INTRODUCTION
Allergic diseases are inflammatory disorders in which susceptible individuals have an aberrant immune response to innocuous environmental antigens. In the 1980s, two main subsets of T helper (Th) cells were defined, with different patterns of cytokine secretion, which lead to different functions. Type1 helper cells (Th1), producing interleukin (IL-2), gamma-interferon (IFN-γ) and tumour necrosis factor-beta (TNF-β) are inductors of delayed-type hypersensitivity reactions. Type2 helper cells (Th2) producing IL-4, IL-5, IL-6 and IL-10 are helpers for B-cell antibody secretion, particularly IgE 1. During the last 15 years, it has largely been shown that allergic inflammation was caused by activated Th2 lymphocytes, leading to IgE production and eosinophil activation 2. Throughout this period, the role of IgE and Th2 cytokines in driving the clinical manifestations of early and late allergic reactions has been extensively studied, but data provided are insufficient to explain the whole immunopathology of allergic diseases. In addition to these effector functions, an immuno-modulatory role has been attributed to IgE molecule 3 and to some of the inflammatory mediators released during the acute phase 4, which may influence the evolution of the immune process that occurs in allergy.
In most allergic individuals, reactions are mediated by IgE isotype, and these patients are said to suffer from IgE-mediated allergy. However, in some patients, allergy may be mediated by mechanisms associated to allergen-specific antibodies other than IgE or may be the result of a Th2 or Th1 cell-mediated process. In these cases it has been said that patients suffer non-IgE mediated allergy. In some instances, the originally IgE-initiated allergy would evolve to a chronic disease in which the inflammatory reaction is mediated by allergen-specific lymphocytes 5. Different evidence showed that in addition to the acute response induced by IgE, Th2 and/or Th1 cells, could later promote a delayed-type hypersensitivity reaction that results in clinical manifestation of different allergic syndromes 6. This results in a mixed form of IgE/non-IgE mediated allergy 7.
One of the unanswered questions in allergy is what causes and what prevents the disease. Epidemiological studies have shown that environmental changes produced in industrialized countries are major contributors in the expansion of allergy. The "hygiene hypothesis" proposed that the sanitized style of life in developed countries results in a reduced microbial exposition during childhood which determines a defective induction of Th1 cells that account for the increasing incidence of allergic disease 8. Most recent evidence suggests that other alterations in the immune response might contribute to the emergence of allergic disease. Presently, it has been proposed that allergy is the result of an improper balance between tolerance and immunity to harmless antigens 9-13. The recent identification of T regulatory (Treg) cells as key regulators in peripheral tolerance to allergens 14, and the role that dendritic cells have on their generation 15, open up new perspectives for the understanding of allergy pathogeny and to the development of more efficient therapies.
This article is a review of scientific and clinic contributions published in recent years about these new aspects of allergic inflammation.
T REGULATORY CELLS
Adaptive immune response is a critical process to protect the host against the attack of pathogens, but this response is sometimes elicited against self-antigens or innocuous substances. Various mechanisms that control and regulate the immune system to avoid or minimize inappropriate reactivity or excessive response to pathogens have been reported. Deletion of autoreactive cells occurs preferentially in the thymus, during T and B cell development and allows the immune system to be tolerant to the most self-antigens 16-18. Peripheral tolerance also include the induction of cell death (apoptosis) or the development of a state of nonresponsiveness (anergy) of specific T lymphocytes to harmless antigens 19,20. However, these mechanisms are not complete, and potentially hazardous self-reactive lymphocytes are present in normal individuals. More recently it has become clear that Treg cells downregulate the activation and expansion of auto-reactive cells 21. Although T-cell mediated suppression was initially associated to autoimmunity control, it has lately been found to play a key role in the control of different immune processes including inflammation, infection, allergy, tumour immunity and transplantation tolerance 22-26 and also seems to be involved in suppression of innate immune pathology 27.
These Treg cells are heterogeneous in phenotype, function and the way of generation. A subset of them are naturally occurring, emerging from the thymus as a defined mature T cell subpopulation (natural Treg cells). Their repertoire of antigen specificities is as broad as that of naïve T cells, and they are capable of recognizing both self and nonself antigens 21. One major limitation in the study of Treg cells activity has been the lack of molecular markers specific for this T cell lineage. Natural Treg cells are CD4+ or CD8+ cells and express on their membrane surface high levels of the IL-2 receptor α chain, (CD25), cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), transforming growth factor β (TGF-β), and glucocorticoid induced tumour necrosis factor receptor (GITR). However, these molecules are also expressed after effectors/memory T cells activation, and are not useful markers to identify them. Secretion of IL-10 and TGF-β is a common feature of natural Treg cells, but is also unspecific. The forkhead/winged helix transcription factor FOXP3 (forkhead box P3), was shown to be specifically expressed by naturally occurring cells and appears to be a master control factor for the development and function of these cells 21. This factor is considered as a good marker for this population but not absolutely specific. Human CD4+ CD25+ Treg cells are able to convey suppressive capacity to conventional CD4+ T cells, thereby generating Th suppressor cells through a process known as "infectious tolerance" 28.
Another population of Treg cells also emerges from thymus, but acquires its suppressive activity in the periphery by specific ways of antigenic stimulation (induced or adaptive Treg cells) 29. Unlike naturally occurring ones, inducible Treg cells have not yet been assigned a surface phenotype based solely on CD4/CD8 or CD25 or CD28 molecules. Instead, the regulatory activity may be distributed among multiple fractions including those defined as CD4+ CD25, CD4+ CD25, CD8+, CD8+ CD28, CD4 CD8 CD25+ CD28 or NKT cells. This heterogeneity most likely reflects both multiple routes of induction and disparate physiological functions. Although it has been shown that the generation of the induced regulatory T cells does not depend on foxp3 expression, this marker can be expressed equally. Inducible Treg cells are subdivided according to cytokines produced: Tr-1, characterized by the secretion of high amounts of IL-10 but only small amounts of transforming growth factor beta (TGF-β) and IFNγ, whilst Th-3 constitutively produce low levels of IL-4, IL-10 and high levels of TGF-β 30.
Both subsets of Treg cells actively control or suppress effector T helper cells and T cytotoxic cells. These cells are activated by antigen-presenting cells such as dendritic cells, or directly by interacting with receptors expressed on endothelial cells or other T cells, but they exert their regulatory activity in a non-specific way. Although many aspects of the mechanisms by which these cells exert their regulatory effects remain to be explained, it is well established that Treg cells suppress immune responses via cell-to-cell interactions and/or the production of interleukin (IL-10) and TGF-β 24,30,31. Some surface molecules have been reported to be responsible for contact mediated suppressive activity: CTLA-4 and TGF-β, but so far there is no general consensus on the mechanism involved 20,24,30-32.
