Component-resolved diagnosis based on the use of well-defined, properly characterised and purified natural and recombinant allergens constitutes a new approach in the diagnosis of venom allergy. Prospective readers may benefit from an up-to-date review on the allergens. The best characterised venom is that of Apis mellifera, whose main allergens are phospholipase A2 (Api m1), hyaluronidase (Api m2) and melittin (Api m4). Additionally, in recent years, new allergens of Vespula vulgaris have been identified and include phospholipase A1 (Ves v1), hyaluronidase (Ves v2) and antigen 5 (Ves v5). Polistes species are becoming an increasing cause of allergy in Europe, although only few allergens have been identified in this venom.
In this review, we evaluate the current knowledge about molecular diagnosis in hymenoptera venom allergy.
Stings by hymenoptera, namely bees, wasps, yellow jackets, hornets and ants, usually cause just local reactions. However, in some cases, they can induce systemic symptoms, and even fatal reactions.1,2
Reactions to hymenoptera venom are also responsible for decreased quality of life and significant anxiety about future stings.1,3 The results of the quality-of-life questionnaire demonstrated that a well-tolerated sting challenge test improves the quality of life of venom-allergic patients by reducing the anticipatory anxiety associated with the fear of being stung.4,5
Diagnosis of hymenoptera venom allergy forms the basis of treatment.6 Venom immunotherapy is the only treatment that addresses the cause of the anaphylactic reaction. It has proven very effective in inducing tolerance, with a protection rate ranging from 75% to 98%.7
Diagnosis of hymenoptera allergy is based on a systemic reaction after a sting, a positive skin test result and detection of specific IgE antibodies. Both skin test and specific IgE frequently reveal multiple sensitisations, which complicates the choice of the venom for immunotherapy (VIT).8 Double-positive results are a common issue when diagnosing Hymenoptera venom allergy based on crude venom extracts, since up to 59% of patients react to both honey bee venom and yellow jacket venom. Moreover, there is an increasing pattern of double sensitisation to Vespula and Polistes in southern European countries.9,10 On the other hand, cases of double positivity of IgE to bee and Vespula venom are often caused by clinically irrelevant cross-reactive antibodies against cross-reacting carbohydrate residues or by homologue allergens expressed in different Hymenoptera venoms.10,11
Component-resolved diagnosis (CRD) based on the use of well-defined, properly characterised and purified natural and recombinant allergens constitutes a new approach in venom allergy diagnosis.12–14
The best characterised venom is that of Apis mellifera, whose main allergens are phospholipase A2 (Api m1), hyaluronidase (Api m2) and melittin (Api m4).2,15 Additionally, allergens of Vespula vulgaris have been identified, as phospholipase A1 (Ves v1), hyaluronidase (Ves v2) and antigen 5 (Ves v5).16Polistes species are becoming an increasing cause of allergy in Europe, although only few allergens have been identified to date.
In this paper, we analyse the current knowledge about molecular diagnosis in hymenoptera venom allergy.
Apis melliferaBee venom is a complex mixture of allergenic proteins with enzymatic function, together with other pharmacologically-active molecules, such as biogenic amines and basic peptides.
