Several studies suggest that early-life exposure to animal allergens constitutes a relevant risk factor for the development of allergic sensitization.
ObjectivesThe aim of the present study was to determine the role of interleukin-33 in children sensitive to cat allergen with allergic rhinitis and/or asthma.
MethodsThe study included 51 children aged 5–18 years, both sexes, allergic to cats. Sensitization to cat allergen was confirmed by skin prick tests or specific IgE. Children were evaluated for the presence of bronchial asthma, atopic dermatitis, allergic rhinitis. A questionnaire evaluating the occurrence of allergic symptoms in children after contact with the cat and dog was performed. Mothers completed a questionnaire regarding cat exposure: during pregnancy and having a cat at home. A blood sample was taken from all children to measure the level of IL-33 in the serum.
ResultsKeeping a cat in the home, once in the past, or having a cat in the home during the mother’s pregnancy, revealed a statistically significant relationship with IL-33 levels in the studied patients. Also, daily contact with a cat during pregnancy affected the level of IL-33. Higher levels of IL-33 were shown in people with hypersensitivity to cat and pollen allergens and cat and other animals. In patients with bronchial asthma higher levels of IL-33 were found than in patients without bronchial asthma.
ConclusionsIncreased serum levels of IL-33 is related with keeping cats during pregnancy and in early childhood and can be associated with the development of asthma in children.
Animals are inseparable companions in human life. At the same time, they are a source of strong allergens. The data available in several studies suggests that early-life exposure to household pets has the capacity to reduce risk for allergic disease,1,2 while other articles prove that exposure to animal allergens constitutes a relevant risk factor for the development of allergic sensitization.3 Cats and dogs are the most common pets living in indoor environments — a survey from 2014, shows that 83% of Poles have a dog in their home and 44% own a cat.4 Exposure to cat allergen in many European countries, where cats are common pets, is virtually universal. Also homes without cats have significant levels of cat allergen, as do day-care centers, and schools.5
An important factor in the induction of allergic inflammation is interleukin-33 (IL-33), a member of the interleukin-1 (IL-1) cytokine family.6 IL-33 needs the specific receptor ST2 (membrane-bound receptor) and Interleukin-1 receptor accessory protein heterodimer for its binding, which instigates the production of different types of cytokines and chemokines that have crucial roles in the exacerbation of allergic diseases and inflammation. IL-33 receptor ST2 is highly expressed in immune cells including macrophages, eosinophils, DCs, mast cells (MCs), basophils, NK cells, innate lymphoid group 2 cells (ILC2s), Th2 lymphocytes and B cells, as well as the endothelium, epithelium cells and fibroblasts.3,7 It was recently reported that myeloid dendritic cells (mDCs) in peripheral blood mononuclear cells in atopic subjects expressed higher levels of ST2 (IL-33 receptor) than those of non-atopic subjects.8 IL-33 acts not only as a Th2-inducing cytokine, but also as a pro-inflammatory cytokine in various other immune responses.9–11 The aim of the present study was to determine the role of interleukin-33 in children sensitive to cat allergen with allergic rhinitis and/or asthma.
MethodsThe study included 51 children aged 5–18 years, both sexes, allergic to cats, under the care of an allergy clinic. They were considered to have a cat allergy if they suffered from one or more of allergy symptoms during contact with the cat; sensitization to cat allergen was confirmed by skin prick tests or specific IgE. All patients were also tested for allergy to house dust mites, mold, pollen, and other animals by skin prick tests or specific IgE. Current child health status assessment was performed by an allergist. Children were evaluated for the presence of bronchial asthma, atopic dermatitis, allergic rhinitis. The definition of asthma was based on the Global Initiative for Asthma (GINA) guidelines.12 Atopic dermatitis was diagnosed according to the revised Hanifin and Rajka criteria.13 The diagnosis of AR was performed according to ARIA guidelines.14 Findings of AR were consistent with one or more of the following symptoms: nasal congestion, runny nose, itchy nose, and sneezing, red and watery eyes. A questionnaire evaluating the occurrence of allergic symptoms in children after contact with the cat and dog was performed, such as: eye symptoms (tearing, redness, pruritus), nasal symptoms (sneezing, nasal itch, runny nose), skin changes, symptoms from the side lower respiratory tract (cough, shortness of breath, wheezing). Mothers completed a questionnaire regarding cat exposure: (a) during pregnancy (permanently at home or periodically in other people who have a cat), (b) having a cat at home in the first two years of the child’s life and over the age of two years.