T REGULATORY CELLS IN ALLERGY
Mucosal epithelium is continuously exposed to a vast quantity of antigens from the air, food and environmental microorganisms. A stable state of immune unresponsiveness to harmless antigens and to self-antigens must be developed by the immune system whilst maintaining their ability to develop appropriate immune responses against pathogens. As in central tolerance, clonal deletion and anergy are operative mechanisms occurring in peripheral lymphoid tissues to control immunity 19,20, but active suppression seems to be a key mechanism to avoid aberrant immune response to innocuous antigens 30.
In recent years, several independent studies on immune response to allergens have revealed that a peripheral repertory of Treg, Th1 and Th2 allergen-specific cells exists in allergic as well as in healthy individuals, and it has been suggested that allergic disease can result from an inappropriate balance between allergen activation of Treg cells and effectors Th2 cells 9-13.
One observation that enables an association between a deficiency in Treg cell and establishment of allergic disease is that mutations in the gene encoding the transcription factor Foxp3, that seriously compromise regulatory T cell function, results in the human syndrome IPEX (immunodysregulation, polyendrocrinopathy and enteropathy, X-linked) characterized by severe autoimmune endocrine pathologies and allergic symptoms that include atopy, eosinophilia, severe eczema and food allergy 33. A similar allergic and autoimmune alteration is observed in Foxp3 mutant mice 34. Furthermore, in a model of murine allergy and systemic autoimmunity, the injection regulatory Treg cells, induced in vitro by Foxp3-retrovirus infection, can control the symptoms 35.
Using a mouse model of hyper IgE response, Curotto de Lafaille et al showed that the transfer of polyclonal Treg cells prevented the development of antigen specific Th2 cells, the formation of germinal centres and class switching to IgE. In the same experimental model, the transfer of Treg cells after the IgE response was established did not substantially decrease IgE levels. These results suggest that Treg cells are important to control Th2/IgE response, but are less effective in eradicating an already established response 26,36. However, another interesting clinical study showed that children who outgrew their allergy to cow's milk proteins had higher frequencies of circulating natural Treg cells compared to children who maintained clinically active allergy 37. In addition, generation of antigen-specific suppressor cells during allergy immunotherapy seemed to be a key immunologic process in the curative efficiency of this treatment 38-41.
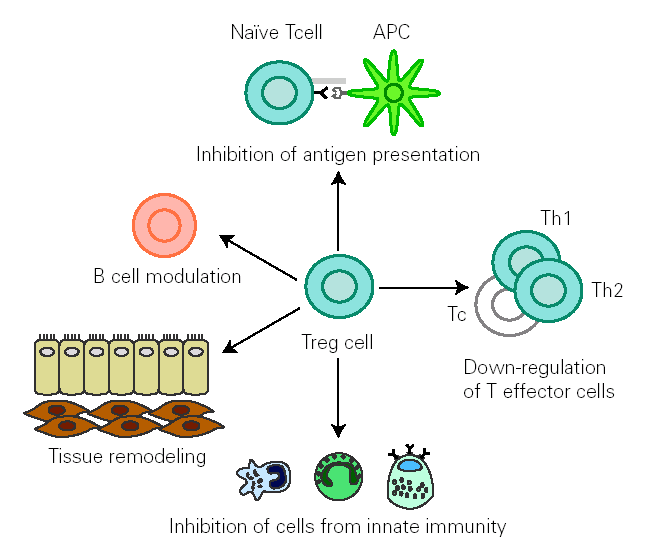
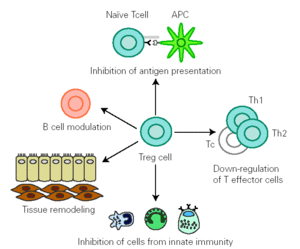
There is growing evidence that Treg cells contribute to the control of allergen-specific immune response not only by regulating activation and proliferation of specific Th and T cytotoxic (Tc) cells, but also in additional ways. These cells suppress antigen presentation that generates effector Th1 and Th2 cells 14. They modulate B cell function 42 and by releasing cytokines they determine antibody class switching. One of the best characterized effects of TGF-β is its ability to stimulate isotype switching to IgA, leading to an inhibition in IgE transcription. This mechanism promotes secretion of high levels of IgA in mucous, which selectively avoids environmental allergen penetration. A decreased synthesis of secretory IgA has been associated to development of allergy 43. In naïve B cells, IL-10 suppresses IgE synthesis whereas promotes IgG4 production 39, but in B cells that are already IgE switched, IL-10 potentiates IgE production 44. IL-10 inhibits macrophage activation and dendritic cell function 45. In addition, Treg cells supress mast cells, basophils and eosinophils and cause tissue remodelling 14 (fig. 1).
Figure 1.--Treg cells regulate allergen specific response at different levels: 1) modulate B cell activation, 2) inhibit IgE production whilst promoting IgA release 3) suppress activation of macrophages, eosinophils, mast cells and basophils 4) inhibit antigen presentation that generates allergen-specific Th1 or Th2 cells and effector responses 5) inhibit Th1,Th2 and Tc activation 6) interact with resident cells remodelling damaged tissue. (Adapted from reference 14.)
The fact that Treg cells are able to suppress a specific immune response without affecting other processes, offers promise for developing new long-term efficient therapies. In this line, IL-6 has been identified as a cytokine potentially determinant of the balance between Treg and Th2 cells. IL-6 can bind to the soluble IL-6R and induce Th2 cells proliferation in the airways. In contrast, through the membrane-bound IL-6R, IL-6 controls CD4+ CD25+ Treg cells development and function as well as the initial stages of the Th2 cells development. In an experimental asthma model, blockade of the soluble IL-6R reduces Th2 cells in the lung, whereas blockade the membrane-bound IL-6R, by specific antibody treatment, induced local expansion of T-regulatory cells, thereby reducing the local number of CD4+ T-effector cells in the lung 46. These data suggest that modulation of IL-6 function might be a useful therapeutic approach in allergic asthma.
The crucial role of Treg cells in immunologic homeostasis has arisen great interest in discovering the mechanisms that regulate the formation of such cells, an active process that is regulated by the dendritic cells (DCs) by means of complex processes that are still largely unknown.