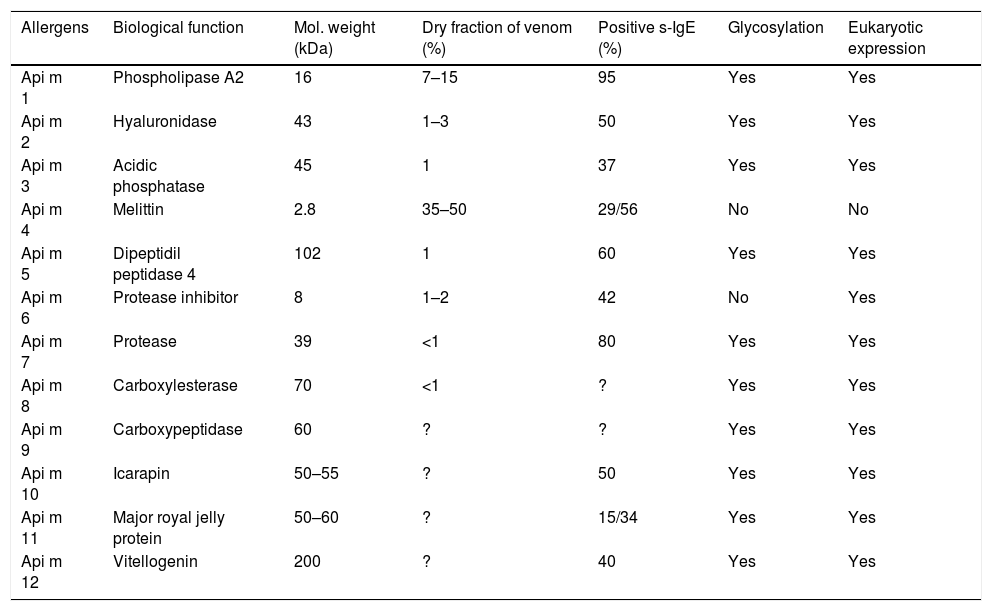
The complete genome sequencing of the bee has allowed the study of the composition of its venom, making it a model for the study of these insects. The most recent proteomic analysis of bee venom reveals that there must be more than 100 different components. Currently, 12 allergens have been identified in the bee, most of them in the venom17,18 (Table 1).
The bee venom allergens.90
| Allergens | Biological function | Mol. weight (kDa) | Dry fraction of venom (%) | Positive s-IgE (%) | Glycosylation | Eukaryotic expression |
|---|---|---|---|---|---|---|
| Api m 1 | Phospholipase A2 | 16 | 7–15 | 95 | Yes | Yes |
| Api m 2 | Hyaluronidase | 43 | 1–3 | 50 | Yes | Yes |
| Api m 3 | Acidic phosphatase | 45 | 1 | 37 | Yes | Yes |
| Api m 4 | Melittin | 2.8 | 35–50 | 29/56 | No | No |
| Api m 5 | Dipeptidil peptidase 4 | 102 | 1 | 60 | Yes | Yes |
| Api m 6 | Protease inhibitor | 8 | 1–2 | 42 | No | Yes |
| Api m 7 | Protease | 39 | <1 | 80 | Yes | Yes |
| Api m 8 | Carboxylesterase | 70 | <1 | ? | Yes | Yes |
| Api m 9 | Carboxypeptidase | 60 | ? | ? | Yes | Yes |
| Api m 10 | Icarapin | 50–55 | ? | 50 | Yes | Yes |
| Api m 11 | Major royal jelly protein | 50–60 | ? | 15/34 | Yes | Yes |
| Api m 12 | Vitellogenin | 200 | ? | 40 | Yes | Yes |
The best-known bee venom allergens so far are phospholipase A2 (Api m 1), hyaluronidase (Api m 2) and melittin peptide (Api m 4), which constitute the majority of the dry weight of venom.
Phospholipase A2 (Api m 1) has been considered the most important and potent allergen of bee venom since 1976.19 It is the most abundant enzyme capable of sensitising the vast majority of patients allergic to bee venom.10,20 Its enzymatic activity causes the hydrolysis of membrane phospholipids, producing a significant increase in arachidonic acid and leukotrienes; these are responsible for bronchoconstriction, mucus production and vascular permeability.21 Phospholipase A2 was cloned in 1989.22 In 1992, it was recombinantly expressed through a prokaryotic system using the E. coli bacteria, achieving an enzymatic activity and skin reactivity similar to the natural purified form.23,24
In the bee venom compound-based diagnosis, it has been observed that use of allergen Api m 1 has different sensitivity according to the technique and sources used.25–28
Technique sensitivities vary ranging from 95.6 to 97% of ADVIA Centaur,10,29 to 61.8–91% of ImmunoCAP,8,20,25–28 being the highest observed prevalence of sensitisation using native Api m 1. In contrast, Immulite technique has shown a sensitivity of 83.1% with the recombinant form of Api m 1.30
Hyaluronidase (Api m 2) is a glycosylated enzyme considered a major allergen of bee venom.20,29 It hydrolyses the hyaluronic acid in the target tissue, enhancing the penetration of the other components of the venom.21
Bee hyaluronidase shares 55% sequence identity with vespid hyaluronidase,32 which may explain the cross-reactivity between them, together with carbohydrate determinants.33
Hyaluronidase was isolated in 198434 and cloned in 1993.35 It is recombinantly produced in prokaryotic (E. coli) and eukaryotic (Baculovirus) systems, the latter having an enzymatic activity and IgE binding capacity similar to that of the natural purified allergen.31
The most abundant peptide of the venom is melittin (Api m 4); with a very low molecular weight it represents the major toxic component of bee venom. Schröder et al. achieved its synthesis in 197136 and in recent years it has been confirmed that melittin is an allergen in highly purified preparations, through the detection of specific IgE antibody by radioallergosorbent test (RAST).37 It is currently available in native and synthetic form.
