Common variable immunodeficiency (CVID) is the most common symptomatic primary immunodeficiency, characterised by low concentration of serum IgG in association with low serum levels of IgA and/or IgM, despite normal to low number of B-cells and variable T-cells abnormalities.1,2 The patients with CVID could present a variety of clinical manifestations, including autoimmunity, malignancy, and recurrent infections, mainly in respiratory and gastrointestinal (GI) systems.1,3,4
Gastrointestinal manifestations in humoral immunodeficiencies could be due to infection, malignancy, inflammatory disorders or autoimmunity.1,5 The incidence of these manifestations ranges from 20% to 60%. Infections and inflammation leading to malabsorptive symptoms could be seen in about 20% of individuals with CVID, while GI infections with Salmonella, Campylobacter, or Giardia occur in about 10% of individuals.1,2,5Giardia lamblia is a common cause of diarrhoea in CVID patients. Treatment with metronidazole is generally effective, but often requires a prolonged course, while patients exhibit a high relapse rate, reflecting the inability of the immunodeficient patient to eradicate this organism.5,6
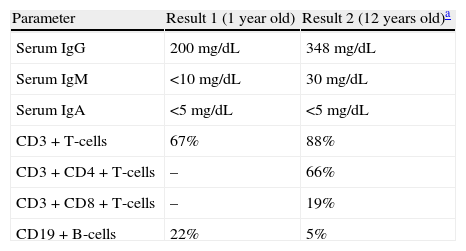
Herein, a 26-year-old woman with CVID is presented, who suffered from persistent chronic diarrhoea and poor weight gain. The patient had a past history of right wrist osteomyelitis caused by pseudomonas at the age of one year. Subsequently, immunological work-up was done for the patient at the same time, as of family history of death in one sibling at the same age with recurrent episodes of bacterial pneumonitis. Quantitative immunoglobulin measurement revealed an IgG of 200mg/dL, IgA <5mg/dL, and IgM <10mg/dl, while lymphocyte enumeration showed normal number of B- and T-cells (Table 1). Further and detailed immunological studies revealed impaired antibody function and absent iso-haemagglutinin titers; hence the diagnosis of hypogammaglobulinaemia was made and monthly intravenous immunoglobulin (IVIG) replacement therapy without prophylactic antibiotics was started. As she continued to have hypogammaglobulinaemia even after four years of age, the diagnosis of CVID was made for her. Eight years later, at the age of nine years, she stopped receiving IVIG; and seven months later, she was admitted to hospital because of unilateral arthritis of left knee, which was treated with empiric antibiotics. IVIG was started again and she has been under regular IVIG therapy since that age.
Laboratory data of the patient with CVID.
| Parameter | Result 1 (1 year old) | Result 2 (12 years old)a |
| Serum IgG | 200mg/dL | 348mg/dL |
| Serum IgM | <10mg/dL | 30mg/dL |
| Serum IgA | <5mg/dL | <5mg/dL |
| CD3+T-cells | 67% | 88% |
| CD3+CD4+T-cells | – | 66% |
| CD3+CD8+T-cells | – | 19% |
| CD19+B-cells | 22% | 5% |
It should be noted that watery diarrhoea started at one year of age, 3–4 episodes per day with fluctuations in severity, but never improved completely until 25 years of age. Failure to thrive was remarkable. Stool exams were always negative for blood or fat. At the age of 15 years, stool exam revealed Giardia lambelia cyst without trophozoite; hence metronidazole was ordered for 3–4 weeks and continued as a prophylactic daily schedule, but unfortunately no relief from diarrhoea was achieved. Giardia lambelia cyst was constantly present in almost all stool exams since 25 years ago and did not improve with metronidazole or tinidazole. Investigations for other opportunistic organisms such as isospora, cryptosporiduasis, trichomoniasis and other parasites were inconclusive. Barium meal showed enhancement of descending colon earlier than ascending colon, while possibility of a fistulous tract was suggested. Subsequent colonoscopy revealed no fistulous tract, but only mucosal thickening due to chronic Giardia colonisation is considered. Specimens were not suggestive for inflammatory bowel diseases.
During 25 years of receiving monthly IVIG, trough IgG level ranged from 300 to 500mg/dL without any effective control of diarrhoea and her weight ranged between 30 and 35kg. Because of frustration about her disabling condition, immunoglobulin therapy via oral consumption was started experimentally with the dose of five grams monthly and continued for previous nine months. Significant and dramatic relief of diarrheal frequency and severity was achieved during this short period of time, while she started to gain weight reaching 37kg that is 4kg weight gained during nine months.
Treatment for antibody deficiency syndromes includes administration of immunoglobulin which may reduce the frequency of infections and autoimmune diseases. However, sometimes GI diseases are not controlled well with IVIG replacement, because these preparations contain IgG which cannot reach the lumen of the intact gut and also contain very little amount of IgA which is a vital component of mucosal defence.5,6 Recent experimental evidence suggests that IVIG could exert an effect more than passive substitution of antibodies could do against pathogenic microbes; it rectifies the defective signalling and induces an optimal functioning of cellular compartment, thus re-establishing immune homeostasis.7 Theoretically, treatment with oral immunoglobulin has not been successful, because IgG is rapidly destroyed before reaching the small intestine. There is an animal study, which showed an induction of oral tolerance by oral administration of IVIG against anti-phospholipid syndrome in naïve mice.5,6,8 The presented case is a unique one, since the patient experienced improvement of chronic diarrhoea following oral administration of IVIG. Our results point to a possible role of oral IVIG in the improvement of chronic diarrhoea in CVID patients, especially in those infested by organisms such as Giardia. However, further original multi-centre studies are needed to test the efficacy of oral administration of immunoglobulin in improvement of diarrhoea severity in those with CVID.
Ethical disclosureConfidentiality of dataThe authors declare that no experiments were performed on humans or animals for this investigation.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflicts of interest and no funding was received.