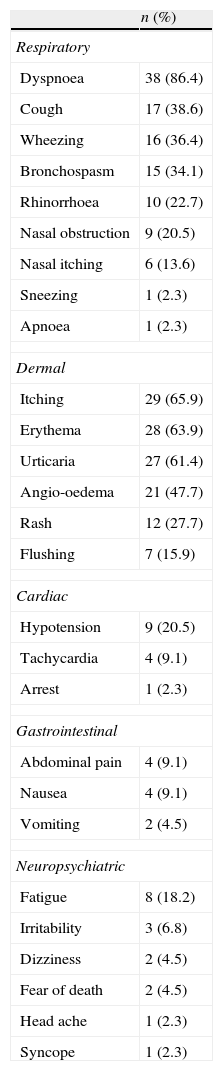
The first and frequently involved system was skin (79.4%). Other first symptoms were in the respiratory system in eight patients (18.3%) and in the gastrointestinal system in one patient (2.3%). Cardiovascular or neuropsychiatric signs were never seen as a first sign. During the course of anaphylaxis, symptoms seen were listed as respiratory (n=42, 95.5%), skin 90.9% (n=40), cardiovascular 20.5% (n=9), neuropsychiatric 25% (n=11), and gastrointestinal 11.4% (n=5). Distribution of the symptoms is shown in Table 3.
Symptoms and findings of anaphylaxis cases.
| n (%) | |
| Respiratory | |
| Dyspnoea | 38 (86.4) |
| Cough | 17 (38.6) |
| Wheezing | 16 (36.4) |
| Bronchospasm | 15 (34.1) |
| Rhinorrhoea | 10 (22.7) |
| Nasal obstruction | 9 (20.5) |
| Nasal itching | 6 (13.6) |
| Sneezing | 1 (2.3) |
| Apnoea | 1 (2.3) |
| Dermal | |
| Itching | 29 (65.9) |
| Erythema | 28 (63.9) |
| Urticaria | 27 (61.4) |
| Angio-oedema | 21 (47.7) |
| Rash | 12 (27.7) |
| Flushing | 7 (15.9) |
| Cardiac | |
| Hypotension | 9 (20.5) |
| Tachycardia | 4 (9.1) |
| Arrest | 1 (2.3) |
| Gastrointestinal | |
| Abdominal pain | 4 (9.1) |
| Nausea | 4 (9.1) |
| Vomiting | 2 (4.5) |
| Neuropsychiatric | |
| Fatigue | 8 (18.2) |
| Irritability | 3 (6.8) |
| Dizziness | 2 (4.5) |
| Fear of death | 2 (4.5) |
| Head ache | 1 (2.3) |
| Syncope | 1 (2.3) |
Initiation with skin involvement followed by respiratory symptoms was the most frequently seen pattern with 33 patients (75%). Starting with respiratory symptoms followed by skin involvement was in five cases (11.4), respiratory symptoms followed by cardiologic symptoms in three cases (6.8%), skin followed by cardiac symptoms in two cases (4.5%) and gastrointestinal followed by skin involvement in one case (2.3%).
Symptoms in asthmatic patientsAnaphylactic symptoms of asthmatic patients were mostly respiratory (94.4%), and cardiovascular (22.2%). In 16 of the patients with asthma, symptoms were seen within the first 30min after exposure (88.9%), while in 20 of the non-asthmatic patients symptoms were seen within the first 30min (76.9%) (p>0.05). Adrenaline was used in 12 asthmatic cases (66.6%) and 11 non-asthmatic cases (42.3%) (p>0.05). There was no relation with presence of asthma and severe anaphylaxis, considering need for adrenaline, duration between onset of symptoms and exposure. However, asthmatic patients more frequently needed beta agonist. Beta agonist was used in 13 asthmatic cases (72%) versus 9 non-asthmatic cases (34%); (p<0.05).