DENDRITIC CELLS IN PERIPHERAL TOLERANCE
DCs are professional antigen presenting cells which display capacity to stimulate naïve T cells and induce primary immune responses or tolerance 47. In addition, DCs imprint tissue-homing specificity on T cells, and thus license effector/memory cells to access anatomical sites most likely to contain their cognate antigen 48.
DCs precursors develop in the bone marrow and migrate into tissues where cells home in and differentiate into resident populations. DCs can be divided into several subtypes on the basis of surface antigen differences and functional potential 49. Many subtypes arise from separate developmental pathways (myeloid or lymphoid), but their phenotype and function are principally modulated by signals that the cells receive from the environment and from T cells 15,47,49,50. DCs in the periphery are mostly immature, characterized by a low-level expression of MHC class II, T cell receptors ligands and costimulatory molecules and are low IL-12 productors. In infectious and inflammatory environment, DCs become mature, expressing high levels of co-stimulatory molecules and receptor ligands. These cells release stimulatory cytokines to prime T cells and determine the outcome of T cell responses Th1 or Th2 15,47,49,50.
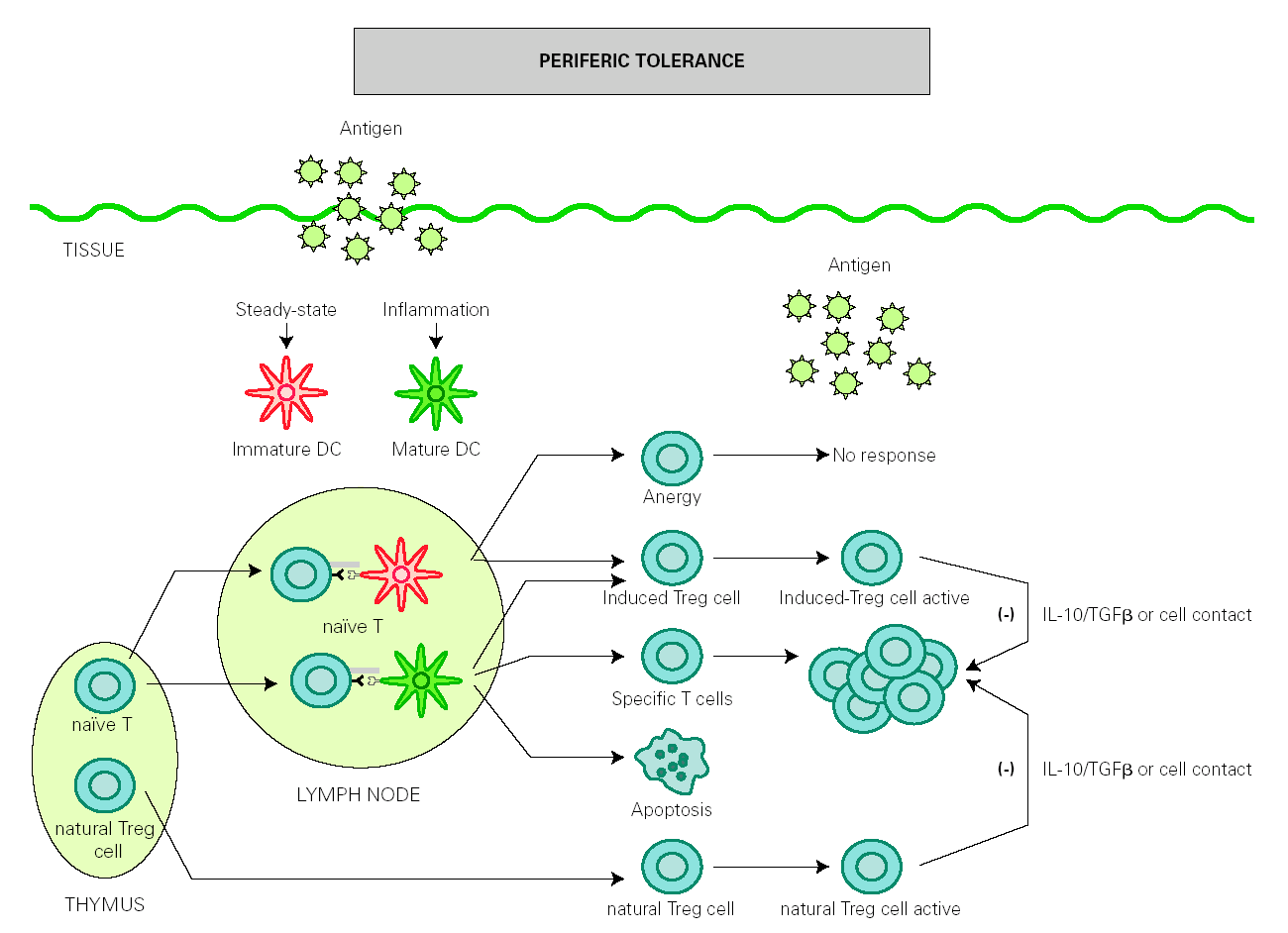
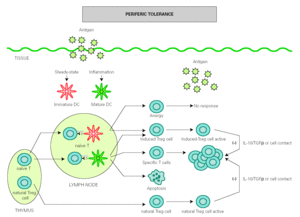
The role of DCs in the induction of peripheral tolerance has been supported, with increasing evidence that they also participate in the differentiation and expansion of Treg 51. One hypothesis on how DCs induce immune response and also inhibits inflammation is that tolerance is mediated by immature or semi-mature DCs, whereas immunity is generated by mature DCs. In the absence of inflammation, DCs remain immature after antigen uptake but are able to migrate to lymph nodes, where they present antigen to naïve T cells under suboptimal conditions that will result in the generation of Treg cells rather than effector T cells 52,53. However, other authors have found that both mature and immature DCs participate in the development of peripheral tolerance 54-56 (fig. 2).
Figure 2.--Dendritic cells control tolerance and immunity in periphery. In steady-state conditions, DCs remain immature but may still capture the antigen and migrate to the lymph nodes to prime naïve cells and induce anergy. Under determined conditions immature/semimature DCs induce naive cells to become specific Treg cells. In presence of signals from infection or inflammation, DCs change to a mature form and prime T cells for different effector functions but also generate specific Treg cells. High levels of antigen promotes T cell apoptosis. Natural Treg cells, emerge from the thymus as a defined mature T cell subpopulation. Both induced Treg and natural Treg are antigen-activated to suppress Tcell effector functions by releasing IL-10 TGFb or by direct contact.
