Since gamma interferon release assays (IGRAs) cannot differentiate between active tuberculosis and latent tuberculosis infection (LTBI), development of rapid and specific diagnosis tools are essential for discriminating between active tuberculosis (TB) from LTBI. Both IGRAs are based on Mycobacterium tuberculosis-specific antigens, namely, early secretory antigenic target 6 (ESAT-6) and 10kDa culture filtrate (CFP-10). The aim of this study was to evaluate the potential value of IL-2 secretion by whole blood cells after stimulation with rESAT-6 and rCFP-10 for discriminating between active and latent tuberculosis.
MethodsInterleukin-2 and IFN-γ were measured after blood stimulation of 90 cases (30 with active TB, 30 with LTBI and 30 healthy controls) with recombinant ESAT-6 and CFP-10. Receiver operating characteristic (ROC) curve analysis was conducted to determine the best IL-2 and IFN-γ result thresholds in discriminating between cases with active or latent TB, and the corresponding sensitivity and specificity were recorded.
ResultsThe IFN-γ release assay demonstrated a good sensitivity and specificity (sensitivity 83–84% and specificity 92%) for diagnosis of tuberculosis. The discrimination performance of IL-2 assay (assessed by the area under ROC curve) between LTBI and patients with active TB were 0.75 and 0.8 following stimulation with rESAT-6 and rCFP-10, respectively. Maximum discrimination was reached at a cut-off of 11.6pg/mL for IL-2 after stimulation with recombinant rESAT-6 with 72% sensitivity and 79% specificity and 10.7pg/mL for IL-2 following stimulation with rCFP-10 with 75% sensitivity and 79% specificity, respectively.
ConclusionThis study demonstrates that rESAT-6 and rCFP-10 can provide a sensitive and specific diagnosis of TB. In addition, it was shown that IL-2 may be serving as a marker for discriminating LTBI and active TB.
The cell-mediated immunity-based in vitro gamma interferon release assay (IGRA) of Mycobacterium tuberculosis-specific antigens has potential as a specific and accurate diagnostic means to detect those individuals with M. tuberculosis infection, especially when compared to the skin test.1,2 According to recent attempts for tuberculosis (TB) eradication, the treatment of latent tuberculosis infection (LTBI) and active TB is urgently required in order to lower and ultimately prevent the further spread of the disease at its present rate.3,4
Since IGRAs cannot differentiate between active and past TB infections or between LTBI and tuberculosis,5,6 a rapid and specific diagnosis tool is essential for discrimination of active TB from LTBI. Based on recent reports,5,7–10 cytokines such Interleukin-2 (IL-2) play a critical role during primary and latent infection; therefore, evaluation of IL-2 could be instrumental in discriminating between active and latent TB infection.
Both IGRAs are based on M. tuberculosis-specific antigens, namely, early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10).11,12 The aim of this study was to evaluate the potential value of IL-2 in stimulated whole blood cells with rESAT-6 and rCFP-10 for the discrimination of active and latent tuberculosis.
Material and methodsM. tuberculosis standard strain H37RV DNA was obtained from the National Research Institute of Tuberculosis and Lung Disease (NRITLD), National Mycobacteriologic Reference Laboratory, Tehran, Iran. Coding sequences of each dominant antigen fragment of ESAT-6 and CFP-10 were amplified according to our previous report.13 Each PCR product was ligated into pTZ57R/T cloning vector after purification using QIA quick PCR purification kit (MBI Fermentas, Lithuania). The ESAT-6 and CFP-10 gene were subcloned into pET32a(+) expression vector (Qiagen, USA) using SalI and BamHI for ESAT-6 and BamHI and HindIII enzymes for CFP-10.
Protein expression and purification of recombinant ESAT-6 and CFP-10The pET32a(+)-ESAT-6 and pET32a(+)-CFP-10 plasmids were transformed into E. coli BL21 DE3 (Novagen, Germany) expression host as described previously.13 Recombinant ESAT-6 and CFP-10 were purified using nickel–nitrilotriacetic acid (Ni2+–NTA) metal affinity chromatography according to the manufacturer's recommendations for purification of proteins under soluble conditions (Qiagen, USA).