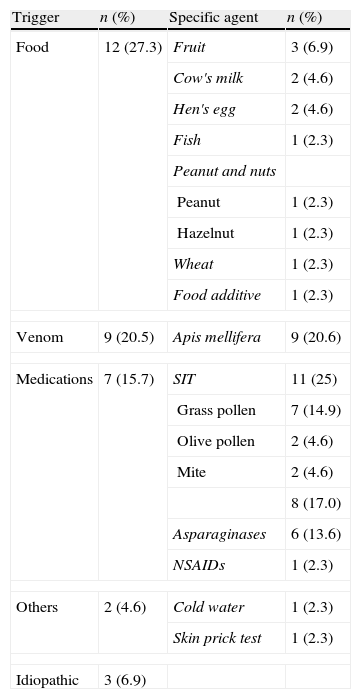
Trigger of anaphylaxisIn 93.2% of anaphylaxis cases, the trigger was detected or strongly suggested. The most frequently seen agent was food which was responsible for 27.3% of cases (n=12). This was followed by specific immunotherapy (SIT) with 25% (n=11), venom (bee sting) with 20.5% (n=9) and medications with 15.7% (n=7). There was also one case of anaphylaxis due to cold water and one case occurred during skin prick test. The trigger could not be determined in three of the cases (6.9%) (Table 4).
Specific causative agent of anaphylaxis.
| Trigger | n (%) | Specific agent | n (%) |
| Food | 12 (27.3) | Fruit | 3 (6.9) |
| Cow's milk | 2 (4.6) | ||
| Hen's egg | 2 (4.6) | ||
| Fish | 1 (2.3) | ||
| Peanut and nuts | |||
| Peanut | 1 (2.3) | ||
| Hazelnut | 1 (2.3) | ||
| Wheat | 1 (2.3) | ||
| Food additive | 1 (2.3) | ||
| Venom | 9 (20.5) | Apis mellifera | 9 (20.6) |
| Medications | 7 (15.7) | SIT | 11 (25) |
| Grass pollen | 7 (14.9) | ||
| Olive pollen | 2 (4.6) | ||
| Mite | 2 (4.6) | ||
| 8 (17.0) | |||
| Asparaginases | 6 (13.6) | ||
| NSAIDs | 1 (2.3) | ||
| Others | 2 (4.6) | Cold water | 1 (2.3) |
| Skin prick test | 1 (2.3) | ||
| Idiopathic | 3 (6.9) | ||
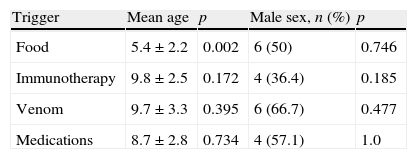
While there were no differences for trigger between girls and boys (p>0.05), food was more frequently the trigger for the younger age group (p<0.05) (Table 5).
Of the children who had anaphylaxis triggered by food, 11 had atopy (91%). For other triggers, atopy was less common (68%) and this was significantly different from anaphylaxis triggered by food (p<0.05). Atopy was significantly less in the medication group (28%) when compared with other triggers (83%) (p<0.05).
The course of anaphylaxisTime lapse between exposure and onset of symptoms: For all causative agents, anaphylactic reaction started 12.5min following exposure on average (minimum 2min, maximum 360min). Time lapse of onset was longer for food, and shorter for medications and venom (p<0.05).
The mean duration for symptoms was 90.1±59.2min (Minimum 30, maximum 240min). Duration of symptoms was longer for food and SIT-triggered anaphylaxis and shorter for medication-triggered cases (p<0.05).
None of the 44 cases in the study group was biphasic. Only one of our patients had cardiopulmonary arrest and needed resuscitation, but the patient was saved.
The setting of anaphylaxisMost of the anaphylaxis happened at home (n=17/44, 38%). Hospital was the second most common place with 15 cases (34%) followed by open area (garden, street) with nine cases (20%), school and sea with one case each (2.3%). Eight of the anaphylaxis cases occurred in hospital were triggered with specific immunotherapy (53.8%), six were triggered with medications (39.6%), and one with skin prick testing (6.6%).
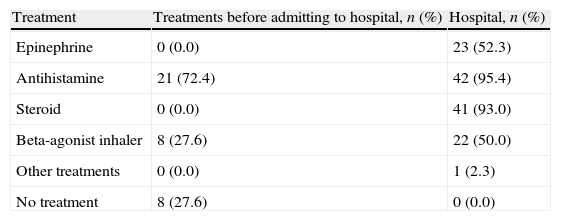
TreatmentFirst aid was given by health care workers (paediatrician, practitioner, nurse, etc.) to the cases which happened in hospital. But outside the hospital, first aid was given by people from other occupations (teachers, family members).
Most of the anaphylaxis cases outside the hospital were first episodes with 22 cases. First aid was given by families to 21 cases (72.4%), and 8 (27.6%) patients did not receive any medical support and admitted to the emergency. Of the outside the hospital cases who had no pre-admittance treatment, two were triggered by food (25%), and six were triggered by bee venom (75%). Except for one case triggered by venom all of them were first episodes (87.5%) (Table 6).
