Cell mediated immunity is suppressed by systemic corticosteroids. Inhaled corticosteroids have been shown to affect parameters including bone metabolism, hypothalamus–pituitary adrenal axis, linear growth, and lead to the development of cataracts. However, it is unclear if high dose inhaled corticosteroid therapy affects cell mediated immunity.
Study objectivesTo evaluate if asthma patients taking high dose inhaled corticosteroids chronically have reduced cell mediated immunity compared to asthma patients not taking inhaled corticosteroids.
MethodsEighteen asthmatic subjects participated in this cross-sectional study. Cell mediated immunity was evaluated in nine patients who had been taking high dose inhaled corticosteroids for >6 months and nine patients not taking inhaled corticosteroids. Cell mediated immunity was evaluated by delayed type hypersensitivity (DTH) skin testing with intradermal placement of candida and tetanus antigens.
ResultsThere was no significant difference in DTH skin test results between the high dose inhaled corticosteroid and no corticosteroid treated asthma group.
ConclusionPatients with asthma taking high dose inhaled corticosteroids chronically (>6 months) did not have significantly greater impaired cell mediated immunity than patients not taking inhaled corticosteroids in this study.
Asthma is a variable and chronic inflammatory disease of the airways. The inflammatory disorder underlying asthma arises from the interaction of various cells including eosinophils, mast cells and T lymphocytes.1 Thus, anti-inflammatory therapy, in the form of inhaled corticosteroids, is key in the management of persistent asthma.2 As the prevalence of asthma has been increasing, so has the use of inhaled corticosteroids, and at higher doses than previously prescribed early in its introduction.
Recent studies have shown that inhaled corticosteroids have systemic effects on bone metabolism,3–8 hypothalamus–pituitary adrenal axis,9–15 on linear growth,16–18 and cataracts.19–21 Oral corticosteroids have an even more potent effect on these parameters, and additionally have been shown to suppress cell mediated immunity, increasing an individual's susceptibility of acquiring an opportunistic infection.22–31
It is unclear at this time whether high dose inhaled corticosteroids also suppress cell mediated immunity. Studies addressing this issue have been conflicting thus far.32–34 Currently there are no data to suggest increased incidence or course of fungal, viral or bacterial infections with the use of inhaled corticosteroids. However, there are rare cases that have been reported of reactivation of tuberculosis or viral infections associated with the use of inhaled corticosteroids.35–37 The US Food and Drug Administration has instructed the manufacturers of oral, injectable and inhaled corticosteroids to include the warning about the possible risk of tuberculosis and viral infections with the use of these agents.38 However, the American Academy of Allergy and Immunology issued a position statement, stating that this warning is unwarranted.39
The objective of our current study was to evaluate if there is a correlation between high dose inhaled corticosteroids and suppression of cell mediated immunity in asthmatics. This was accomplished by determining if there was a statistically significant higher incidence of impaired cellular immunity (as reflected in anergy or lower cumulative skin test scores to DTH testing) in patients with asthma who have already been receiving high dose inhaled fluticasone for >6 months compared to an age-matched control asthma population not on high dose inhaled corticosteroids.
Materials and methodsStudy designEighteen volunteers with asthma between the ages of 42 and 67 were recruited for this cross-sectional study. Informed consent was obtained from each subject prior to entry into the study and the protocol was approved by the local Institutional Review Board (IRB B, VA Promise No. 0035). Exclusion criteria included subjects who have received systemic corticosteroids or immunosupressants in the past 6 months, patients with chronic diseases that may influence cell mediated immunity (autoimmune disease, malignancy or immunodeficiency), or patients with a significant infection in the past 6 months.
Group A consisted of nine asthma patients who had been compliant with taking high dose inhaled corticosteroids (defined as fluticasone/salmeterol 500/50μg twice daily or fluticasone propionate 220μg two puffs twice daily) for at least 6 months duration.
Group B consisted of nine volunteers with mild intermittent asthma who had been treated with albuterol as needed only, without exposure to inhaled corticosteroids.
