Mucociliary clearance (MCC) is impaired due to chronic inflammation in allergic rhinitis. Our aim was to evaluate MCC in children with allergic rhinitis, to determine its relationship with disease severity and evaluate MCC change after nasal irrigation.
Materials and methodsSaccharin test was performed in 51 patients with allergic rhinitis and in 50 controls. Nasal irrigation was performed to the patients and saccharin test was repeated at the 10th minute. Total nasal symptom score (TNSS) and visual analogue scale (VAS) results were recorded. Patients were divided into mild/moderate-severe groups according to TNSS, VAS, and ARIA guidelines. Nasal MCC time (NMCCT) of the patients and the controls and NMCCT before and after nasal irrigation of the patients were compared. Correlations between NMCCT and TNSS/VAS were evaluated. NMCCTs of the mild and moderate-severe groups were compared. The cut-off values were calculated to discriminate the patient group.
ResultsThe mean NMCCT of the patient group was higher than the controls. Mean NMCCTs were different between before and after irrigation. NMCCT was higher in uncontrolled/moderate-severe groups than in controlled/mild groups. NMCCT correlated positively with VAS and TNSS. The sensitivity and specificity of NMCCT>535s were found to be 86.27% and 94%, respectively.
ConclusionsIn children with allergic rhinitis, the prolongation of MCC may be identified with the easily applicable saccharin test, the deterioration in MCC increases as disease severity increases. Nasal irrigation is important in children with allergic rhinitis to improve MCC.
Allergic rhinitis is a chronic disease in children with a prevalence of 2–25% and leads to impaired quality of life. It is the most common form of non-infectious rhinitis. In allergic rhinitis, IgE-mediated immune response to allergens occurs. Allergic rhinitis is diagnosed by the presence of nasal symptoms, and the presence of ocular symptoms is frequently accompanied by the presence of an allergic response.1–3
Mucociliary clearance (MCC) is a very important protection system for the protection of the nose from harmful molecules and allergens. With this system, the mucus formed in the nasal mucosa is mobilized by the movement of the nasal ciliates, and the particles are delivered through the nose to the nasopharynx through this movement. The particle that reaches the nasopharynx is either swallowed or removed orally.4
MCC can be evaluated by various methods. Some of these methods are the evaluation with indigo carmine dye, evaluation with gamma scintigraphy, and evaluation with saccharin test.5–7 The saccharin test is distinguished from other tests because it is easy to be applied in outpatient settings as well as being cheap and non-invasive. The saccharin test is based on the time elapsed between the placement of the saccharin piece in the anterior part of the inferior turbinate and the feeling of the sweet taste in the mouth, and which may be achieved within minutes.7
In allergic rhinitis patients, deterioration of the structure of the cilia, as well as changes in mucus viscosity due to long-term chronic inflammation, has been shown. Mechanical obstruction may also lead to mucus stagnation, causing pericellular compression and nasal MCC deterioration. With the deterioration of MCC, removal of allergens from the nasal cavity becomes difficult, contact with allergens increases, and therefore treatment success decreases.4,8
The aim of our study was to evaluate MCC by saccharin test in children with allergic rhinitis, to determine its relationship with disease severity, and to evaluate MCC change after nasal irrigation.
Materials and methodsParticipantsPatients between 6 and 18 years of age diagnosed with allergic rhinitis were recruited as the patient group, and patients who presented to our clinic but were not diagnosed with allergic diseases by diagnostic tests or patients who had allergic diseases other than the involvement of the respiratory system were recruited as the control group. Those who received intranasal, inhaled or systemic steroids in the previous two months; antihistamines in the previous week; nasal or systemic decongestants in the previous two days; who had upper or lower respiratory tract infection in the previous month; visually remarkable nasal septum deviation or nasal deformity; those with a history of nasal surgery or trauma; an additional inflammatory, metabolic, endocrine, psychiatric disorder; and those with passive smoking exposure at home, and who had comorbid asthma were excluded from the study. Patients who had adenoid vegetations according to their self-report or previous otolaryngology examination notes or patients who were never evaluated for adenoid vegetations were excluded from the study. Some patients with allergic rhinitis were not on intranasal steroid treatment for the previous two months because they were either mildly symptomatic at presentation or non-adherent to their treatment.