Different mature DCs subsets have been referred to participate in the induction of Th1, Th2 or Treg cells 15,49,57-59. Dendritic cells type 1 (DC1), that produce IL-12 and express high levels of costimulatory molecules, induce a Th1 response associated with the production of IFNγ and IL-2. Dendritic cell type 2 (DC2) induces a Th2 differentiation with major production of IL-4, IL-5, and IL-13, by a mechanism independent of IL-4 or IL-12 but probably mediated by IL-10 or by the absence of IL-12. Dendritic regulatory cells (DC) secrete high levels of IL-10 and induce production of Treg cells.
Understanding the mechanisms implied in the induction of an aberrant Th2 by DC2 in allergic individuals is an important step towards designing new therapies in allergy. Molecular regulation of Th2 by dendritic cells is currently the subject of intensive research 15.
Data from human DCs derived by in vitro culture indicate that functional subsets DC1 and DC2 would arise from different plasmacytoid or myeloid bone marrow precursors 57,60. However, results from studies in mice are conflicting and showed that functional DC1 and DC2 subsets may have both types of precursors. It seems that the differentiation signals that determine different functional subpopulations of DCs mainly proceed from stromal cell and from inflammatory cells in the tissues 49,61,62. A novel cytokine produced by human epithelial, stromal and mast cells, the human thymic stromal lymphopoitin (TSLP), exerts an effect on DCs to promote specific Th2 cell differentiation and recruitment, control T cell activation and B cell differentiation 63-65. TSLP expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity 66, and DC2 are also elevated in peripheral blood of patients with asthma 67. TSLP was also highly expressed by keratinocytes from patients with atopic dermatitis 68. These results suggest that TSLP is a key initiator factor in allergic disease and a target for a curative therapeutic approach.
Interestingly some of the inflammatory mediators produced during the allergic response, such as cysteinyl leukotrienes 69, prostaglandin E2 70, prostaglandin D2 71 and histamine 72, enhance the proallergic priming function of DC2. Other soluble mediators such as IL-10, tryptophan and its metabolites 73 and vitamin D3 74 interact with DCs to induce a tolerogenic response.
Interaction between DCs and T cells through engagement of costimulatory molecules provide critical signals for an efficient generation of effector or Treg cell response. Interaction of dendritic B7-1 and B7-2 with CD28 on naïve T cells provides a strong activation that induces effector cells 75. Interaction of the TNF receptor family members OX40 and their ligand prevents tolerance, and is critical for both Th1 and Th2 responses in allergic inflammation 76. Mature DCs that selectively express MHC class II-associated invariant chain peptide (CLIP) favour naïve T cell polarization toward Th2 77. Signals induced via inducible costimulator (ICOS) expressed on T cells and their ligand on DCs costimulate the development of both Th2-driven inflammation and Treg-cell-mediated tolerance 78,79, suggesting that this distinct process could be related. It has been proposed that the development of Th2 cells in allergic diseases may represent an aberration of Treg cell development 78,79.
The high level of functional plasticity presented by dendritic cells towards environmental factors may allow their therapeutic modulation to change their function from pro-inflammatory to tolerant. Through the formation of these dendritic cells, specific Treg cells could be generated to act as suppressors of the T cells responsible for allergic disease, so opening up new hopes for the design of cures 80.
THE HYGIENE HYPOTHESIS AND Treg CELLS
Epidemiological studies in the late 80's have revealed that the increase in allergy prevalence in the developed countries is directly linked to a reduction in microbial infections during childhood, establishing the hygiene hypothesis 81.
After the definition of the two mains subsets of Th cells and considering the ability of Th1 responses to counter-regulate Th2 reactivity, it was proposed that exposure to microorganisms in early life may play a key role in stimulating the maturation of the immune system away from the Th2, a birth-predominating-profile, towards a dominant Th1 phenotype 8. According to the hygiene hypothesis, a sanitized style of life results in a failure to promote pathways that inhibit the development of a Th2 response to allergens. In the past few years numerous studies have proved a direct correlation between widespread vaccination, improvements in standards of hygiene, or extensive use of antibiotics and the dysregulation of Th2 responsiveness that typifies allergy 82,83.
However, this hypothesis does not explain the parallel increase in the prevalence of diseases associated to inadequate production of Th1 cells such as autoimmune or inflammatory bowel diseases, suggesting that the microbial protection against allergy is not just the result of an immune response deviation. At the present time, it is proposed that any type of immune stimulations by pathogens (both Th1 or Th2 polarizing) induce Treg cells that control aberrant immune responses. It has also been proposed that microbiota from the digestive tract induce Treg cells that prevent allergy, and endogenous microbiota alteration has been linked to disease development 84. In accordance with the current view of cellular and molecular mechanisms underlying in allergy, a new concept in the hygiene hypothesis has emerged. It has been proposed that all types of microbial stimulation (both Th1 and Th2 polarizing) induce regulatory cells that control immune responsiveness. A drop in Treg production takes place as a consequence of the reduction in contact with microorganisms, originating a failure in the inhibition of a T specific response against innocuous antigens such as allergens or autoantigens 82,85,86.
IGE SYNTHESIS IN ALLERGY
Once generated, Th2 cell activates B cells to induce an immunoglobulin class switching and its transformation into plasmatic cells secretors of specific IgE. In healthy persons, synthesis of IgE by these cells occurs at a low rate compared to that of other antibodies, indicating that IgE synthesis is a highly regulated process.
Although an increase in Th2 lymphocytes may result in elevated IgE synthesis, not all allergic patients show the same susceptibility to present this alteration. A high percentage of them showed very high serum levels of total and specific IgE in association to their symptomatology, with a clear genetic and/or personal predisposition. However, some patients develop monospecific IgE inductor of symptoms with normal serum IgE levels. In the first case, the high production has been associated to genetic polymorphisms of factors which regulate the IgE synthesis 87,88. Class switching to IgE is preceded by the production of gemline transcripts driven by the IL-4-responsive Ie promoter. In recent years new factors have been involved in negative regulation of IgE production by down-regulating Ie transcription. Their deficit produces IgE and IgG1 hipersecretion, and thus they are candidates for being altered in atopic allergy 89,90. IL-21 is a cytokine recently implicated in the down-regulation of IgE switching, and mice deficient in the receptor for IL-21 have higher production of the immunoglobulin IgE but lower IgG1 than wild-type animals 91. At experimental level, the injection of IL-21 reduces in vivo IgE production, without affecting the production of other immunoglobulins 92. The selective control of IgE synthesis that IL-21 exerts, points to a possible therapeutic use of this cytokine.