PatientsIn this study we included 30 patients with active TB infection, 30 with LTBI, and 30 healthy individuals. Patients were recruited from the infectious diseases ward at the Masih Daneshvari Hospital, affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran. All enrolled individuals gave their written informed consent. Individuals were classified as having active TB when the diagnosis was confirmed by positive M. tuberculosis culture from sputum specimens. Individuals who had positive TST were selected and if they had positive QuantiFERON-TB Gold In-Tube test (QFT-G-IT) in the absence of symptomatic, microbiological, or radiological evidence of active tuberculosis were entered into the study and classified as having LTBI. Healthy controls were BCG-vaccinated individuals with no known exposure to M. tuberculosis and a negative response to the QFT-G-IT. We did not include in the study individuals who had positive human immunodeficiency virus screening result.
The study received approval from the Ethical Committee of Tehran University of medical sciences (100676).
Whole blood stimulationAbout 5ml of whole blood was collected from all study participants into heparinised tubes. The recombinant ESAT-6 and CFP-10 proteins were used at a final concentration of 10μg/mL.14
The diluted antigens (100μL at 20μg/mL) as well as the medium (unstimulated control) and positive control, were then seeded into 96-well plates in triplicate, after which plates were frozen at −80°C until the day of whole blood assay (WBA). The positive control was phytohaemagglutinin (PHA) and was used at a final concentration of 5μg/mL. On the day of WBA, pre-frozen antigen plates were allowed to thaw, and then 100μl of the blood added into each well containing the antigens or controls.
The plates were placed at 37°C in a humidified 5% CO2 incubator for three days. Culture supernatants were used for IFN-γ and IL-2 quantification.
IFN-γ and IL-2 detectionIFN-γ and IL-2 secretion were measured in the culture supernatants in the whole blood assay stimulated with antigens using IFN-γ ELISA kit and Human IL-2 ELISA kit (Mabtech AB, Sweden).
Flat-bottomed 96-well ELISA plates (Nunc Maxisorp, Thermo Fisher Scientific, United Kingdom) were coated with 2μg/mL coating antibody (Mabtech AB, Sweden) and was incubated overnight at 4°C. Wells were washed twice with 200μL/well sterile phosphate buffered saline (PBS) and blocked for 1h at room temperature with 200μL/well PBS with 0.05% Tween 20 containing 0.1% BSA. After incubation with whole blood assay supernatants for 2h plates were developed with 1μg/ml biotin-conjugated Ab (Mabtech) for 1h at room temperature and then streptavidin–HRP (Mabtech) diluted 1:1000 in PBS for 1h at room temperature. Wells were then washed six times and 100μL/well of tetramethylbenzidine (TMB) substrate (Sigma, United Kingdom) was added. Plates were allowed to develop for 20min before adding 100μL/well of 0.5M H2SO4. Plates were read immediately on an ELISA reader at a 450-nm wavelength.
Statistical analysisThe differences in levels of biomarkers among groups were analysed using non-parametric analysis of variance with the Kruskal–Wallis test.
Receiver operating characteristic (ROC) curve analysis was conducted to determine the best IL-2 and IFN-γ result thresholds in discriminating between cases with active or latent TB, relatively to a specific M. tuberculosis antigen ESAT-6 and CFP-10, and the corresponding sensitivity and specificity were reported. The area under the ROC curve (AUC) and 95% confidence interval (CI) were also calculated. Cut-offs were estimated at various sensitivities and specificities and at the maximum Youden's index (YI), i.e. sensitivity×specificity−1.15 Individual concentrations of IL-2 detected in the plasma from antigen-stimulated culture minus the concentration in the respective control plasma were used for this determination.
Statistical analysis was performed using the statistical software STATA 11 (StataCorp, College Station, TX, USA) and p value<0.05 was considered statistically significant.
ResultsIn this study, rESAT-6 and rCFP-10 were cloned, expressed successfully and used as M. tuberculosis stimulators. The antigen-specific levels of IFN-γ and IL-2 were evaluated in all 90 subjects included in the study. Thirty individuals were classified as uninfected, 30 as LTBI and 30 as active TB cases (Table 1).