Treatments before admitting to hospital and in hospital.
| Treatment | Treatments before admitting to hospital, n (%) | Hospital, n (%) |
| Epinephrine | 0 (0.0) | 23 (52.3) |
| Antihistamine | 21 (72.4) | 42 (95.4) |
| Steroid | 0 (0.0) | 41 (93.0) |
| Beta-agonist inhaler | 8 (27.6) | 22 (50.0) |
| Other treatments | 0 (0.0) | 1 (2.3) |
| No treatment | 8 (27.6) | 0 (0.0) |
Thirty six of the patients were first episodes (81.8%). Of the remaining eight cases, seven had their second episode, and one case was the third episode, and thus recurrent cases (18.2%). Recurrent episodes were mostly triggered by venom (n=5, 62.5%), others were triggered by medications, food and cold water. Recurrences were due to the same trigger.
Prophylaxis of anaphylaxisAll anaphylaxis cases were consulted by health workers regarding the protection from future allergic reactions.
All patients who had anaphylaxis triggered by specific immunotherapy had their immunotherapy program stopped. For patients in the medication group, medications were stopped and families were informed about them. Mostly antihistaminic drugs were prescribed (n=30, 68.2%). For eight patients, antihistaminic and beta agonist were prescribed together (n=8, 18.2%). Only 15 anaphylaxis cases were prescribed epinephrine auto injector (34.1%). Eight of them had venom triggered anaphylaxis, 6 was food triggered and 1 was cold water triggered.
DiscussionWhile there are a lot of studies on adult anaphylaxis, studies regarding childhood anaphylaxis are limited.6 This study would give some important information on anaphylaxis since it has a paediatric population and was done at a referral hospital with nearly 200,000 outpatients and around 15,000 allergy outpatients annually in the largest paediatric hospital of Turkey.
Our study has not only given the data on anaphylaxis cases of a large population, but also has reached some significant findings on food triggered anaphylaxis, pointing to a different frequency of triggers. It was also shown that asthma, which is considered as a risk factor for fatal anaphylaxis, has not increased the severity of anaphylaxis.
Although anaphylactic reactions may be seen in all age groups, they most frequently occur in the under five age group. Mean age was found as 2.4 and 3.8 years in two studies in Australia,7,8 and five years (from 3 months to 12 years) in Germany.9 Our study group had an older mean age, which may be due to late exposure of triggers (SIT, medications and hymenoptera).
Anaphylaxis is more common in males. In our study, for each age group there was a male dominance, although not significant. In other studies, it is found to be more frequent in males, in Australia 63% and 60%,7,8 in Germany 58%,9 in Italy 65%10 and in USA 56%.11
It is controversial that atopy increases the risk for anaphylaxis.12,13 Most of our patients had comorbid allergic diseases. This may favour the suggestion that atopy plays a role in anaphylaxis. Food- triggered anaphylaxis cases were atopic significantly more (91%) when compared to medication-triggered cases (p<0.05). There are a number of studies with similar results.6,7,9,10
Childhood anaphylaxis, in contrast to adults, usually occurs at home as well as in the hospital.5,7–9,11 The setting was 38% home, 34% hospital and 20% open area like garden or street. A high percentage of hospital cases may be due to types of triggers such as SIT, and skin prick test.
The most frequent trigger in childhood anaphylaxis is food, followed by insect bite, blood products, SIT, latex, vaccines and radiocontrast materials. However, exercise-triggered and idiopathic anaphylaxis described in adults are very rare in children.6–10,14 Food-induced anaphylaxis, which is the most frequent trigger, is usually seen in younger children.15 While food was the major trigger, it was not as high as expected (27.3%). That can be explained by the fact that serious food allergy is a rare condition in our country.
An anaphylactic reaction is one of the alarming adverse effects of SIT. Although a systemic reaction can follow a biphasic course, its incidence, its outcome and the risk factors for its development are unknown in patients treated with SIT. In one study 453 patients received 21,022 immunotherapy injections and 131 anaphylactic reactions occurred.16,17 Mehl et al. reported SIT to be the third most common trigger (12%) following food (57%) and insect stings (13%).9 In our study, SIT is the second most common (25%) trigger. In contrast, a considerable number of reports have not mentioned SIT as a major trigger.7,10,15,18–22 SIT was unexpectedly high as a trigger in our study. The high number found in our study may be due to the high number of SIT performed in our clinic, which was 5700 shots annually.