Some individuals have normal total IgE levels and negative values for allergen-specific IgE whilst presenting allergic symptoms. Clinical studies strongly suggest that there might be local IgE production in skin and in intestinal and respiratory mucosa. Local IgE production can be detected in nasal mucosa 93, brochoalveolar lavage 94 and stool 95, and their levels are not necessarily correlated to those found in blood. In addition, it has been found that mast cells and basophilic cell lines may contact with B lymphocytes and, in presence of IL-4, can provide the cell contact signals that are required for IgE synthesis, independently from Th2 cells 96. These observations provide an explanation for the fact that serum IgE measures and cutaneous tests do not correlate well with allergic symptoms 93,97-99. Detection of specific IgE in secretions and stool would have diagnostic value in allergies with negative cutaneous tests and RAST.
Akdis et al have proposed that a predominant Th1 apoptosis skews the immune response to surviving Th2 cell in patients with elevated IgE synthesis rates, whereas a disturbed balance towards allergen-specific Th2 cell instead of Treg cell characterizes the response in monoalergic patients 100. Other authors suggest that the altered mechanism contributing to increased IgE levels may be a deficient apoptosis rate of B cells after the switch to IgE. They also suggest that IgG4-switched B memory cells might occasionally undergo a secondary switch to IgE on chronic allergen exposure 101.
IGE EFFECTOR FUNCTIONS
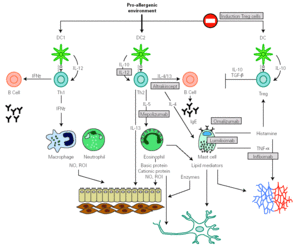
Specific IgE antibodies directed against allergens bind to high-affinity Fc receptor (FCÎRI), constitutively expressed on mast cell and basophils. Upon re-exposure to a multivalent allergen, attached IgE is cross-linked and cells become activated. Within minutes of allergen re-exposure, cells release a series of preformed mediators, which leads to the acute phase characteristic of the allergic reaction. Activation also results in the synthesis and secretion of lipid mediators and cytokines (fig. 3) that participate in the influx of new inflammatory cells, causing late-phase response and promoting the inflammatory process. In addition to these well-known functions, IgE activates other processes that participate in the support and evolution of the allergic inflammation by binding to FCÎRI and to low affinity receptors FCÎRII (CD23).
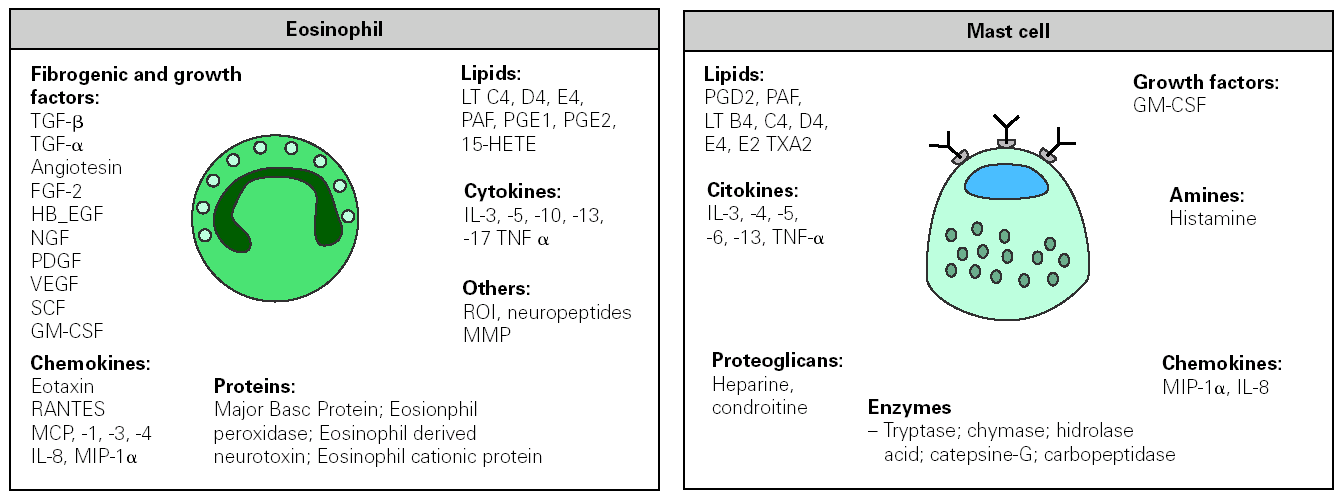
Figure 3.--Inflammatory mediators released by eosinophil and mast cell following their activation in an allergic response. FGF, fibroblast growth factor; HB-EGF, hepain-binding epidermal growth factor; NGF, nerve growth factor; PDGF, platelet-derived growth factor, VEGF, vascular endothelial growth factor; GM-CSF, granulocyte/macrophage-colony stimulating factor; MIP macrophage inflammatory protein; MMP metalloproteinase. MCP monocyte chemotactic protein; PAF, platelet activating factor; RANTES, regulated on activation normal T-cell expressed and secreted; SCF, stem cell factor; TNF tumour necrosis factor; ROI reactive oxygen species; HETE, hydroxy-eicosatetranoic acid; PG, prostaglandin; LT, leukotrienes; TX tromboxan.
FCÎRI aggregation has recently been understood to generate a mixture of positive and negative intracellular signals 3,102,103. Monomeric IgE through FCÎRI regulates positively the expression of this receptor on mast cells and basophils, prevents them from apoptosis, induces NFkB activation and, under some circumstances, may induce cytokine secretion. This regulation leads to a permanent cell sensitization that maintains the capacity of these cells to mount an immediate response after a new contact with an allergen 104. Bound IgE to FCÎRI, expressed on professional antigenpresenting cells and on mast cells and eosinophils, mediates allergen internalization and specifically amplify the inflammatory response, but also takes part in the afferent phase of the immune response to allergens. After antigen-IgE capture, DCs migrate to local lymphoid tissue where they present antigen to naïve cells, widen the spectrum of epitopes recognized by IgE and enhance the immune response. When DCs are located in an inflammatory environment different from the initial one, new specific T helper cells will be generated with different effector functions, contributing to the appearance of new symptoms. In addition to professional antigen presenting cells, mast cells and activated eosinophils internalize allergens through the IgE attached to the receptor and subsequently act as antigen-presenting cells activating the specific T effector cells, helping to perpetuate the process 4,105.
Mast cells and basophils also express low-affinity and, under specific conditions, high-affinity IgG receptors. When co-engaging these receptors with FCÎRI, IgG antibodies can amplify or dampen IgE-induced mast cell activation 3,4.