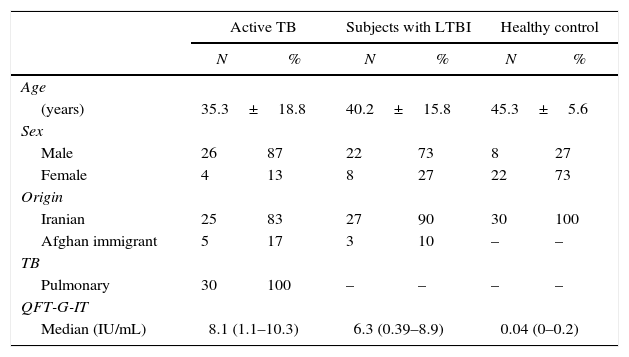
Demographic characteristics of enrolled individuals.
| Active TB | Subjects with LTBI | Healthy control | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Age | ||||||
| (years) | 35.3±18.8 | 40.2±15.8 | 45.3±5.6 | |||
| Sex | ||||||
| Male | 26 | 87 | 22 | 73 | 8 | 27 |
| Female | 4 | 13 | 8 | 27 | 22 | 73 |
| Origin | ||||||
| Iranian | 25 | 83 | 27 | 90 | 30 | 100 |
| Afghan immigrant | 5 | 17 | 3 | 10 | – | – |
| TB | ||||||
| Pulmonary | 30 | 100 | – | – | – | – |
| QFT-G-IT | ||||||
| Median (IU/mL) | 8.1 (1.1–10.3) | 6.3 (0.39–8.9) | 0.04 (0–0.2) | |||
TB: tuberculosis; LTBI: latent tuberculosis infection; QFT-G-IT: QuantiFERON-TB Gold In Tube.
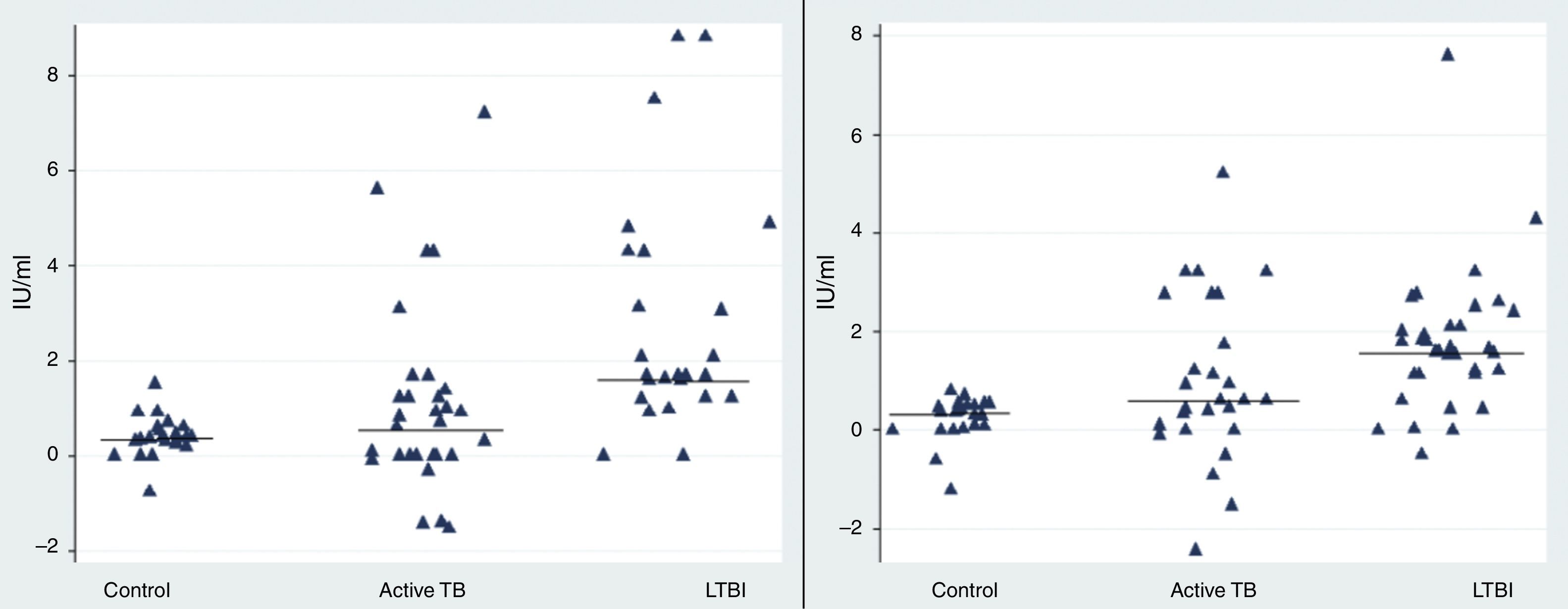
Our results revealed a statistical difference between the median IFN-γ levels of the LTBI and control groups after stimulation with rESAT-6 and rCFP-10 antigen (p value=0.05 and 0.028, respectively) (Fig. 1). The median level of IFN-γ following stimulation with rESAT-6 in active TB group and LTBI group was 0.61IU/mL (0.2–1.3) and 1.68IU/mL (1.2–4.2), respectively. In addition, the median level of IFN-γ was 0.62IU/mL (0.2–2.5) and 1.84IU/mL (0.43–2.5) following stimulation with rCFP-10 in active TB group and LTBI group, respectively.