All children were also assessed for the occurrence of the above-mentioned allergic symptoms after contact with the dog. Mothers completed analogous questionnaires regarding contact with the dog during pregnancy and after childbirth.
Information about having other animals at home was also collected and species of pet possessed (rabbit, hamster, guinea pig, fish, birds).
A blood sample was taken from all children, to measure the level of IL-33 in the serum. Commercial enzyme-linked immunosorbent assays (ELISA) were applied to measure serum levels of IL-33 (Biorbyt, USA). The assay was conducted using the protocols recommended by the manufacturers (standard range: 15.6–1000pg/ml sensitivity: 4.14pg/ml).
The study was approved by the Ethical Committee of the Medical University, Lodz, Poland; RNN/39/14/KE; written consent was obtained from all the subjects before the study.
Statistical analysisThe investigated traits were described by way of measures of location — arithmetic or geometric mean, along with measures of dispersion — standard deviation, 95% confidence interval and minimum and maximum values. The categorical variables were depicted by using absolute numbers and percentages.
Multivariate linear regression (for numerical dependent variables such as IL-33 measurements) or logistic regression models (for binary dependent variables such as allergy symptoms or having an animal in the domicile) were performed in order to test statistical relationships. Independent variables for the regression equations were selected after having performed stepwise regression procedures. When dealing with non-normally distributed variables, robust standard errors (i.e., sandwich estimators) were used within a specific regression model. The IL-33 measurements had been natural log transformed (ln(IL-33)) prior to running the analyses. Specific variables that revealed the perfect prediction (i.e., when each study participant or none of them displayed a specific trait) were excluded from a regression equation. All the regression models were controlled for the studied patients’ age and gender.
A level of P<0.05 was considered statistically significant. All the statistical computations were carried out by means of Stata/Special Edition, release 14.2 (StataCorp LP, College Station, Texas, USA).
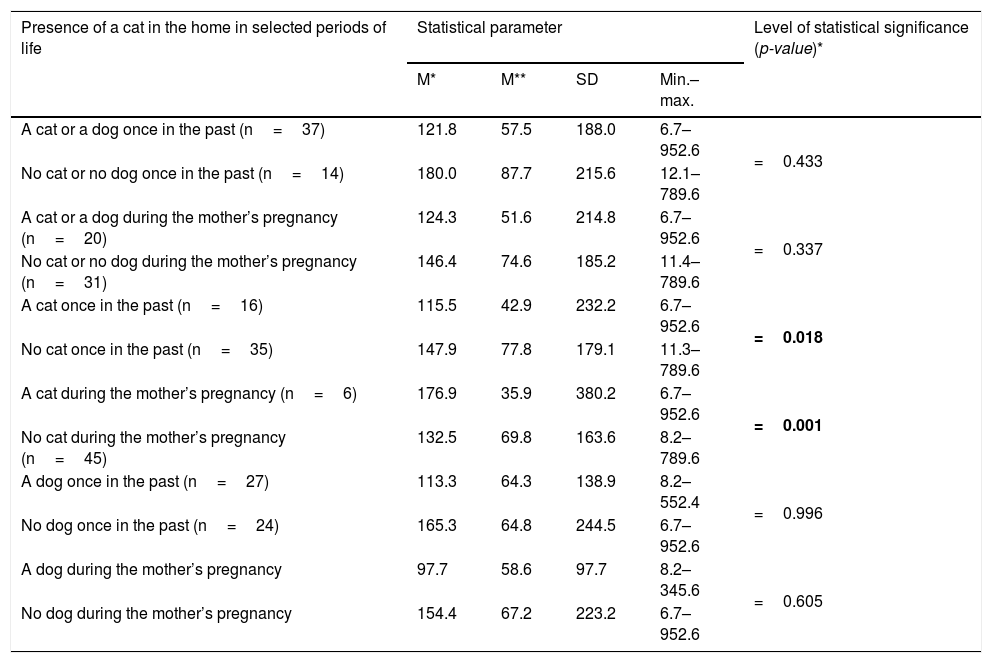
ResultsBaseline characteristics of the studied patients are presented in Table 1. Keeping a cat in the home, once in the past, or having a cat in the home during the mother’s pregnancy, revealed a statistically significant relationship with IL-33 levels in the studied patients (p=0.018, and p=0.001 respectively) (Table 2). Moreover, daily contact with a cat during pregnancy, even if there was no cat at home, affected the level of IL-33 (p=0.006); this relationship was not demonstrated for the less frequent contact with the cat.