Binding of IgE to CD23 on B cells facilitates allergen presentation to T cells, resulting in preferent immune responses to the allergen and exercising a positive feedback control. However crosslinking of CD23 results in the down-regulation of IgE synthesis 3.
IL-4 was shown to induce expression of CD23 on the surface of intestinal epithelial cells, allowing rapid internalization and transcytosis of IgE-allergen complexes from intestinal lumen to the underlying tissue to stimulate local hypersensitivity reactions 106.
MAST CELLS IN ADAPTIVE IMMUNE RESPONSE
Mast cells are widely recognized as immune effector cells that initiate innate immunity against pathogens. They are essential in host defense against helmints and also are the major effectors of IgE-associated allergic disorders 107. Recently it has been hypothesized that mast cells also contribute to the initiation and regulation of adaptive immune responses 4,107. Through the release of mediators, mast cells modulate different immune processes. That is the case of histamine, the main proinflammatory mediator of these cells. This molecule participates in the early-phase of allergic response, primarily binding to H1 receptor. In addition to these effects histamine, regulates several essential events in the immune response 108,109. Through its H1, H3 and H4 receptors expressed on DCs, histamine acts as a positive stimuli that increases antigen presentation, proinflammatory cytokine production and promotes Th1 differentiation 14. On the contrary, through the H2 receptor histamine inhibits antigen presentation and acts as a suppressor of IL-12 production whereas enhances IL-10 release which polarize a Th2 response 72,110-112. Histamine can also bind to receptors expressed on lymphocytes and regulates antigen-specific Th1 and Th2 cell as well as related antibody isotype response 113. Binding of histamine to the H4 receptor produces a chemotactic action for mast cells and eosinophils, favoring their extravasation and activation 114,115. As has been previously mentioned, prostaglandin, E2 prostaglandin D2 and cysteinyl leukotrienes are potent inductors of DC2. In addition mast cells can act as antigen presenting cells.
Activated mast cells liberate different type Th2 cytokines and determine an environment where dendritic cells promote differentiation of new Th2 lymphocytes, favoring the allergic inflammatory process 107. By releasing TNFα, mast cell induces expression of endothelial adhesion molecules and, through MIP-1α, it attracts a chemokine responsible for T-lymphocyte recruitment to the inflamed tissue. Moreover, by liberating IL-3, IL-5 and GM-CSF mast cells promote production of eosinophils in the bone marrow and contribute to their infiltration and activation. Mast cells also express Toll-like receptors, which may further accentuate their role in the immune-inflammatory response 107.
TH2 CELLS IN ALLERGIC INFLAMMATION
Once CD4+ T lymphocyte has been primed for a determined allergen, it proliferates and differentiates into effector T cells or memory cells. Then cells leave lymphatic tissue and enter the blood stream besides resting until their migration to the anatomic place where the allergen is found. In the tissue, DCs and the local macrophages present small peptide fragments (epitopes) in conjunction with major histocompatibility complex (MHC) class II, to the Th2 cells, leading to T cell proliferation and activation. As a consequence, T cells release cytokines and increase their affinity for the tissue matrix, being retained inside the affected tissue while the antigen persists. Th2 cell activation promotes a delayed hypersensitivity reaction in which the main effector cell is eosinophil, and presents different characteristics in function of affected tissue. This cellular response is responsible for late phase reaction and underlies most of the chronic allergic process, playing an essential role in the physiopathology of eosinophilic gastroenteropathies and in allergic asthma 7,116.
It is assumed that allergen specific T lymphocytes express on their surface adhesion molecules, which permit their selective migration into the organ where the sensitization was produced. However, specific T lymphocytes to digestive allergens have been seen to express on their surface cutaneous lymphocyte antigen (CLA), a skin-specific homing receptor 117,118. This justifies the emergence of inflammatory symptoms on the skin after the ingestion of food allergens.
It is important to emphasize that for induction of immediate response the presence of the complete allergen is required, while the activation of the T lymphocyte only needs the presence of determined peptides 119. It has been shown that the delivery of synthetic peptides representing T cell epitopes of the allergen, but lacking of IgE epitopes, could induce strong T cell- mediated inflammatory responses 120,121. This response was induced in the absence of preceding IgE-mediated events. These observations reveal that T lymphocytes can be activated and induce an allergic syndrome through IgE non-dependant mechanisms. It is possible that this process has parallelism with the mechanisms by which some drugs are capable of directly attaching the receptor of different types of T cells and activating them, through mechanisms dependant on MHCII without the need for a previous sensitization 122,123. In this line it has been observed that during the digestive process, peptides which do not contain epitopes for the IgE but preserve those recognized by T cells are generated 124. It has been proposed that this digestive peptides may be able to induce both local and systemic inflammatory response, mediated solely by the activation of T lymphocytes, in absence of IgE 119.
Additionally, when administered systemically at low dose, peptides may induce long-lasting hyporesponsiveness in the T cell compartment through a mechanism that is associated with induction of IL-10. In the same way, the use of peptides-based allergen preparation for immuntherapy results in the induction of tolerance, but gives rise to adverse reactions in a high proportion of patients 13.
Th2 lymphocyte activation induces the release of cytokines taking part in the inflammatory response through different actions. Thus, IL-4 in conjunction with TNFα released by mast cells, participates in the expression of endothelial adhesion molecules, which permit the attachment of eosinophils to the endothelium, an essential phase to their subsequent extravasation. IL-5 contributes to the differentiation of the eosinophils and basophils in bone marrow, promotes the release of substances chemotactic to these cells (eotaxin, eotaxin2 eotaxin3 and RANTES) and maximizes their action 125. But this interleukin also activates the eosinophil in absence of IgE, which justifies the appearance of symptoms due to the activation with specific peptides for Th2 receptors. IL-4 promote the synthesis of local and systemic IgE and inhibit the effector response of the macrophage; under such conditions new sensitizations produced will be Th2 type. IL-13 stimulates mucus secretion and fibrosis that contributes to the perpetuation of tissue damage. IL-9 is a stimulator of the proliferation and differentiation of mast cells and eosinophils 126.
IL-17E (also named IL-25), is a new identified interleukin, mainly produced by Th2 lymphocytes that seemed to be directly implicated in Th2-associated allergic inflammation. IL-25 is a potent inductor of IL-4, IL-5, and IL-13 production and its infusion in mice reproduces Th2-associated pathologies 127. It is interesting that in an in vitro study it has been observed that the major cell type that responds to IL-25 by liberating Th2 cytokines appears to be a non-T/non-B cell population, expressing MHCII and CD11c, which is a characteristic of accessory cells 128.