The rESAT-6 and rCFP-10 stimulated expression of IFN-γ in patients with different groups. IFN-γ release obtained after incubation with a mixture of rESAT-6 (left panel) and rCFP-10 (right panel) in three different groups of subjects: patients with active tuberculosis, subjects with LTBI and healthy non-infected controls, respectively. Horizontal bars indicate the medians of the IFN-γ values of the respective population.
A statistically significant difference was found between the median IFN-γ levels of the both antigens tested: rESAT-6 (p value=0.001) and rCFP-10 (p value=0.002) when compared with the TB disease and control groups (Fig. 1).
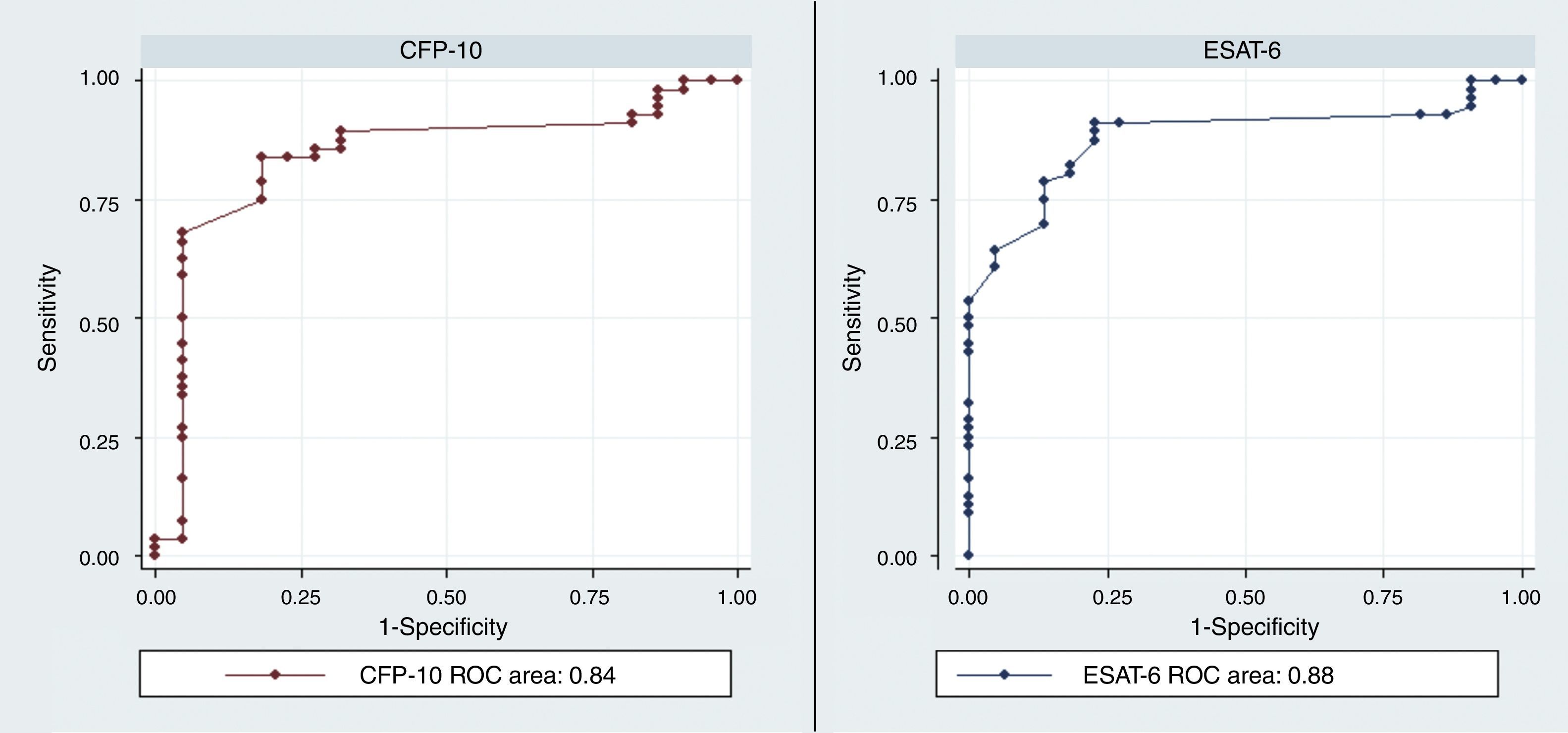
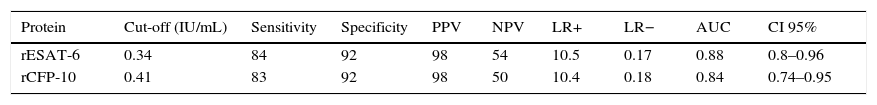
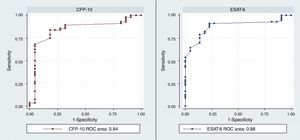
The diagnostic ability of the ESAT-6 and CFP-10 demonstrated a good discriminatory power in detecting patients with TB and LTBI infection from those without the infection who were BCG vaccinated. These results demonstrated a good sensitivity and specificity in detecting patients with TB and LTBI infection (Table 2). The AUCs for ESAT-6 and CFP-10 were 0.88 and 0.84, respectively (Fig. 2).