Procedure – saccharin testSaccharin test was performed to evaluate mucociliary clearance in 51 patients who were diagnosed with allergic rhinitis according to the ARIA guidelines, and in 51 patients in the control group. At room temperature, the participants were asked to sit in the sitting position and tilt their heads 10 degrees backwards. A saccharin tablet 1mm3 in size was placed in the medial segment of the inferior nasal turbinate. Patients were asked not to sneeze and swallow while the saccharin tablet was in place. If this happened, the test was terminated. The patients were told to swallow once every 30s. The time elapsed between the moment when they felt the saccharin taste and the start of the test was recorded in seconds. This time was defined as nasal MCC time (NMCCT).
Procedure – nasal irrigationNasal irrigation was performed only to patients with allergic rhinitis through the compressible nasal douching bottle. Sinus Rinse Pediatric (120mL bottle) (Abfen Farma, Ankara, Turkey) was applied for nasal irrigation and at least half of the bottle was applied during douching. Patients with allergic rhinitis underwent saccharin test again at the 10th minute after nasal irrigation.
Procedure – assessment of symptomsTotal Nasal Symptom Score (TNSS) of the patient group was recorded. Children self-assessed their TNSS, which is used to rate the symptom severity for nasal obstruction, rhinorrhea, sneezing, ocular pruritis, and postnasal drip on a scale of 0 (absent) to 3 (severe). Patients with TNSS≥6 were classified as moderate-severe, and those with TNSS<6 were classified as mild allergic rhinitis.9
The patients were asked to mark the number between 0 (no symptoms) - 10 (severe symptom) on the visual analogue scale (VAS), considering their symptoms of allergic rhinitis in the last month. Patients with a VAS score of ≤5 were grouped as mild, and patients with a VAS score of ≥6 were grouped as severe allergic rhinitis.10
According to the ARIA guidelines, the patients were grouped as mild allergic rhinitis (normal sleep, normal daily activities, sports, normal school and work life, no troublesome symptoms), or as moderate/severe allergic rhinitis (abnormal sleep, impairment of daily activities, sport, abnormal school and work life, troublesome symptoms).1
Statistical analysisWe did a priori power analysis in order to determine the minimum number of participants needed for the study using G*Power 3.1.9.2, and the analysis showed that in order to identify an effect size of 0.50 (moderate effect size), with an alpha of 0.05 and 0.80 power, we would need a total of 51 participants per group. All the remaining analyses were performed by using IBM SPSS Statistics version 22.0 software package. Mean/median NMCCT values of the patient and control groups, and the mean/median NMCCT values before and after nasal irrigation were compared with dependent samples t-test. The correlation between NMCCT and TNSS and VAS scores was evaluated by Pearson/Spearman correlation analyses. The mean/median NMCCT values between the allergic rhinitis severity groups were compared by independent samples t-test. The cut-off value to discriminate the patient group was calculated by ROC analysis. p value of <0.05 was considered statistically significant.
Ethical approvalAll participants and their parents gave written informed consent before the study. The study was approved by the local ethics committee.
ResultsThe mean age of the patients was 9.65±2.70 years and the mean age of the control group was 9.56±2.53 years. 47.5% of the patients were female. Age and gender were not statistically different between the patient and the control groups. (p=0.867, p=0.761, respectively).
Forty-three of the patients (84.31%) had no adenoid vegetations according to their otolaryngology examination notes, and eight of the patients (15.68%) reported that they were told by their otolaryngology specialist that they had no adenoid vegetations.