The activation of the eosinophils provokes its degranulation and the liberation of inflammation mediators, some common and some different to those released by mast cell 129 (fig. 3). Once activated, the eosinophil expresses MHCII and costimulator molecules enable it to become an antigen presenting cell, feeding back the inflammatory process 105. The main difference is that the eosinophils granules do not contain histamine but release molecules toxic to the tissue, such as the major basic protein, and the eosinophil cationic protein, or different peroxidases and hydrolases or oxygen reactive species. These mediators degrade cells in the tissue, rendering a dysfunction of the affected organ. On the other hand, eosinophils liberate fibrogenic factors and growth factors, which take part in tissue repairing. When the damage is sustained, the repairing response may be excessive and provoke a fibrotic syndrome that contributes significantly to the syndrome perpetuation.
TH1 CELLS IN ALLERGIC INFLAMMATION
The contribution of Th1 cells in the pathology of allergy is more controversial since, conceptually, Th1 cytokines act as a counter-regulating Th2 response 130. In addition, the experimental induction of a Th1 response against respiratory allergens elicits an inflammatory process in which macrophages and neutrophils are the effector cells. This exclusive Th1 response does not produce any manifestation of allergic asthma. When Th2 cells are inhibited, the presence of specific Th1 cells reduce allergic inflammation, but the concurrent expression of Th1 and Th2 interleukins exacerbates the symptoms, mainly in chronic processes. This suggests that once a Th2 cell response has been established, Th1 counter-regulation is more complex.
Patients with atopic dermatitis exhibit a biphasic helper T-cell pattern in which Th2 immune response appears early in the acute phase, but progresses to a Th1 profile as chronic lesions emerge. An eczematous injury is caused in which essentially IgE does not take part 131. The pathophisiological base of this change has recently been described 132. Skin lesions contain increased levels of epidermal Langerhans cell and dendritic precursor CD2 cells, which express the FcÎRI receptor and possess the ability to internalize the antigen to present their peptides to a local Th2 cell. The resulting activation of Th2 cells promotes IgE expression and induces a cell mediated response in which eosinophils are the effector cells. DCs also migrate to the lymphatic tissue, sensitizing new lymphocytes to a Th2 phenotype. In this initial phase, patients showed TSLP greatly augmented in the skin which favors the differentiation of immature DCs to CD2. After the attachment of IgE on DCs, different interleukins and chemokines are released, which specifically attract IDECs (inflammatory dendritic epidermal cell) to the site of inflammation. This type of DC is not found in normal skin, and is characterized by the expression of a large amount of costimulatory molecules and FcÎRI. Cross-link of IgE receptor on the IDEC surface induces the release of high amounts of IL-12 and IL-18, and that promotes the generation of specific Th1 lymphocytes against the initial antigen. Later, specific Th1 activation in the skin or in other tissues is responsible for a cellular response mainly mediated by macrophages and neutrophils that change the clinical expression of the disease.
Few inflammatory diseases have been associated with an exclusive induction of a specific Th1 response against external antigens. Among them, celiac disease is a pathology in which a Th1 and T citototoxic (Tc) response is produced against the gliadin fraction of wheat, barley, rye and oats 133. Several groups have described the activation of Th1 lymphocytes mediated by different drugs, which give place to a delayed hypersensitivity reaction 122. In the Heiner Syndrome, a chronic pulmonary inflammatory process is produced, associated to the presence of precipitins specific against milk proteins 134. Chronic inhalation of large amounts of some antigens induces allergic alveolitis mediated by high IgG levels, such as in Farmer's lung (actinomyces and molds), or pigeon breeder's disease (bird droppings) 5.
T CD8 + CELLS IN ALLERGY
Different studies support evidence for the contribution of T cytotoxic (Tc) cells in the pathogenesis of allergic diseases. Tc have been divided into two subsets that secrete Th1 or Th2 cytokines (Tc1 or Tc2 cells respectively), however both induce similar inflammatory reactions including cytotoxicity and induction of delayed type hypersensitivity reaction with edema and granulocytic infiltration. In mice, Tc1 and Tc2 inhibit the IgE response 135-137. Nevertheless, its contribution seems to be different in humans. Hence, in atopic dermatitis allergen-specific Tc cells have been found to be elevated in blood and a cytotoxic activity has been detected in skin lesions that correlates with the intensity of the pathology 138,139. Besides, in allergic contact dermatitis against small peptides (haptens) there is a high percentage of Tc1 cells in injured tissues that show citotoxic activity against keratinocytes 140,141.
In allergic asthma, Tc cells accumulate in lung after provocation with allergens and during acute episodes. In cases of fatal asthma, a Tc infiltration in peribronquial tissue has been found. Other authors functionally divide Tc cells as effector or memory cells and describe that Tc2 type effectors contribute in a significant way to asthma through IL-13 production 142.
It has also been found that TSLP, in association with CD40L, induces the formation of pro-allergenic Tc1 cells that contribute to the tissue inflammation 143.
NEW THERAPEUTICAL PERSPECTIVES ON ALLERGIC DISEASE
Traditional treatment for allergy disorders includes drugs that prevent effects produced by mediators of inflammation or control inflammation in a non-specific way, to provide short time relief from disease symptoms. Long term use of these palliative therapies is sometimes associated with adverse collateral effects.