Cell mediated immunity was compared between volunteers of group A and group B by DTH skin testing. To account for differences that may be related to age, each volunteer in group A was age matched within 3 years to a volunteer in group B, resulting in a comparable spread of age between the two groups.
DTH skin testing and interpretationDTH skin testing was performed in our study using the standard Mantoux technique. Intradermal injections of 0.1mL of candida (Candin; Allermed Laboratories; San Diego, CA, USA) and tetanus (Tetanus Toxoid USP; Aventis Pasteur) were placed on the volar aspect of the forearm. Results were read by a single blinded individual for all subjects 48h after placement using the ballpoint pen technique to measure the induration at each site. Orthogonal diameters were measured, and the average of these diameters was recorded. A mean induration of ≥2mm was considered positive. A cumulative skin test score was calculated by adding the mean diameters of positive reactions.
Statistical analysisThe non-parametric Wilcoxon rank sum test and the parametric t test were both used to compute p values for comparing means between the inhaled corticosteroid group (group A) and the albuterol only (group B) in total skin test score for candida and tetanus individually, total skin test score for candida plus tetanus, as well as difference in incidence of anergy. A statistically significant result was defined as p<0.05. Means±SEM are reported.
ResultsEighteen volunteer subjects completed this study. Subjects ranged in age from 42 to 67 years (mean, 57.11±6.6). One subject in the study was lost to follow up. There were no significant adverse events experienced in the 18 subjects who completed the study. The most common complaint was mild pruritis at the site of skin testing.
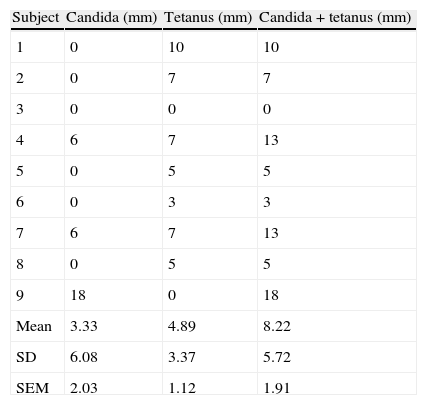
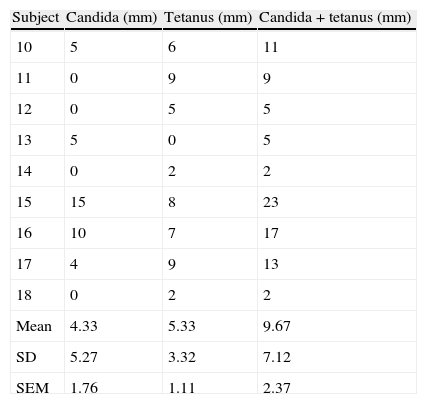
Tables 1 and 2 depict the sum of mean orthogonal diameters for candida and tetanus skin test score individually as well as for the candida plus tetanus total skin test score for the high dose inhaled corticosteroid group (group A) and no inhaled corticosteroid group (group B), respectively. The mean candida skin test score of 3.33±2.03mm and 4.33±1.76 in the inhaled corticosteroid group and albuterol only group, respectively, was not significantly different statistically (p=0.714 using t-test, p=0.594 using Wilcoxon rank sum test). The mean tetanus skin test score of 4.89±1.12 and 5.33±1.11 in the inhaled corticosteroid group and albuterol only group, respectively, was also not significantly different statistically (p=0.782 using t-test, p=0.721 using Wilcoxon rank sum test). Comparison of the mean candida plus tetanus total skin test score of 8.22±1.91 and 9.67±2.37 in the inhaled corticosteroid group and albuterol only group, respectively, showed no statistically significant difference (p=0.642 using t-test, p=0.824 using Wilcoxon rank sum test). Finally, there was no significant difference in the incidence of anergy between the two study groups. The largest mean difference in standard deviation (SD) units is a mean difference of 0.225 SD units for candida plus tetanus. To confirm that this difference is statistically significant with 80% power would require a sample size in excess of 300.