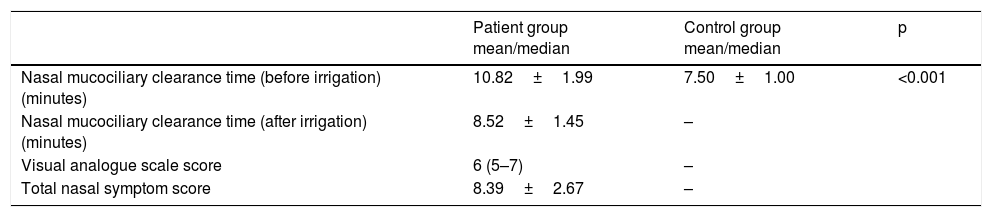
The mean first NMCCT value of the patient and the control groups, the mean NMCCT value of the patients after nasal irrigation; the mean/median VAS and TNSS scores of the patients are presented in Table 1. The mean NMCCT value was significantly higher in the patients than the control group (p<0.001). The mean NMCCT value before the nasal irrigation was 10.82±1.99min, and the mean NMCCT value after nasal irrigation was 8.52±1.45min. The mean NMCCT values of the patients before nasal irrigation were significantly higher than the mean NMCCT value after nasal irrigation (p<0.001).
Nasal mucociliary clearance time before and after nasal irrigation, and visual analogue scale and TNSS scores of the patients.
| Patient group mean/median | Control group mean/median | p | |
|---|---|---|---|
| Nasal mucociliary clearance time (before irrigation) (minutes) | 10.82±1.99 | 7.50±1.00 | <0.001 |
| Nasal mucociliary clearance time (after irrigation) (minutes) | 8.52±1.45 | – | |
| Visual analogue scale score | 6 (5–7) | – | |
| Total nasal symptom score | 8.39±2.67 | – |
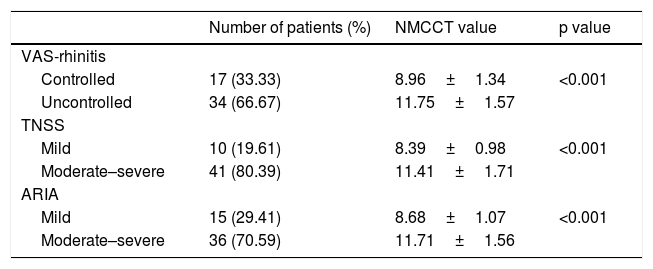
When the mean NMCCT was compared between the VAS and TNSS scores and ARIA severity groups, NMCCT was significantly higher in the uncontrolled or moderate-severe groups than in the controlled and mild groups (Table 2).
Comparison of the mean nasal mucociliary clearance time between the disease severity groups.
| Number of patients (%) | NMCCT value | p value | |
|---|---|---|---|
| VAS-rhinitis | |||
| Controlled | 17 (33.33) | 8.96±1.34 | <0.001 |
| Uncontrolled | 34 (66.67) | 11.75±1.57 | |
| TNSS | |||
| Mild | 10 (19.61) | 8.39±0.98 | <0.001 |
| Moderate–severe | 41 (80.39) | 11.41±1.71 | |
| ARIA | |||
| Mild | 15 (29.41) | 8.68±1.07 | <0.001 |
| Moderate–severe | 36 (70.59) | 11.71±1.56 |
NMCCT values correlated positively with VAS and TNSS scores (rho=0.764, p<0.001 and r=0.833, p<0.001, respectively).