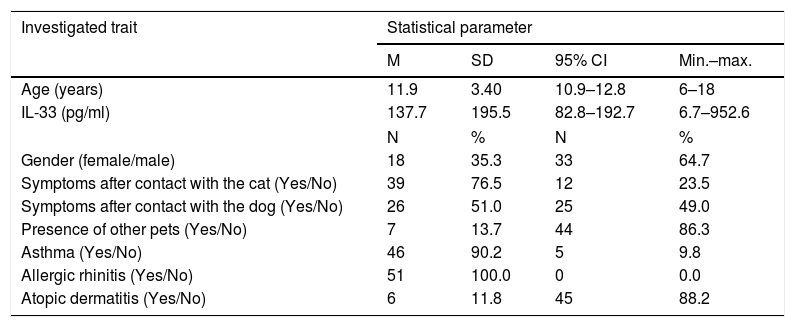
Baseline characteristics of the studied patients (n=51).
| Investigated trait | Statistical parameter | |||
|---|---|---|---|---|
| M | SD | 95% CI | Min.–max. | |
| Age (years) | 11.9 | 3.40 | 10.9–12.8 | 6–18 |
| IL-33 (pg/ml) | 137.7 | 195.5 | 82.8–192.7 | 6.7–952.6 |
| N | % | N | % | |
| Gender (female/male) | 18 | 35.3 | 33 | 64.7 |
| Symptoms after contact with the cat (Yes/No) | 39 | 76.5 | 12 | 23.5 |
| Symptoms after contact with the dog (Yes/No) | 26 | 51.0 | 25 | 49.0 |
| Presence of other pets (Yes/No) | 7 | 13.7 | 44 | 86.3 |
| Asthma (Yes/No) | 46 | 90.2 | 5 | 9.8 |
| Allergic rhinitis (Yes/No) | 51 | 100.0 | 0 | 0.0 |
| Atopic dermatitis (Yes/No) | 6 | 11.8 | 45 | 88.2 |
Associations between IL-33 levels in the studied patients and presence of cat/dog in their domicile once in the past and during pregnancy.
| Presence of a cat in the home in selected periods of life | Statistical parameter | Level of statistical significance (p-value)* | |||
|---|---|---|---|---|---|
| M* | M** | SD | Min.–max. | ||
| A cat or a dog once in the past (n=37) | 121.8 | 57.5 | 188.0 | 6.7–952.6 | =0.433 |
| No cat or no dog once in the past (n=14) | 180.0 | 87.7 | 215.6 | 12.1–789.6 | |
| A cat or a dog during the mother’s pregnancy (n=20) | 124.3 | 51.6 | 214.8 | 6.7–952.6 | =0.337 |
| No cat or no dog during the mother’s pregnancy (n=31) | 146.4 | 74.6 | 185.2 | 11.4–789.6 | |
| A cat once in the past (n=16) | 115.5 | 42.9 | 232.2 | 6.7–952.6 | =0.018 |
| No cat once in the past (n=35) | 147.9 | 77.8 | 179.1 | 11.3–789.6 | |
| A cat during the mother’s pregnancy (n=6) | 176.9 | 35.9 | 380.2 | 6.7–952.6 | =0.001 |
| No cat during the mother’s pregnancy (n=45) | 132.5 | 69.8 | 163.6 | 8.2–789.6 | |
| A dog once in the past (n=27) | 113.3 | 64.3 | 138.9 | 8.2–552.4 | =0.996 |
| No dog once in the past (n=24) | 165.3 | 64.8 | 244.5 | 6.7–952.6 | |
| A dog during the mother’s pregnancy | 97.7 | 58.6 | 97.7 | 8.2–345.6 | =0.605 |
| No dog during the mother’s pregnancy | 154.4 | 67.2 | 223.2 | 6.7–952.6 | |
Significance of bold values: p<0.005.