It is considered a minor allergen with sensitisation prevalence ranging around 22.9%–29%.20,37 However, it has recently been described as a major allergen in a population (69 patients) in the south of Spain with allergy to A. mellifera venom whose prevalence of sensitisation to Api m 4 was of 53.6%. Sensitisation to Api m 4 has behaved as a biomarker in patients with poor tolerance to the start of the bee venom immunotherapy with very low s-IgE values.29
Use of IgE-Api m 4 as the single discrimination criterion demonstrated different ways of being allergic to bee venom. Patients with sIgE-Api m 4 ≥0.98kU/L had more severe reactions with stings, higher skin sensitivity, and baseline sIgE-Apis-rApi m 1 than patients with sIgE-Api m 4 <0.98kU/L. Patients with high sensitisation to melittin experienced 41% of the SRs during the build-up phase, and the sting challenge success rate was 82% in the second year.38
In recent years, advances in molecular characterisation have made it possible to identify other less abundant proteins in the venom, some had already been described as potentially allergenic, such as acid phosphatase (Api m 3). Its prevalence of sensitisation to purified native allergen is 60% in sera from patients allergic to bee venom, which decreases to 37% when the recombinant protein is used.39 However, in a recent publication, sensitivity to the recombinant protein was detected in 50% of patients studied.20Dipeptidyl Peptidase 4 (Api m 5) has been identified as one of the proteins of higher molecular weight (102kDa) of bee venom20 and appears to be responsible for the cross-reactivity between bees and wasps.40 It is a glycosylated enzyme, with a described prevalence of sensitisation to the recombinant molecule of 58.3%. A new protein, Icarapin (Api m 10) has proven to be a genuine bee venom allergen with high diagnostic and therapeutic potential, being considered as a marker of failure in bee venom immunotherapy, although the presence of this allergen detected in therapeutic extracts of bee venom remains controversial.20,41,42
Api m 6, namely a Protease Inhibitor, and Api m 4 are the only unglycosylated allergens of bee venom, and are considered as minor allergens. The rate of recognition of Api m 6 in its natural purified form is 40%, using the Immunoblot method43; however, only 26% of allergic patients show specific IgE reactivity to the recombinant protein.43,44Api m 7, namely a protease with unknown enzymatic activity, was detected by means of specific IgE in 80% of the studied serum, using a immunoblot assay.45 There are few data on Carboxylesterase (Api m 8) and carboxypeptidase (Api m 9); to our knowledge, a recombinant protein Api m8 captured sIgE present in 46% of sera from 28 allergic patients, by ELISA method.46 One of the most recently characterised bee allergens is Major royal jelly protein (Api m11), which presents two isoallergens: MRJP 8 and MRJP 9. The prevalence of sensitisation in its natural form is 50% for MRJP8, and 60% for MRJP 9. It seems that the contribution of carbohydrates is present, as in their recombinant form they show a much lower power of sensitisation, being 15% and 34%, respectively.47
Vitellogenin (Api m 12), is the highest molecular weight protein of bee venom known. It has recently been shown that approximately 40% of patients allergic to bee venom showed specific IgE to the recombinant protein.48 On the other hand, Api m 12 may be involved in cross-reactivity with vespids due to its similarities with an allergen that is also present in this venom (Ves v 6).
Vespula and PolistesGenus VespulaV. vulgaris, known as the common wasp, is found in various regions of the world. It is sometimes known as the European wasp; this name is also used for the species Vespula germanica, which is also known as the German wasp. Sometimes it is referred to as the “common yellow-jacket”, too.1
The extraordinary adaptation skills of V. vulgaris enable it to live in a wide range of habitats.