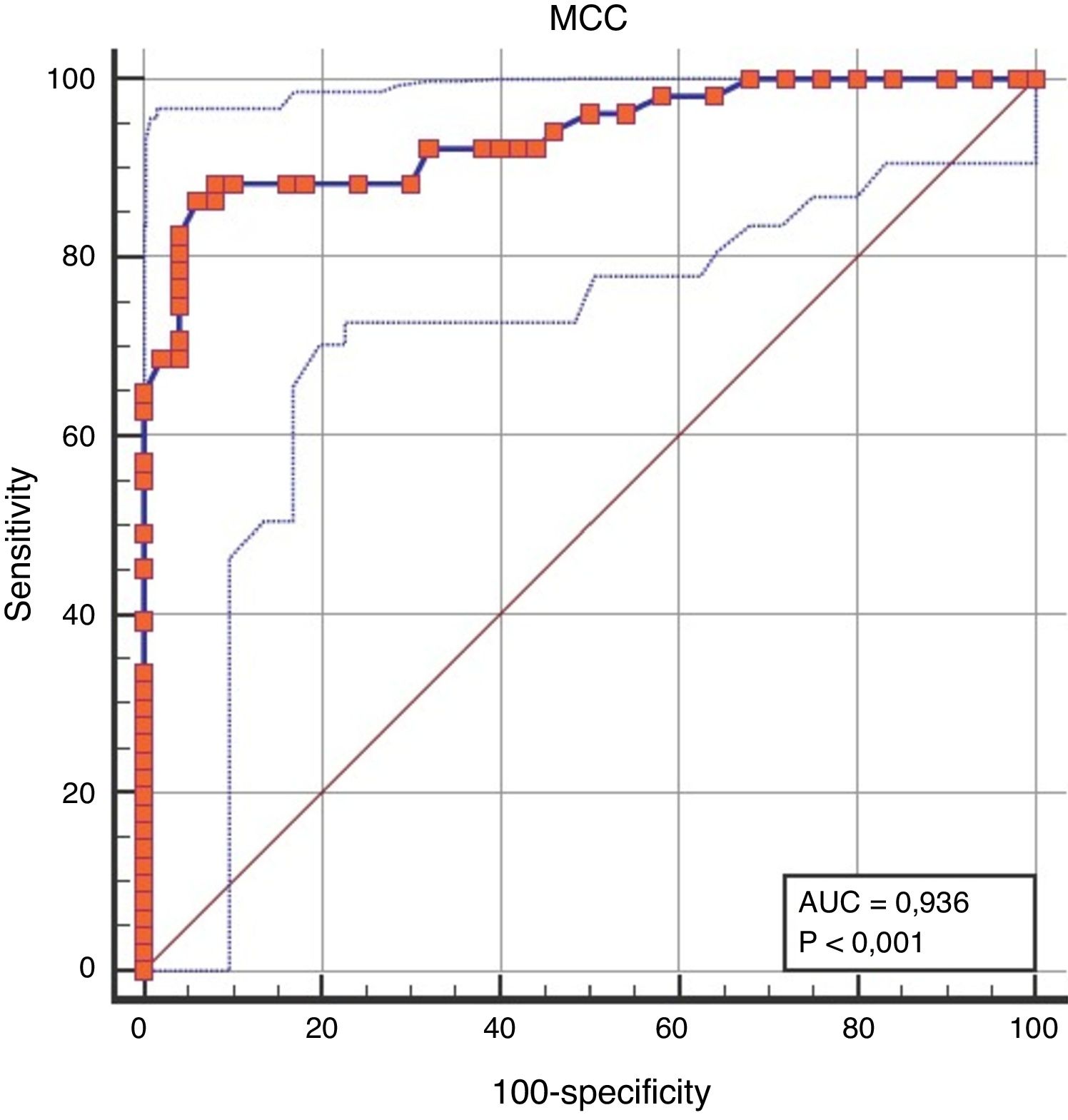
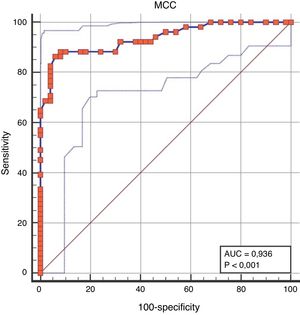
When the discriminative performance of NMCCT to discriminate the patient group from the control group was evaluated by ROC analysis, the cut-off value of NMCCT>8.96min was found to have a sensitivity of 86.27%, and a specificity of 94% (AUC=0.936, p<0.0001) (Fig. 1).
DiscussionMCC is a mechanism for protection from allergens and pathogens, which may be impaired due to mucus changes, number and structural as well as molecular defects of the cilia and mechanical obstruction. In this study, it was shown that NMCCT in pediatric patients with allergic rhinitis was longer than the healthy controls, and that NMCCT was associated with disease severity, and that NMCCT improved after nasal irrigation.