The discriminatory power of IFN-γ after stimulation with rESAT-6 and rCFP-10 in detecting patients with TB (both active and latent) and controls.
| Protein | Cut-off (IU/mL) | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | AUC | CI 95% |
|---|---|---|---|---|---|---|---|---|---|
| rESAT-6 | 0.34 | 84 | 92 | 98 | 54 | 10.5 | 0.17 | 0.88 | 0.8–0.96 |
| rCFP-10 | 0.41 | 83 | 92 | 98 | 50 | 10.4 | 0.18 | 0.84 | 0.74–0.95 |
PPV: positive predictive values; NPV: negative predictive values; LR: likelihood ratio; AUC: area under the curve.
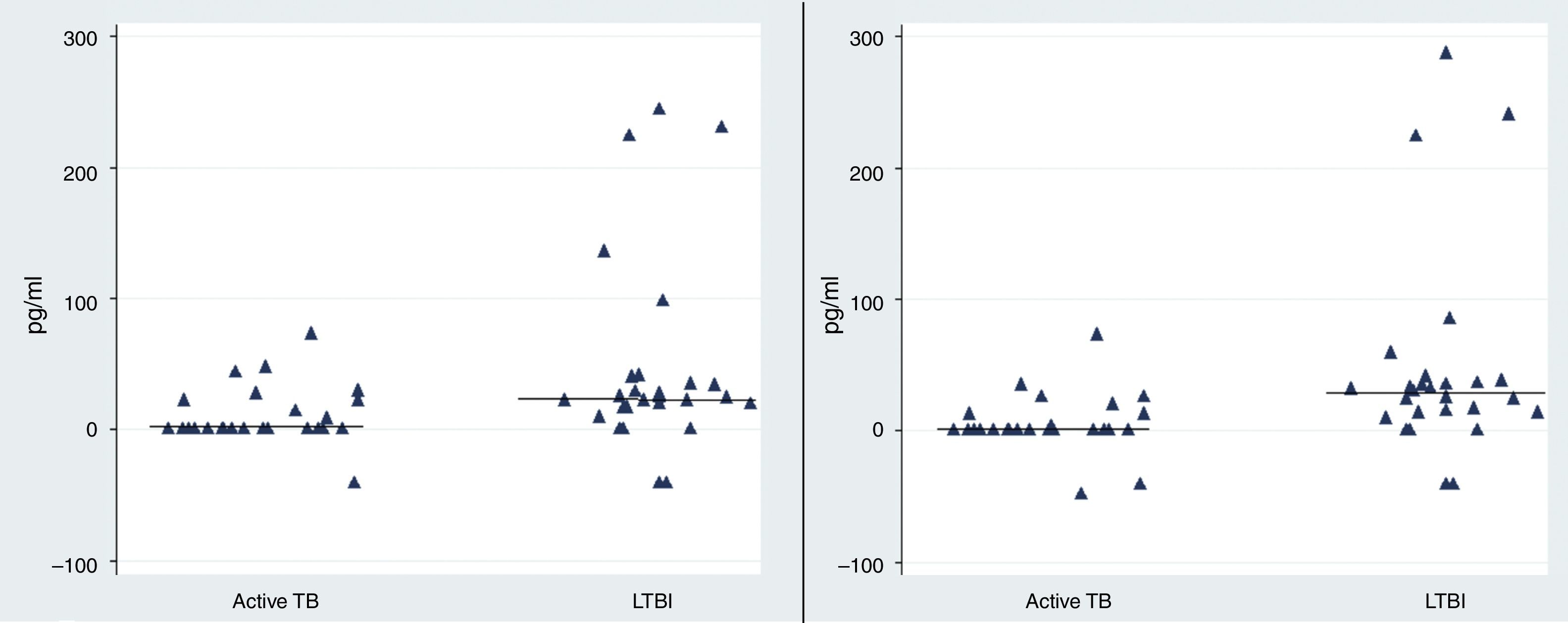
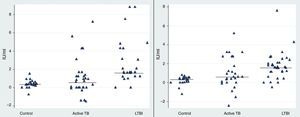
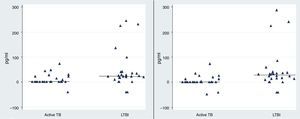
Observing the level of IL-2 released after 72h of incubation, we found that the level of IL-2 were significantly higher in LTBI group than in patients with active TB infection following stimulation with rESAT-6 (p value=0.002) and following stimulation with rCFP-10 (p value=0.001), using an unpaired Student's t-test) (Fig. 3).