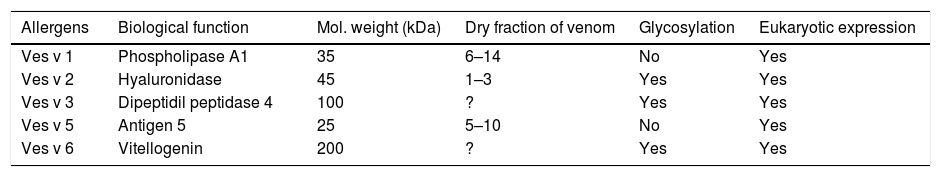
Prominent yellow-jacket venom (YJV) allergens include phospholipase A1 (Ves v 1), hyaluronidase (Ves v 2.0101), and antigen 5 (Ves v 5), a protein of unknown function but very abundant in the venom.16,32,49–51 Recently, a second inactive hyaluronidase (Ves v 2.0201), carrying an inactivating mutation in the active site of the enzyme, was identified in YJV which interestingly seems to be the predominant isoform52–57 (Table 2).
The vespula venom allergens.18
| Allergens | Biological function | Mol. weight (kDa) | Dry fraction of venom | Glycosylation | Eukaryotic expression |
|---|---|---|---|---|---|
| Ves v 1 | Phospholipase A1 | 35 | 6–14 | No | Yes |
| Ves v 2 | Hyaluronidase | 45 | 1–3 | Yes | Yes |
| Ves v 3 | Dipeptidil peptidase 4 | 100 | ? | Yes | Yes |
| Ves v 5 | Antigen 5 | 25 | 5–10 | No | Yes |
| Ves v 6 | Vitellogenin | 200 | ? | Yes | Yes |
The assessment using CRD improves the differential diagnosis among true double-sensitisation and cross-reactivity.6,8,58,59 Recently, rVes v 5-spiked, a Yellow Jacket Venom (YJV) ImmunoCAP enhanced by spiking with rVes v5, demonstrated a more reliable detection of Yellow-Jacket Venom (YJV) sensitisation in patients with insect venom allergy, improving the sensitivity to 96.8%.14,51 Previously it was observed that IgE reactivity was higher to the major YJV allergen Ves v 5 than to YJV extract, and sometimes IgE reactivity was only detectable by Ves v 5 ImmunoCAP.51 In a study involving 59 allergic individuals from a Mediterranean region, the determination of specific IgE against phospholipases and antigen 5s, seems to be sufficient to discriminate the source of sensitisation in vespid-allergic individuals, although regional phenotypes may arise. Interestingly, in this group of Mediterranean patients, very low levels of antigen 5s were seen in about 48% of patients (12/25 cases).14
Lately, the dipeptidyl peptidase 4 enzymes allergen C (Api m 5) and its vespid homologue Ves v 3 have been described as a novel class of hymenoptera venom enzymes.40
Api m 12 and Ves v 6 belong to the family of vitellogenins, are present in most oviparous animals, and represent the first vitellogenin allergens identified in hymenoptera venoms. Sensitisation to Api m 12 and Ves v 6 without interference of cross-reactive carbohydrate determinants in a population of Honey Bee Venom (HBV)- and YJV-allergic patients was addressed by specific IgE (sIgE) measurement. The obtained data suggested a relevant role for Api m 12 and Ves v 6 as sensitising venom components and as a novel cross-reactive class of homologous allergens in hymenoptera venoms potentially responsible for double positive test results with HBV and YJV, apart from cross-reactive carbohydrate determinants (CCD).48
Cross-reactive carbohydrate determinantsAnti-N-Glycan cross-reactive carbohydrate determinant (CCD) IgE antibodies, i.e. against epitopes of common glycoproteins, are induced by pollen exposure and insect stings may interfere with in vitro tests for allergy diagnosis, however its clinical relevance seems null.6
Genus PolistesThe Polistes genus belongs to the Polistinae subfamily within the Vespidae family. Different species of Polistes are spread in Europe, mostly in the Mediterranean area, the most commons of which are Polistes dominulus and Polistes gallicus and, to a lesser extent, Polistes nimphus. Other species – Polistes exclamans, Polistes annularis, Polistes metricus, Polistes apachus and Polistes fuscatus – live in North America.60,61 Nonetheless, there is evidence that the European species P. dominulus has spread to the United States.62
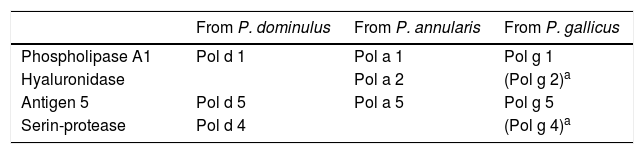
Polistes venom composition counts with four major allergens: phospholipase A1, hyaluronidase, antigen 5 and a protease (Table 3).60,63,45
Phospholipase and antigen 5 are strong allergens. Antigen 5 is considered to be species specific and, consequently, the major cause of the different immunological response between American and European venoms. Protease is considered a weak sensitiser in North American Polistes venom, while it is a significant allergen in European counterparts and, therefore, can also explain the low cross-reactivity between American and European venoms. Hyaluronidase has lower allergenic activity and it is responsible for the main cross-reactivity among venom from different species (vespids and also A. mellifera).32,60,64