In patients with allergic rhinitis, changes in mucus density and deterioration of the ciliary structure due to chronic inflammation, or mechanical obstruction due to nasal obstruction have been shown to cause impaired MCC.11,12 It has been shown that IgE mediated inflammation caused by allergic inflammation causes more severe deterioration in MCC than non-allergic inflammation.13 In accordance with studies showing that NMCCT is longer than the control group in patients with allergic rhinitis,13–15 in our study in children with allergic rhinitis, NMCCT was found to be higher compared to the control group. In contrast to our study, to the best of our knowledge, all of the previous studies comparing the NMCCT of allergic rhinitis patients with healthy controls were undertaken in adults, and therefore, in this respect, the current study may be the first conducted among children with allergic rhinitis.
NMCCT may vary depending on the degree of inflammation and the severity of allergic rhinitis. The only adult study in the literature evaluating the association of MCC with allergic rhinitis disease severity reported that as the deterioration of MCC increased, the disease severity also increased.13 In our study, we also demonstrated that as the deterioration of MCC increased, the severity of allergic rhinitis increased as well.
Adenoid hypertrophy may cause MCC impairment by increased inflammation due to bacterial overload as well as an increase in mucus concentration by mechanical obstruction.16,17 In our study, patients with adenoid hypertrophy were excluded from the study, and therefore the effect of adenoid hypertrophy on MCC was not a confounder, and the net effect of allergic rhinitis on MCC could be demonstrated. Since not all patients had an otolaryngology examination note, and the researchers relied on their self-report about the presence of adenoid hypertrophy, this may be considered a limitation of the study. However, a great majority of the patients (84.3%) had an otolaryngology examination note, so this limitation was at least to some extent rectified.
Nasal irrigation may be used as adjunctive therapy in patients with allergic rhinitis. By removing airborne particles and aeroallergens through nasal irrigation, other treatments of allergic rhinitis may be augmented.18 In the study of Satdhabudha et al., nasal irrigation with both hypertonic saline and isotonic saline were found to improve NMCCT in pediatric patients with allergic rhinitis.19 In our study, nasal irrigation was performed only with isotonic saline, and a significant improvement in NMCCT was observed after nasal irrigation.
In our study, the cut-off value to discriminate the children with allergic rhinitis from the control group was also calculated, and to the best of our knowledge, our study is the first study in which such a cut-off value has been reported in the literature.
In the literature, nasal cilia structural changes, goblet cell hyperplasia and vascular congestion have been reported in children with passive smoking exposure.20 In a previous study, it was shown that passive smoking exposure in children might cause an increase in NMCCT.21 Due to the reports of the effects of passive smoking exposure on NMCCT, the exclusion of children with passive smoking exposure is one of the other strengths of our study.
Mucociliary velocity (MCV), which may be calculated by dividing the distance between the upper medial incisor and soft palate inferior edge by NMCCT, may be helpful in objectively assessing MCC without being influenced by the interpersonal changes in the length of the nasal cavity.16,22 One of the limitations of our study is that nasal MCV was not calculated. Since the length of the nasal cavity may only be measured during surgery, MCV could not be calculated in our study. In addition, children as young as six years old may have had difficulties in describing the taste sensation of the saccharin tablet. However, only three children in the patient group and only six children in the control group were six years old. Therefore, although this is another limitation of the study, we believe that they have not negatively affected the results. Another limitation of the study is related to the cross-sectional study design which precluded the necessary time frame to observe the positive effect of nasal irrigation on NMCCT.
In conclusion, in children with allergic rhinitis, MCC may be assessed by the inexpensive and easily applicable saccharin test. NMCCT is prolonged in pediatric allergic rhinitis patients, and the deterioration in MCC increases as allergic rhinitis severity increases. In children with allergic rhinitis, nasal irrigation treatment can improve the MCC. Our study is important because it is the first study to determine the cut-off value of NMCCT to discriminate patients with allergic rhinitis from the control group, and it is also the first study to evaluate the association of MCC with the disease severity in children with allergic rhinitis.
Conflict of interestThe authors have no conflict of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.