The rESAT-6 and rCFP-10 stimulated expression of IL-2 in patients with active tuberculosis and individuals with LTBI. IL-2 release obtained after 72h of incubation with a mixture of rESAT-6 (left panel) and rCFP-10 (right panel) in different groups of subjects: patients with active tuberculosis and subjects with LTBI, respectively. Horizontal bars indicate the medians of the IL-2 values of the respective population.
The median level of IL-2 following stimulation with rESAT-6 in the active TB group and LTBI group were 0.3pg/mL (0.2–21.9) and 24.5pg/mL (14.3–40), respectively. In addition, the median levels of IL-2 were 0.3pg/mL (0.2–7.6) and 27.4pg/mL (12.3–38.4) following stimulation with rCFP-10 in active TB group and LTBI group, respectively. No secretion of IL-2 was detected in the control group (median: 0pg/mL (0–0.08) (data not shown)).
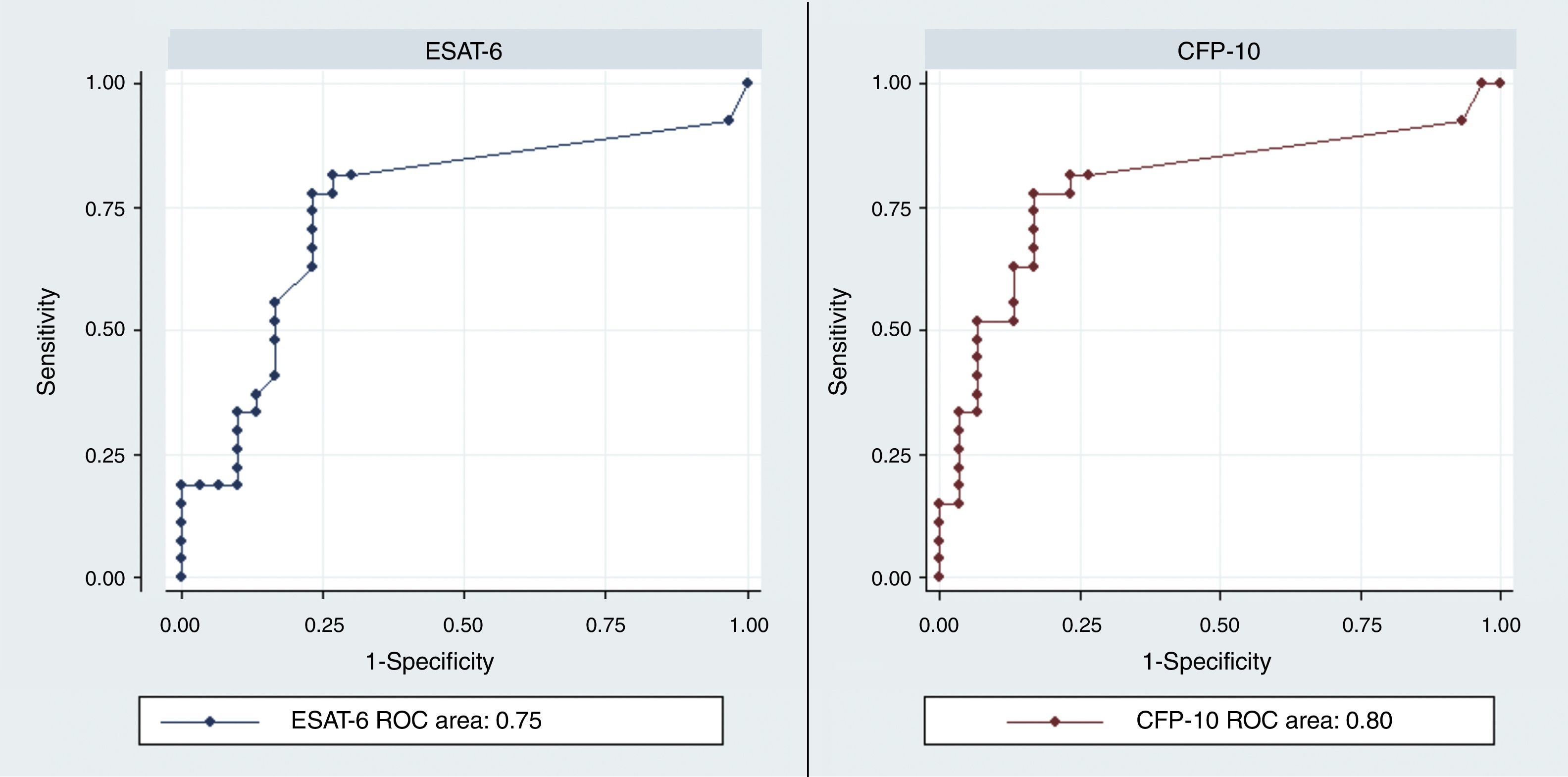
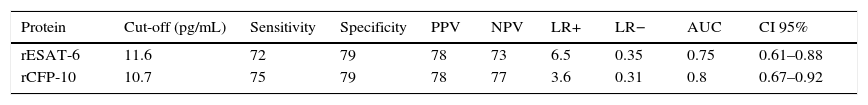
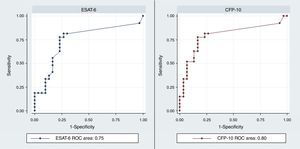