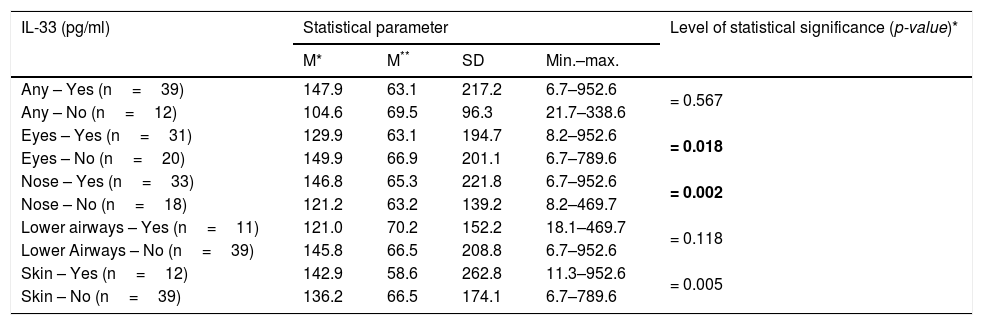
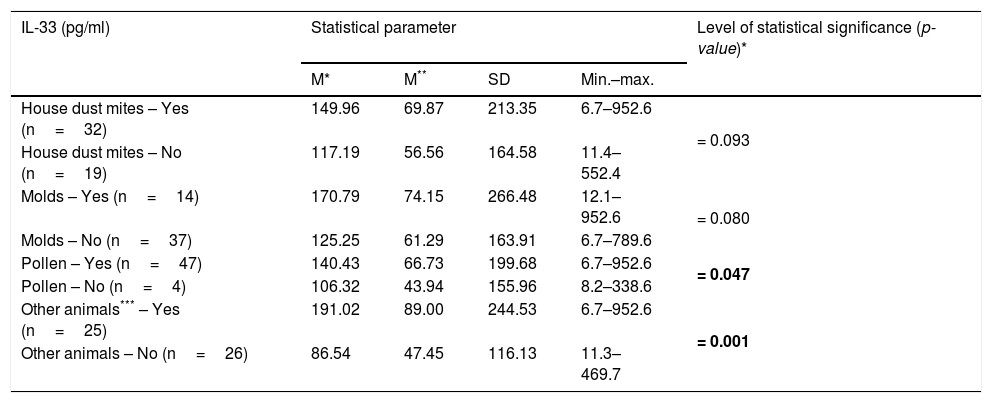
There was a significant relationship between the level of IL-33 and the occurrence of eye and nose symptoms after contact with the cat (p=0.018 and p=0.002 respectively) (Table 3). There was no statistically significant relationship between the level of IL-33 and the occurrence of any symptoms after contact with the cat in children with additional allergy to dust mites (p=0.356), mold (p=0.575), pollen (p=0.122), and other animals (p=0.390). Persons allergic to cat’s fur and house dust mite significantly less often reported allergic symptoms from the lower respiratory tract after contact with the cat in comparison to people sensitized to cat fur only, OR=0.13 (95% CI: 0.02–0.85) (p=0.033). Higher levels of IL-33 were shown in people with hypersensitivity to cat and pollen allergens (p=0.047) and cat and other animals (p=0.001), at the same time no such relationship was found in people with hypersensitivity to house dust mite (p=0.093) and molds (p=0.080) (Table 4).
Associations between IL-33 levels in the studied patients and presence of allergy symptoms after contact with the cat.
| IL-33 (pg/ml) | Statistical parameter | Level of statistical significance (p-value)* | |||
|---|---|---|---|---|---|
| M* | M** | SD | Min.–max. | ||
| Any – Yes (n=39) | 147.9 | 63.1 | 217.2 | 6.7–952.6 | = 0.567 |
| Any – No (n=12) | 104.6 | 69.5 | 96.3 | 21.7–338.6 | |
| Eyes – Yes (n=31) | 129.9 | 63.1 | 194.7 | 8.2–952.6 | = 0.018 |
| Eyes – No (n=20) | 149.9 | 66.9 | 201.1 | 6.7–789.6 | |
| Nose – Yes (n=33) | 146.8 | 65.3 | 221.8 | 6.7–952.6 | = 0.002 |
| Nose – No (n=18) | 121.2 | 63.2 | 139.2 | 8.2–469.7 | |
| Lower airways – Yes (n=11) | 121.0 | 70.2 | 152.2 | 18.1–469.7 | = 0.118 |
| Lower Airways – No (n=39) | 145.8 | 66.5 | 208.8 | 6.7–952.6 | |
| Skin – Yes (n=12) | 142.9 | 58.6 | 262.8 | 11.3–952.6 | = 0.005 |
| Skin – No (n=39) | 136.2 | 66.5 | 174.1 | 6.7–789.6 | |
All the analyses were controlled for the studied patients’ age and gender, along with the remaining symptoms.
Significance of bold values: p<0.005.