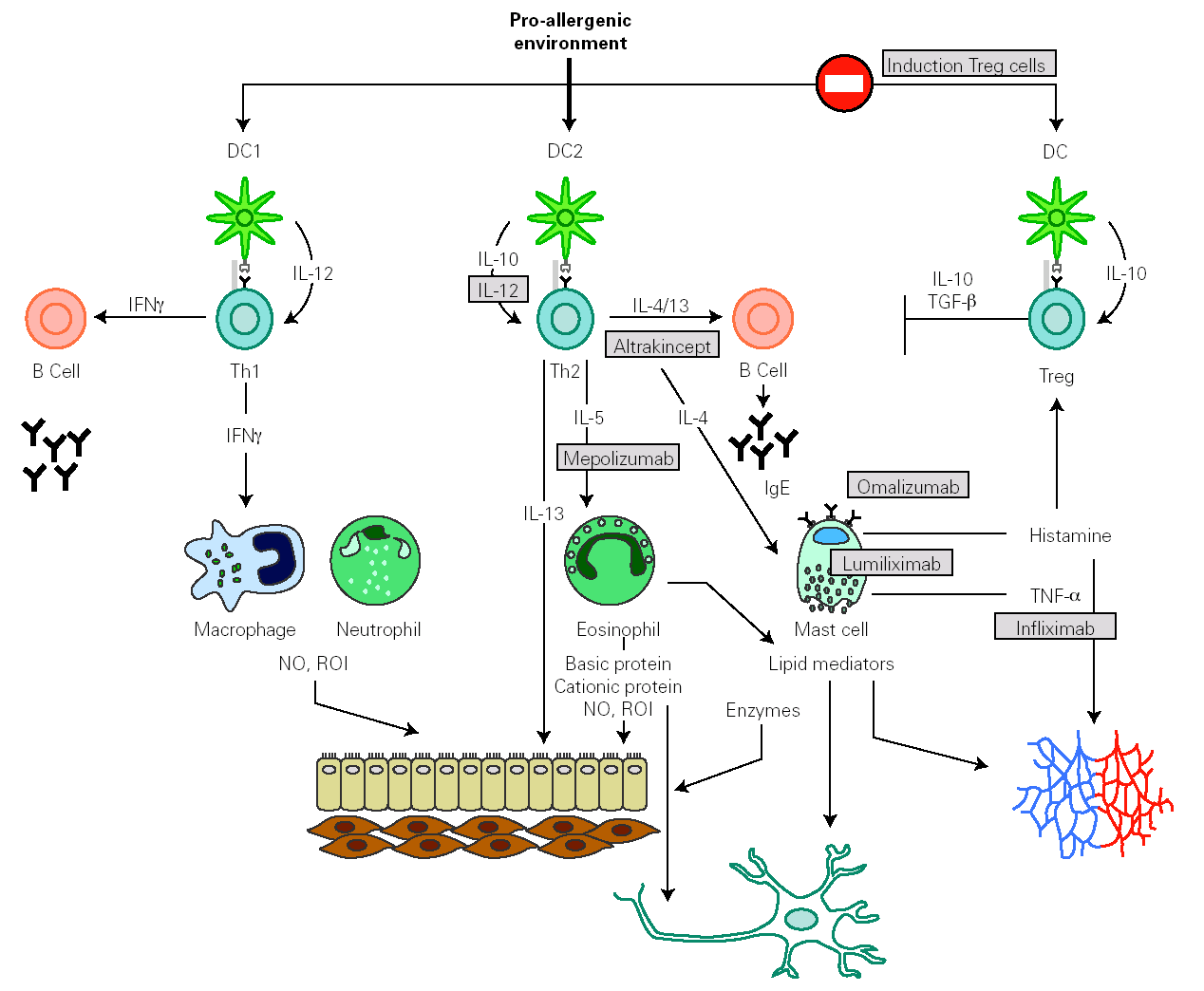
Identification of effector molecules that drive the inflammatory process in allergy has lead to the development of biological therapies directed to specifically block allergic pathways, or to enhance their natural regulatory mechanisms (fig. 4) 144,145. The first biological agent used to treat allergy is a humanized monoclonal antibody, targeted to the C3 domain of the heavy chains of human IgE (omalizumab). It has been shown to be efficient in depleting circulating and tissue IgE, downregulating FcÎRI on different effector and antigen presenting cells and in reducing the influx of eosinophils into the airway lumen. This effect suggests that a significant component of the eosinophilia in allergic asthma is dependent on IgE 146. Another therapeutic strategy to block IgE action consists of the administration of a primatized monoclonal antibody directed against CD23. This molecule seemed to reduce antigen presentation and Th2 response, but their therapeutical efficacy is still under study.
Figure 4.--Main inflammatory pathway implied in the allergic response that presently are susceptible to be specifically modulated by biological therapies.
Some human monoclonal antibodies have been generated to target different Th2 cytokines. In the case of mepolizumab, a humanized monoclonal antibody against IL-5, its administration produces a strong and sustained reduction of circulating and airway eosinophil numbers. Despite these effects, early and late asthmatic reaction to allergen provocation was not affected.
Weekly nebulisation of altrakincept, a recombinant of human soluble IL-4 receptor, prevented lung function decline and asthma exacerbations after abrupt withdrawal of inhaled corticosteroids.
Therapies based on the administration of IL-12 exert beneficial effects on allergic symptoms but might produce important side effects.
The recent identification of novel cytokines such as IL-6, IL-21, IL-25 or TSLP, highly implicated in the immune process underlying allergy, offers new targets to control allergic inflammation.
The evidence that in allergic diseases Treg cell function might be impaired, focuses current research on the design of therapies that generate an allergen-specific regulatory T-cell population with the potential to provide long-term improvement of disease symptoms. Some strategies are based on the modification of dendritic cell function to obtain specific Treg cells 13,80. Other strategies exploit the fact that immunotherapy promotes Treg cells generation in a natural way and look for adjuvants from bacteria or helmints to potentiate this process 85,147. For example, the treatment of mice with a killed Mycobacterium vaccae-suspension gives rise to allergen-specific Treg cells, that confer protection against airway inflammation 148.
It has been observed that glucocorticoid treatment induces the generation of IL-10 producing cells with regulatory properties. The association of this drug with vitamin D3 149 or β2 agonists 150 clearly increases the number of regulatory cells induced. These findings are being exploited to develop new curative therapies.
CONCLUSIONS
Presently the allergic disease is considered as the result of an inappropriate balance between allergen activation of regulatory T cells and effector T helper 2 cells in susceptible individuals, a process in which dendritic cells are key players. This lack of regulation in the immune response leads to an ongoing inflammatory process, in which different immune processes may follow one and other and accumulate over time. The presence of the allergen not only induces the activation of an effector response responsible for clinical manifestations, but also promotes an immunomodulatory process which may determine the evolution of the disease. In addition, every patient shows a genetic and environmental susceptibility to activating these responses. This leads to a high variability in the immune response that predominate in the inflammatory process, and the disease may be present with a wide range of clinical manifestations.
Recently some biological therapies, that specifically control inflammatory pathways in allergy, have been shown to be effective in processes like asthma but their therapeutical efficacy is still under study. Current research strategies seek to exploit recent knowledge on Treg and dendritic cells to induce specific tolerance against allergens that allow a long-lasting control of allergic disease.
ACKNOWLEDGMENTS
I would like to thank Maria Flora Martín Muñoz for her critical review of the manuscript, and Julia Barnes for revising the English translation.
Correspodence:
M.T. Montero
Servicio de Bioquímica-Investigación
Hospital Ramón y Cajal
Ctra. Colmenar, km 9
28034 Madrid. Spain
E-mail: teresa.montero@hrc.es