The discrimination performance (assessed by the area under ROC curve) between LTBI and active TB group were 0.75 and 0.8 for IL-2 following stimulation with rESAT-6 and rCFP-10, respectively (Fig. 4). Maximum discrimination was reached at a cut-off of 11.6pg/mL for IL-2 after stimulation with recombinant rESAT-6 with 72% sensitivity and 79% specificity and 10.7pg/mL for IL-2 following stimulation with rCFP-10 with 75% sensitivity and 79% specificity, respectively (Table 3).
The discriminatory power of Il-2 after stimulation with rESAT-6 and rCFP-10 in detecting patients with active TB and LTBI.
| Protein | Cut-off (pg/mL) | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | AUC | CI 95% |
|---|---|---|---|---|---|---|---|---|---|
| rESAT-6 | 11.6 | 72 | 79 | 78 | 73 | 6.5 | 0.35 | 0.75 | 0.61–0.88 |
| rCFP-10 | 10.7 | 75 | 79 | 78 | 77 | 3.6 | 0.31 | 0.8 | 0.67–0.92 |
PPV: positive predictive values; NPV: negative predictive values; LR: likelihood ratio; AUC: area under the curve.
Seventeen percent and 50% of subjects with active TB and LTBI have IL-2 response of more than two cut-offs following stimulation with rESAT-6. In addition, IL-2 response of more than two cut-offs following stimulation with rCFP-10 was found in 13% of subjects with active TB and 60% of subjects with LTBI.