Associations between IL-33 levels in the studied patients and presence of co-allergy.
| IL-33 (pg/ml) | Statistical parameter | Level of statistical significance (p-value)* | |||
|---|---|---|---|---|---|
| M* | M** | SD | Min.–max. | ||
| House dust mites – Yes (n=32) | 149.96 | 69.87 | 213.35 | 6.7–952.6 | = 0.093 |
| House dust mites – No (n=19) | 117.19 | 56.56 | 164.58 | 11.4–552.4 | |
| Molds – Yes (n=14) | 170.79 | 74.15 | 266.48 | 12.1–952.6 | = 0.080 |
| Molds – No (n=37) | 125.25 | 61.29 | 163.91 | 6.7–789.6 | |
| Pollen – Yes (n=47) | 140.43 | 66.73 | 199.68 | 6.7–952.6 | = 0.047 |
| Pollen – No (n=4) | 106.32 | 43.94 | 155.96 | 8.2–338.6 | |
| Other animals*** – Yes (n=25) | 191.02 | 89.00 | 244.53 | 6.7–952.6 | = 0.001 |
| Other animals – No (n=26) | 86.54 | 47.45 | 116.13 | 11.3–469.7 | |
All the analyses were controlled for the studied patients’ age and gender, along with the remaining allergy.
Significance of bold values: p<0.005.
Statistically significantly higher levels of IL-33 in the asthmatic subjects were seen as compared to the non-asthmatic ones (p<0.001); the presence of atopic dermatitis did not show any statistically significant relationships with IL-33 levels in the studied patients (p=0.124).
Keeping the dog and other animals at home, once in the past, or possessing the dog and other animals at home during the mother’s pregnancy, did not show any statistically significant relationship with IL-33 levels in the studied patients (Table 2).
DiscussionIL-33 plays an important role in allergy and inflammation. Our study showed a positive correlation between having a cat at home in early childhood and during maternal pregnancy and serum level of IL-33 in the examined patients. Daily contact with the cat during pregnancy affected the level of IL-33 even if the cat was not permanently at home. We found higher serum levels of IL-33 in children with bronchial asthma. Higher levels of IL-33 were also seen in children allergic to cats with hypersensitivity to pollen allergens and other animals.
Recently, IL-33 has been considered as an emerging key factor in the development of allergic diseases.7 The cat allergen, as well as other allergens, including house dust mite are involved in several allergic diseases, such as asthma and AR. Notably, IL-33 expression is up-regulated in the bronchial mucosa of asthmatic patients related to disease severity.15,16 Interleukin-33 (IL-33) appears to be a potent inducer of Th2 immune response. This occurs when IL-33 binds and activates its receptor, the membrane ST2 (ST2L) in mast cells, dendritic cells, basophils, eosinophils, innate lymphoids and Th2 cells, leading to the release of these cytokines and intensifying allergic inflammation. Polymorphisms in the IL-33 and IL1RL1 can act as protective or risk factors for asthma and/or allergy in humans.17 It was shown that bronchial epithelium is an important reservoir of IL-33 in the lung and that IL-33 expression is elevated in the airways of bronchial asthma along with disease severity.6,18 In allergic and non-allergic children with severe treatment-refractory asthma, levels of IL-33 were increased in multi-sensitized children and correlated with IgE levels to dust mite, ryegrass, and fungi but not cat, ragweed, or food sources.19 Many authors have shown higher levels of IL-33 in asthmatic patients.20–22 Ravanetti et al. demonstrated that IL-33 was necessary to drive asthma exacerbations.23 In addition to promoting Th2 inflammation, Zoltowska et al. suggest a role for the IL-33/ST2 pathway for the induction of peripheral inflammation and mucus production that causes airway hyperresponsiveness (AHR) in the peripheral lung in a house dust mite mouse model of asthma.24 Our results suggest that higher levels of serum IL-33 could be reported in children with asthma who are allergic to cat when compared to non-asthmatic children. We showed that keeping a cat during pregnancy or the first years of a child’s life at home is conducive to bronchial asthma.
Gasiuniene et al. proved that IL-33 is increased in asthma patients, particularly in some phenotypes: allergic asthma and eosinophilic asthma.22 Hesselmar et al. observed fewer allergic manifestations (any of asthma, allergic rhinoconjunctivitis, or eczema) with increasing number of household cats and dogs during the first year of life.25 In our study, the conclusions are different. There was no correlation between allergic symptoms and number of household cats and dogs during the first year of life, however our study was not powered to determine such a relationship.