The different species of Polistes in Europe show a great similarity in the sequences of their venom allergens, but it is lower when compared to American species. Thus, cross-reactivity is higher within European paper wasp venoms than between European Polistes and their American counterparts.11,61,63,65 Pantera et al. showed a high degree of similarity of the amino acid composition of the major allergens from P. gallicus and P. dominulus (Pol g 5 and Pol d 5: 98% of identity) versus a lower degree of similarity between P. gallicus and P. annularis (Pol g 5 and Pol a 5: 85% of identity; Pol g 1 and Pol a 1: 75.6% of identity).60 The amino acid sequence similarities between Polistes and other vespid allergens are lower than among Vespa, Dolichovespula and Vespula species and are responsible of cross-reactivity between Polistes and other vespids32: Pol g 5 and Pol a 5 have respectively 58.7% and 56.8% identity with Ves v 5; Pol g 1 shows 29% of identity with Ves v 1.60 Since the Polistes genus is the most distantly related in the Vespidae family and since there is only partial cross-reactivity between European and American paper wasp venoms, it is crucial, for diagnosis and therapy of Polistes allergy, to identify the venom that produced the reaction.60,63,65 In particular, true double sensitisation should be distinguished from cross-reactivity in patients with double positivity to Vespula and Polistes major allergens through recognition of the causative allergen molecules.59,66 In Polistes allergy diagnosis, crucial recombinant allergenic molecules lack, such as phospholipase A1. Also, in an important number of cases component-resolved diagnosis is not sufficient to distinguish true double sensitisation from cross-reactivity and, for a correct diagnosis and therapy, CAP-inhibition or specific immunotherapy with two venoms is still necessary.66 Indeed, Monsalve et al. documented that the determination of IgEs against both phospholipase and antigen 5 is sufficient to discriminate sensitisation to Polistes or Vespula venom in 69% of allergic patients with double sensitisation.14 Galindo-Bonilla et al. observed that phospholipase was a major allergen in their study population whereas antigen 5 was found to be a minor allergen component and that the detection of IgEs against Pol d 1, Pol d 5, Ves v 1 and Ves v 5 could determine the venom of immunotherapy in the majority of the patients.67
The issue of double sensitisationApart from CCDs, double-positivity in venom allergic patients had been largely attributed to IgE directed against either hyaluronidases (Api m 2 and Ves v 2),55 vitellogenins (Api m 12 and Ves v 6) or dipeptidyl peptidase 4 (Api m 5 and Ves v 3).40 Recent studies indicate that the cross-reactivity between the hyaluronidases on protein level is limited33,54 which is due to the absence of surface areas that have a significant degree of identity.56
Since specific IgE directed against both, CCD and protein epitopes might be present, the detection of CCD-specific IgE does not allow the exclusion of sensitisation to protein epitopes of multiple venoms.57 The only exceptions are the venoms of the paper wasps (Polistinae) which show no immunologically detectable CCD-reactivity.57 The use of species-specific CCD-free allergens facilitates the exclusion of cross-reactivity due to protein epitopes of homologous allergens present in HBV and YJV.2
In southern parts of Europe, V. germanica and P. dominulus are evenly distributed.52
In addition to their established importance in North America and Mediterranean regions of Europe, paper wasps, especially Polistes dominula, increasingly spread all over Europe as well as in the US from the warmer to the more moderate climate zones. Cross-reactivity between Polistinae and Vespinae (especially Vespula species) venoms is frequently observed14,59 independent of CCD-reactivity due to homologue allergens: phospholipase A1 and antigen 5.58
However, in the coming years, the field of molecular IgE diagnostics for the elucidation of multiple sensitisation to Hymenoptera venoms will face additional challenges that need better solutions.