DiscussionTuberculosis control programmes in developing countries are greatly haunted by low case detection rates of LTBI.16 A variety of technologies have been used for the diagnosis of TB, including medical imaging (e.g., chest radiography), microbiology tests (e.g., sputum smear microscopy), histopathology, and immune-based tests (e.g., serologic, antibody detection tests, antigen detection tests, interferon-γ release assays, and skin tests).17 Although detection of acid-fast bacilli or mycobacterial cultures provides indicative values, it cannot serve as a definite diagnostic method due to its complicated procedures. Among these, the skin test is the most widely used screening approach. However, the main drawback of this method is the lack of specificity due to the cross-reactivity with proteins present in other mycobacteria, such as M. bovis BCG.18
The identification of regions of the M. tuberculosis genome that are not present in BCG provides a unique opportunity to develop new highly specific diagnostic reagents. The 10-kDa culture filtrate protein (CFP-10) and 6-kDa early secreted antigen target (ESAT-6) are located in the region of difference-1 (RD-1) of the virulent M. tuberculosis genome but are absent in all BCG strains.19 It has been suggested that polyfunctional T-cells for ESAT-6 or CFP-10 play an important role in control of M. tuberculosis infection.20 Although detection of CFP-10 and ESAT-6 proteins can be used for the early and specific diagnosis of TB and for distinguishing between M. tuberculosis-infected and BCG-vaccinated groups, their value in detection of LTBI remains less clear.21–23
Several papers using ESAT-6/CFP-10 derivatives in IFN-γ release assays have mostly used three kinds of diagnostic reagents such as recombinant individual proteins or fusion protein, pool of overlapping peptides and selected non-overlapping multi-epitopic peptides;24–26 although they do not lead to successful differentiation between active TB and LTBI.24
Measurement of multiple cytokines may help identify potential biomarkers for differentiating active TB from LTBI.27
Our study demonstrated the importance of establishing an efficient diagnostic method, based on the IL-2 detection after stimulation of blood with rESAT-6 and rCFP-10 for discrimination of latent TB infection and TB disease. Our data are in agreement with the recent observation by Sargentini et al.28 and Wang et al.10 describing that detection of IL-2 after stimulation with M. tuberculosis antigens may discriminate individuals with latent infection from patients affected by active TB.
When there is no definite gold standard for the diagnosis of LTBI, the IL-2 release assay in addition to IGRA can improve the ability of IGRA to identify individuals with recently acquired LTBI.5,10,29–31
According to the previous meta-analysis, IL-2 is a valid marker for the diagnosis of LTBI.5 IL-2 is promoting T-cell replication and is essential for cellular immunity and granuloma formation. Although IFN-γ is predominantly produced by effector memory T-cells, IL-2 is mostly produced by central memory T-cells.32,33 The secretion of IL-2 is secreted from cells also secreting IFN-γ in both the early and the later stages of infection and when the antigen load has declined.9 Dual IFN-γ/IL-2-secreting cells in active phase of TB can support their own expansion because IL-2 is a potent T cell growth factor. The presence of these cells in active TB when the antigen load is high may therefore suggest their involvement in the initiation phase of the immune response. IL-2 in LTBI or when the antigen load is reduced or cleared may reflect its function in the termination of T cell responses. This proposed signalling function augments the growth and survival of regulatory T cells that control inflammatory responses.9
The meta-analysis by Menzies et al. reported a pooled sensitivity of 80% and polled specificity of 96% after stimulation with QuantiFERON®-TB Gold In-Tube that used ESAT-6 with CFP-10 for diagnosing LTBI in healthy and immune-suppressed persons.11 Compared with these reports, our estimated sensitivity and specificity for diagnosis of TB was very good (sensitivity 83–84% and specificity 92%). ROC analyses demonstrated sensitivity of 72% and specificity of 79%, in discriminating between latent and active TB, considering response to rESAT-6 by IL-2 release assay for a cut-off of 11.6pg/mL. In addition, sensitivity of 75% and specificity of 79% was demonstrated after stimulation with rCFP-10 protein.
We presented both the Positive Likelihood Ratios (PLR) and Negative Likelihood Ratios (NLR) as measures of diagnostic accuracy. Likelihood ratios of >10 or <0.1 indicated high accuracy. A PLR value of >10 for IFN-γ assay after stimulation with both recombinant proteins suggests that patients with TB have an approximately >10-fold higher chance of being positive with this test. This likelihood was 6.5 and 3.6 for IL-2 after stimulation with both recombinant proteins.
Since no laboratory tool is currently available to distinguish between individuals in the process of progressing from latent TB infection towards active disease, determination of the interferon-gamma and interleukin-2 T-cell signature might provide rapid tool for clinical management of patient.34,35 Our study confirmed that IL-2 release assay has the ability to identify individuals with LTBI. However, the relative small sample size may have influenced the result of the analysis due to low numbers of individuals in each category.
In conclusion, this study demonstrates that rESAT-6 and rCFP-10 can provide a sensitive and specific diagnosis of TB. In addition, it was shown that IL-2 could be serving as a marker for discriminating LTBI and active TB. Since the immunology of TB is complex, and understanding LTBI is even more challenging, a large scale study will be needed to establish the usefulness of this biomarker.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest.
This work was supported by a grant from the Tehran University of Medical Sciences, Tehran, Iran, with project grant number (92-01-88-22252).