According to Hesselmar et al. there is a dose-response association, with fewer allergic manifestations (any of asthma, allergic rhinoconjunctivitis, or eczema) with increasing number of household cats and dogs, as well as pollens, during the first year of life.25 In our study, higher levels of IL-33 were seen in children with hypersensitivity to cat and pollen allergens or other animals. Luo et al. demonstrated that pet keeping in childhood was positively associated with asthma and allergy.26 The cat exposure in the first year of life was significantly and independently associated with current wheezing and current asthma and current rhinoconjunctivitis at the age of seven.27 However, Lodge et al. concluded that exposure to cats or dogs at birth showed a moderate reduction in risk of wheeze and hay fever after seven years of age.28 Brunekreef et al. proved among children (6–7 years of age), that cat exposure in the first year of life was associated with current symptoms of asthma, wheeze, rhinoconjunctivitis, and eczema, especially in less-affluent countries.29 Collin et al. determined that pet ownership during pregnancy and childhood in birth cohort was consistently associated with a reduced risk of aeroallergen sensitization and atopic asthma at the age of seven, but tended to be associated (particularly for rabbits and rodents) with an increased risk of non-atopic asthma.30
IL-33 and ILC2s may be critical for maintaining epithelial integrity and tissue homeostasis,31 they therefore play important roles in the skin barrier dysfunction.2 In some studies, IL-33 and ST2 levels are elevated in epidermis of AD patients.2,32 IL-33 down-regulates the β-defensin 2 expression in human primary keratinocytes33 and influences the susceptibility to bacterial superinfection in acute allergic eczema of AD.2 In addition, allergen exposure and filaggrin deficiency amplify the expression of ST2 and IL-33 in BMMC (bone marrow mononuclear cells).34 Seltmann et al. have reported that IL-33 down-regulates filaggrin expression in keratinocytes and impairs the skin barrier.35 Nygaard showed that serum levels of IL-33 were significantly elevated in AD patients compared with controls.36 In the skin of patients with atopic dermatitis, the expression of IL-33 is increased in epidermal keratinocytes.37,38 However, the mechanism by which the expression of IL-33 is regulated in bronchial epithelial cell and epidermal keratinocytes remains unknown.38–40 However, in our study the presence of atopic dermatitis did not show any relationship with serum IL-33 levels in the studied patients.
In intermittent AR patients who are sensitive to tree and/or grass pollen, serum IL-33 is up-regulated and correlates with disease severity.41 Interestingly, a study including 24 Japanese cedar (JC) pollinosis patients and 14 HDM-sensitized patients with AR found that IL-33 protein is not detected in the serum, but IL-33 level is increased in sinus mucosa and significantly correlated with the total nasal symptom score.42 After ragweed exposure, mice showed aggravated AR symptoms, nasal Th2 activation, increased level of serum ragweed-specific IgE and the infiltration of eosinophils and basophils in the nasal mucosa, which were not showed in IL-33- and ST2-deficient mice.43 In our study, all children were diagnosed with allergic rhinitis, which is why the disease was not considered in the statistical analysis.
Our study has some limitations, including the small number of subjects. It also lacks a control group with children who are not sensitized to cat living and not living with cat. Therefore, it is very difficult to sort out the role of a single cytokine and its relationship to the environment in a real-world setting, where the environmental exposures are based on retrospective history and numerous other confounding exposures exist.
The effect of pet exposure on the risk of children developing allergic disease remains controversial. Our study showed a statistically significant relationship between having a cat at home during maternal pregnancy and in early childhood and the level of IL-33 in the examined patients. We also showed higher levels of IL-33 in people with bronchial asthma. In order to better understand the impact of animal ownership on the development of allergic diseases in children, further research is needed. Understanding the effects of having animals in pregnancy and early childhood can better inform about prevention strategies.
Authors’ contributionK. Smejda: literature search, study design, analysis of data, manuscript preparation.
A. Borkowska: literature search, data collection, laboratory markings.
J. Jerzynska: literature search, data collection, study design, manuscript preparation, review of manuscript.
A. Brzozowska: data collection, study design, analysis of data.
W. Stelmach: literature search, study design, analysis of data.
I. Stelmach: literature search, data collection, study design, manuscript preparation, review of manuscript.
Conflict of interestAll authors state no conflict of interests.
Financial informationThis study was funded by grant 503/2-056-01/503-21-001-18, and 503/6-029-01/503-01 from the Medical University of Lodz, Poland.
Trial registrationNot applicable.