DTH skin test results (millimetres of induration) evaluating cell mediated immunity in the inhaled corticosteroid group.
| Subject | Candida (mm) | Tetanus (mm) | Candida+tetanus (mm) |
| 1 | 0 | 10 | 10 |
| 2 | 0 | 7 | 7 |
| 3 | 0 | 0 | 0 |
| 4 | 6 | 7 | 13 |
| 5 | 0 | 5 | 5 |
| 6 | 0 | 3 | 3 |
| 7 | 6 | 7 | 13 |
| 8 | 0 | 5 | 5 |
| 9 | 18 | 0 | 18 |
| Mean | 3.33 | 4.89 | 8.22 |
| SD | 6.08 | 3.37 | 5.72 |
| SEM | 2.03 | 1.12 | 1.91 |
DTH skin test results (millimetres of induration) evaluating cell mediated immunity in the no inhaled corticosteroid group.
| Subject | Candida (mm) | Tetanus (mm) | Candida+tetanus (mm) |
| 10 | 5 | 6 | 11 |
| 11 | 0 | 9 | 9 |
| 12 | 0 | 5 | 5 |
| 13 | 5 | 0 | 5 |
| 14 | 0 | 2 | 2 |
| 15 | 15 | 8 | 23 |
| 16 | 10 | 7 | 17 |
| 17 | 4 | 9 | 13 |
| 18 | 0 | 2 | 2 |
| Mean | 4.33 | 5.33 | 9.67 |
| SD | 5.27 | 3.32 | 7.12 |
| SEM | 1.76 | 1.11 | 2.37 |
Inhaled corticosteroids have been demonstrated to have adverse effects on bone metabolism, hypothalamus–pituitary adrenal axis, linear growth, and cataracts, like systemic corticosteroids, but to a lesser degree. However, studies evaluating whether inhaled corticosteroids impair cell mediated immunity have been conflicting and inconclusive thus far. An early study by Levy et al. found no suppression of cell mediated immunity in children with asthma treated with lower dose inhaled corticosteroids.32 Cell mediated immunity was evaluated with immune parameters including differential white blood count, T and B cell numbers, T helper and suppressor counts, T cell mitogenic transformation, and IL-1 and IL-2 secretion.32 Recently, two studies evaluated the effects of high dose inhaled corticosteroids on cell mediated immunity. Sharma et al. showed suppression of cell mediated immunity determined by delayed hypersensitivity skin testing (using Multi-test) in nine healthy patients after 4 weeks of high dose inhaled fluticasone compared to baseline.33 However, this study was faulted for using Multi-test, as the American Thoracic Society does not recommend multipuncture devices for DTH testing due to the possibility of administering inconsistent amounts of antigen (17). England et al. conversely showed no suppression of cell mediated immunity determined by delayed hypersensitivity skin testing (using standard Mantoux technique) in 18 healthy subjects after 4 weeks of high dose inhaled fluticasone compared to baseline testing.34 However, it is important to evaluate DTH testing in asthmatics (not healthy volunteers) receiving inhaled corticosteroids for a longer duration.
In our present study, patients with asthma treated with high dose inhaled corticosteroids for at least 6 months did not have reduced cellular immunity in comparison to asthma patients taking albuterol as needed only without inhaled corticosteroids. There was no statistically significant difference for cell mediated immunity by DTH testing using the Mantoux technique. At this time, there are no data to suggest increased incidence or altered course of fungal, viral or bacterial infections with the use of inhaled corticosteroids. There are only rare case reports of reactivation of tuberculosis or viral infections associated with the use of inhaled corticosteroids.35–37 Our findings are in agreement with those of England et al. who found no suppression of cell mediated immunity after 28 days of treatment with high dose inhaled corticosteroids. However, we do acknowledge that there is a wide spread of values obtained in our study, the significance of which is unclear due to the small number of subjects. Further studies with larger numbers of asthma patients followed prospectively with DTH testing both before and after 6 months of therapy may be warranted.
FundingResearch conducted at VA Greater Los Angeles Healthcare System; Los Angeles, CA. Research non-funded and approved by local IRB Board.
Conflicts of interestDr. Lee and Dr. Klaustermeyer have no conflicts of interest to disclose.
The authors would like to thank Jeffrey Gornbein, DrPH, SBCC for statistical computation support.