Other hymenopteraBumblebee venom closely resembles honeybee venom and has two allergens of known sequence, phospholipase A2, and a protease. The honeybee and bumblebee venom phospholipases A1 show no sequence identity,68 which differs in its specificity of the catalytic mechanism. The bumblebee has gained significantly in importance, since it is increasingly used for pollination in greenhouses.69
Although for many years bee venom has been used to treat patients who experienced systemic reactions to bumblebee, due to the existence of some studies which warranted its efficacy, it has been recently reported that Bombus terrestris and bee show a low or very low degree of cross-reactivity. Bumblebee phospholipase A2 has shown a cross-reactivity of just 53% with bee phospholipase A2.70
Therefore, bee venom immunotherapy could be not as effective for this kind of patients as it was reported in the past. Cruz et al. described two phenotypes of patients allergic to bumblebee: 1) patients who had not been exposed to bumblebee and that were sensitised to bee venom; 2) patients who were primarily sensitised to bumblebee and that worked in greenhouses. For the latter kind of patients, the European Academy of Allergology and Clinical Immunology recommends using specific bumblebee venom immunotherapy.71
Severino et al. reported 17 patients who had suffered from a severe reaction after Vespa crabro (VC) sting and proved skin and CAP positive also to Vespula. In 11/17 patients, Vespula venom completely inhibited IgE binding to VC venom, whereas VC venom inhibited binding to Vespula venom only partially (<75%). In six subjects the CAP-inhibition provided inconclusive results and their sera were analysed by immunoblotting. The SDS-PAGE identified hyaluronidase, phospholipase A1 and antigen 5 as the main proteins of the venoms.72 With these results, it seems probable that patients with allergy to VC shall benefit from vespula venom immunotherapy.73
In the absence of commercial Vespa orientalis venom, the practice of treating patients allergic to this insect with available commercial venoms seems to be efficacious and Vespula venom is probably responsible for this effect.72
Moreover, fire ant venoms show high similarity with vespid venoms and contain a phospholipase A1 and an antigen 5. Varying from all other known Hymenoptera venoms, the major allergens of the Myrmecia venom are small peptides (pilosulins) which partially form homo- or heterodimers,73 but additionally, phospholipase and hyaluronidase activity have been reported.
In South-eastern Australia, ant sting allergy is relatively common being predominantly due to Myrmecia pilosula (Jack Jumper Ant, JJA). JJA venom immunotherapy has been shown to be effective in preventing anaphylaxis to the sting of the JJA.74
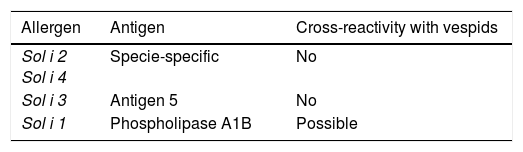
In Asia, there seems to be an increasing importance of fire ants. As beforehand stated, venom allergens of fire ants and jack jumper ants have been characterised and studied elsewhere (Table 4). Some studies of Pachycondyla venoms have been fulfilled, with the major allergen Pac c 3, being related to Sol i 3 from fire ants. Although ants seem to share some common proteins in venoms, each group appears to have a number of possibly unique components.75
Solenopsis allergens.