Symptoms in anaphylaxis may differ from a mild skin rash to fatal reactions. These symptoms may be seen in any order but usually start with skin lesions as in our study (79.4%) followed by other symptoms.6,10,11,23,24
In a retrospective study which was done in Australia, skin symptoms were 97%, respiratory symptoms were 97%, gastrointestinal symptoms were 29% and cardiovascular symptoms were 17%.7 In our study, respiratory symptoms were the most frequent (95.5%). Skin symptoms were usually followed by respiratory symptoms. This pattern was consistent with other studies.7,11 Cardiovascular symptoms, which are common in adults, were not frequent in our study, probably due to the younger age group.25
The association between asthma and anaphylaxis has not been established clearly.26,27 In a study where 526,406 patients with asthma were followed for 10 years, after adjustment for age, sex, race/ethnicity, comorbidities, and immunotherapy, asthma was associated with a 5.2-fold (95% confidence interval, 4.7- to 5.6-fold) increased hazard of anaphylactic shock28 Almost half of our patients were asthmatic, but occurrence of asthma did not affect the course of anaphylaxis.
In most of the children who are experiencing anaphylaxis, onset of the symptoms is 5–30min following the exposure.3,10 In our study, mean onset was 12.5min following the exposure which is longer in food triggered anaphylaxis and shorter in medication and venom-triggered ones.
Anaphylactic reactions usually relieve within 1–2h, but occasionally despite the proper treatment, symptoms may last for hours to days with periods without any symptom.29,30 In our study the mean duration of symptoms with all triggers was found to be around one hour. The longest lasted for 4h, which was triggered by food. Food triggered anaphylaxis cases were differed from other triggers by late onset, longer duration of symptoms being seen in atopic children.
Most of our cases had their first episode (81.8%), while 18.2% had recurrent episodes. Recurrence rates of anaphylaxis in previous studies are reported as between 13 and 31%.10,11,22,31 There was no difference in mean age, sex, and being atopic or asthmatic between children with only one episode and those with recurrent episodes of anaphylaxis.9,11
The death rate due to anaphylaxis is reported to be 0.65–2.00% in United States and 1–3 death per million people annually are expected.32 Only one of our cases experienced cardiopulmonary arrest but did not die. This case was an eight-year-old male patient who had anaphylaxis <>8min following SIT. He was intubated and followed-up in the intensive care unit and needed multiple doses of adrenalin.
The first medical intervention was usually antihistamines and beta-agonists given by families. Patients that were admitted to the hospital without any prior treatment or intervention were 27.6%. Although three patients had been prescribed epinephrine auto injector, one of them did not buy the medication and two patients could not use the preparation because it was expired. This demonstrates that despite the important role of families in anaphylaxis management, they have insufficient knowledge and awareness. Similar results have been shown in a number of studies.7,9
A favourable outcome of anaphylaxis depends on the rapidity of adequate initial management and epinephrine injection. Unfortunately, medical treatment in the hospital involved epinephrine in only 52.2% of the cases, which is an indication of the insufficient case management of anaphylaxis. Studies indicate that even paediatricians may have problems in the diagnosis and treatment of anaphylaxis.33–35 In a study carried out in Australia, anaphylaxis was treated by epinephrine injection in 68.7% of cases, or by antihistamines in 14.1%. Epinephrine was the first treatment for the most severe reaction in only 43.9%, while antihistamines were given first in 43.5%.36
All patients at risk for recurrence in the community should be equipped with one or more epinephrine auto-injectors; a written, personalised anaphylaxis emergency action plan; and up-to-date medical identification.37–39 The use of an epinephrine auto-injector was not sufficient in our study. In spite of the history of anaphylaxis, prophylactic epinephrine auto-injector was prescribed to only 15 children. This was probably due to the lack of knowledge on anaphylaxis and<> epinephrine auto-injector has not been a readily available medication on the market in Turkey.
In conclusion, anaphylactic cases frequently have accompanying allergic diseases, but this does not cause more severe episodes, as in our asthma cases. Food anaphylaxis was the most common, and was seen in atopic children and the younger age group. Onset was late and duration of symptoms was long. Our SIT-triggered episodes were more frequent than reported elsewhere. Use of epinephrine and auto-injector was low, which is a sign of insufficient management of anaphylaxis.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interests to declare.