| Allergen | Antigen | Cross-reactivity with vespids |
|---|---|---|
| Sol i 2 Sol i 4 | Specie-specific | No |
| Sol i 3 | Antigen 5 | No |
| Sol i 1 | Phospholipase A1B | Possible |
| Algorithm of venom immunotherapy decision6,90 | |||
|---|---|---|---|
| Complete extracts | Apis, Vespula, Polistes | ||
| Component resolved diagnosis | rApi m 1, rVes v 1, rVes v 5, rPol d 5 | ||
| rApi m 1 | rApi m 1+rVes v 1 and/or rVes v 5/rPol d 5 | rVes v 1, rVes v 5, rPol d 5 | |
| Immunotherapy candidate | Bee venom | Bee+Vespula/Polistes venom | Vespula/Polistes |
CRD has the potential to establish individual sensitisation profiles6,8,14,67,76 and contributes by improving discrimination between sensitisation and genuine allergy.6,67,76 Moreover, CRD enables an individual risk assessment of severity and possibly prediction of persistence,6,67,76 and may expedite the selection of patients for VIT.
Given all CRD potentialities, it may generate a great deal of data whose clinical relevance could be elusive, and shall be used as a complementary diagnostic tool, linked to patient's history, skin-prick testing, and specific-IgE determination, rather than a first-line choice, principally in difficult cases.67,76 Furthermore, additional components will surely optimise both the performance and suitability of CRD in venom allergy diagnosis, because despite the efficiency of VIT, the biomarkers associated with clinical efficacy remain elusive.
Component-resolved diagnosis application in mast cell activation syndromes and hymenoptera venom allergyInsect stings are the leading known cause of anaphylaxis (after idiopathic causes) among patients with systemic mastocytosis (SM), and mainly appear (89%) in indolent systemic mastocytosis (ISM).77,78 Hymenoptera venom anaphylaxis (HVA) episodes are much more frequent in patients without skin involvement (ISMs−) than in those with skin lesions (ISMs+) (67% vs 15.4%).77
Immunotherapy in mast cell activation syndromes (MCAS) is only recommended for those patients who demonstrate sensitisation mediated by IgE following skin tests and/or the determination of a specific IgE in vitro.79–81 In up to 15% of the patients with SM, no IgE mechanism is observed following the determination of serum IgE in a complete extract.82,83 Therefore, studies have been conducted using new diagnostic techniques: basophil-activation tests have shown contradictory results.84,85 Component-based determination of serum specific IgE antibodies to major Hymenoptera venom allergens among cases with a negative allergy study by means of skin tests and CAP determinations was carried out in a study with 14 patients.84 They found positive results (>0.35kU/L) in 7 of 11 patients (64%) who were studied by the ADVIA Centaur® system (ADVIA) (Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA) with the use of natural (n) allergens (nApi m1, nApi m2, nPol d1, nPol d2, nVes v1, and nVes v5), but none of the three who were studied by ImmunoCAP (Thermo Fisher Scientific, Inc) with the use of recombinant (r) antigens (rApi m1, rPol d5, and rVes v5) showed a positive result. All tests were performed in ALK-Abelló (Madrid, Spain), using venom extracts from ALK-Abelló, Source Materials, Inc. (Spring Mills, PA, USA) except for nApi m1 (SIGMA, St Louis, MO, USA). These differences suggest the potentially greater sensitivity of biotinylated natural allergens subjected to ADVIA analyses versus recombinant allergens in component-based allergy testing reported by other groups,14 but further demonstration is needed in future prospective studies.
Patients with ISMs− may often present with normal baseline tryptase values. This fact, together with the absence of skin lesions, the similarity between these patients’ symptoms and those observed in allergic processes, and the absence of mast cells aggregates (the only major diagnostic criteria accepted by the current WHO classification) in the early stages of ISM, makes the diagnosis, especially of ISMs−, complex in many cases, and can only be established in specialised monographic networks that have all of the resources needed to provide a diagnosis. The Spanish Network on Mastocytosis (REMA), based on the comprehensive study of a large number of cases, has developed a Score that is capable of predicting the clonality (the probability that a patient with MCAS has of suffering from an ISMs− or a clonal-MCAS) with high sensitivity and specificity, based only on demographic data (gender), symptoms and signs during the acute episodes and the serum baseline tryptase level.83–89 In patients with a Score ≥2 (suspicion of clonality) it is recommendable to start the appropriate treatment and to conduct a study of the main complications of the mast cell disease. The application of this Score in patients with hymenoptera venom anaphylaxis should be the first step in their management to detect possible underlying diseases.90
ConclusionsIn the last years recombinant technologies have represented a great advance in the diagnosis of hymenoptera venom allergy as they provide a profile of sensitisation to specific allergens. Molecular diagnosis helps to discriminate between true sensitisation and cross-reactivity in multiple-venom sensitisation, which is crucial in order to prescribe the appropriate venom immunotherapy. It has also been useful in cases of undetectable sensitisation, due to the higher sensitivity than sIgE to the whole venom.
Hymenoptera venom is a complex mixture of proteins and carbohydrates, although only a small part of them are relevant in the diagnosis of venom allergy. Cross-reactive carbohydrate determinants (CCD) are present in hymenoptera venom, especially in bee venom, and are an important cause of cross-reactivity between them. Recombinant CCD-free allergens solve this problem and allow the differentiation between true sensitisation and cross-reactivity.
Bee venom has been the best characterised by recombinant technologies and currently 12 allergens have been identified. Moreover, several major allergens such as Api m 1, Api m 2, Api m 3, Api m 5 and Api m 10 have been described, which taken together show a diagnostic sensitivity of 95%.20 Apart from CCDs, other allergens have been identified as being responsible for cross-reactivity between bee and wasp venoms, like Api m 2 (hyaluronidase), Api m 5 (dipeptidyl peptidase IV) and Api m 12 (vitellogenin).
Vespid venoms have been less studied, and currently only a few allergens have been described, being phospholipase A1 and Antigen 5 the main allergens. CCDs are responsible for cross-reactivity between bee and wasp venoms, and also between Vespula and Polistes venoms.
Polistes species are very common in southern European countries, and they are spreading all over Europe. European Polistes venom has shown significant differences from American species.
Of special interest is the fact that there seems to be regional patterns in the vespid sensitisation profile, as in the Mediterranean area the sensitisation to phospholipase A1 is predominant compared to antigen 5 sensitisation in contrast with other European regions.3,14
Further investigations are needed in order to identify new allergens especially in vespid venoms, including Polistes which is becoming an increasing cause of allergy in Europe. New studies shall be carried out, to show the role of molecular allergens in the assessment of venom immunotherapy and their utility to identify risk profiles for severe reactions after field stings or venom immunotherapy or even to prevent treatment failures.89,90
Key points- -
Molecular diagnosis helps to discriminate between true sensitisation and cross-reactivity.
- -
It can also be useful in cases of undetectable sensitisation.
- -
Complex patterns of allergen sensitisation are present in bee venom allergic patients.
- -
Regional patterns of vespid allergens sensitisation have been found in the Mediterranean area.
- -
New investigations are needed to find new allergens specially in vespid venoms.
- -
More studies are needed to analyse the utility of recombinant technologies in the monitoring of venom immunotherapy and in the identification of high risk profiles.
- -
New recombinant molecules are needed to improve the diagnosis of allergy to vespid venoms.
The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Sources of supportThis work was partially supported by a grant from Comunidad de Madrid S2010/BMD-2502 MITIC
Teresa Alfaya Arias received lecture fees from Alk-Abelló and a research grant from Diater; Elisa Boni received lecture fees from Allergy Therapeutics; Arantza Vega received lecture fees from Alk-Abelló, Leti, Diater, Mundipharma and Novartis; Darío Antolín-Amérigo has received lecture fees from ALK-Abelló, Allergy Therapeutics, AstraZeneca, GlaxoSmithKline and Mundipharma, a research grant from Merck-Serono-Fundación 2000, educational grants from Merck and Pfizer and honoraria for articles from Meda and Mundipharma. María José Sánchez-González received fees from ALK-Abelló, AstraZeneca and Chiesi.
Conflict of interestMercedes Rodríguez-Rodríguez, José Barbarroja-Escudero, Berta Ruiz-León, Carmen Moreno-Aguilar, Leticia Sánchez Morillas, David González-de-Olano and Melchor Álvarez-Mon have no conflicts of interest.
All authors declare that have been involved in writing the manuscript and reviewing it before submission.