The most reliable method to diagnose food allergy or to determine tolerance is the oral food challenge.
ObjectivesThe aim of this study was to describe the open oral food challenge applied to children with suspicion of cow's milk allergy mediated by immunoglobulin E, and evaluate the relation between the clinical history and skin prick test with the challenge outcomes.
Patients and methodsForty-six children (median age 13.8 months), with clinical history of immediate reactions to cow's milk and positive skin prick test, underwent an open oral food challenge with cow's milk.
ResultsThe challenge was positive in 41.3%. Cutaneous reactions were the most common (73.7%), followed by respiratory (57.9%) and gastrointestinal reactions (36.8%). According to the severity of the reactions, 57.9%, 36.8% and 5.3% had mild, moderate and severe reactions, respectively. Oral antihistamine was sufficient as treatment in all positive cases. A higher frequency of positive skin prick test with total milk and casein was observed in children with positive oral food challenge. There was a significant agreement between the reactions reported by the family history and those observed during the challenge for 68.4% of children with positive results (Kappa=0.728; p<0.001).
ConclusionsThe method was considered suitable for children up to three years of age, and is safe and easy to perform. There was a significant correlation between the clinical history and the challenge outcomes. A positive skin prick test with total milk and casein was significantly associated with positive challenge results.
Food allergy is defined as an adverse immune response to food proteins. Based on the immunological mechanism involved in the reaction, it may be further classified in: (a) immunoglobulin E (IgE)-mediated; (b) non-IgE-mediated (mostly cell-mediated); (c) mixed.1,2
The IgE-mediated reactions occur within minutes to 2h after allergen exposure. The symptoms include cutaneous manifestations (urticaria, pruritus, angio-oedema, erythema), gastrointestinal (itching and pruritus of the lips, mouth and tongue, nausea, vomiting, diarrhoea), respiratory (rhinoconjunctivitis, sneezing, wheezing, cough and laryngeal oedema) and systemic syndrome (anaphylaxis with hypotension, respiratory distress and shock).2–4
Cow's milk allergy (CMA) is the most common food allergy in infants, affecting 2–3% of children under one-year of age.5,6 CMA is habitually transitory and the majority of children acquire tolerance from the age of three years.7,8
The most reliable method to diagnose CMA or to determine tolerance is the oral food challenge (OFC).9,10 There are three types of OFC: double-blind; placebo controlled (DBPC); single-blind; and open.11
It is reported that only about one-third of suspected food allergies result in a positive challenge.12 Considering the practical aspects of the open challenge, it may be the first choice when the need for OFC is established, especially in children under three years of age.11,13
Studies evaluating open OFC in an evidence-based manner are extremely rare, and there is no standardised method.
The aim of this study was to describe the open OFC applied to children under three years of age with suspicion of IgE-mediated CMA followed in a specialised service. The second goal was to evaluate the relationship between the clinical history and the skin test with the OFC outcomes.
MethodsPatients (23 male and 23 female; median age 13.8 months, ranging from 5.7 to 29 months) admitted or followed up in allergy outpatient clinics were recruited from the Federal University of São Paulo, and the Federal University of Sergipe from December 2007 to November 2009 (cross-sectional study). Inclusion criteria comprised the restriction of cow's milk proteins from the diet due to reported history of suggestive symptoms of IgE-mediated CMA, and positive skin prick test to cow's milk. All of them underwent open OFC with cow's milk. This study was approved by the ethical committee of both universities and signed informed consent was obtained from parents prior to the study.
At the first visit, clinical history was obtained, detailed physical examination was performed and the risks and benefits of the OFC were discussed with parents. Afterwards, they were instructed to follow a CM-free diet for at least two weeks prior to the OFC. The instructions were given by a dietician, and included reading labels, cross-contamination possibilities to identify foods that could contain milk protein in their composition.
Mothers who were breastfeeding their infants were also instructed to follow elimination diet.13–15 Children on treatment with oral antihistamines and oral/inhaled beta-agonists were instructed to discontinue use for at least five to seven days, and 12–24h before the OFC, respectively.16
On the day of the OFC, children were fasted for at least 2h. First, we checked whether the children had been properly prepared for the test and then they were examined in detail, emphasizing the cutaneous, respiratory, and gastrointestinal systems. Children had to be free from fever, signs or symptoms of acute infections, runny nose, cough and wheezing.14 Skin lesions unrelated to cow's milk did not prevent the testing.
Exclusion criteria concerned children with previous history of anaphylaxis associated with CM, presenting with acute infections or inflammatory processes, patients who had not followed the instructions for the test or those who did not improve after exclusion diet.13
Children underwent skin prick test (SPT) as standardised by Sampson.16 Food extracts of total milk (10mg/mL), alpha-lactalbumin (5%), beta-lactoglobulin (5%) and casein (5%) (Diater Laboratorios S.A., Madrid) were applied by the puncture technique. Histamine (1mg/mL) and saline were used as positive and negative controls, respectively. Food allergens eliciting wheals at least 3mm larger than those induced by the negative control were considered positive. Tests were performed in an inpatient setting with close medical supervision, and emergency support available for the treatment of possible severe reactions. The materials for OFC preparation included powder CM (3.4% protein, diluted to 13%), water, disposable cups and spoons, measuring spoon, disposable syringe (20mL), sticker and pen for identification and tray.
The patients received up to 100mL of CM offered in increasing doses of 1, 4, 10, 15, 20, 25 and 25mL, equivalent to 0.03, 0.14, 0.34, 0.51, 0.68, 0.85 and 0.85g of protein, respectively, at intervals of 15–20min.17
Before starting the feeding and before each dose was administered children were examined. Their vital signs, lungs and skin were evaluated and recorded.
Challenges were interrupted when objective signs and symptoms indicated a positive response. After the last dose, children without reactions were observed for 2h.13,14,16
The manifestations considered related to IgE-mediated CMA were: generalised urticaria, rash, angio-oedema, pruritus, repeated nausea and/or vomiting, itching mouth, sneezing, rubbing of nose and/or eyes, watery eyes, coughing and wheezing.18,19 Challenges were considered positive when symptoms were severe, reproducible or persistent; when more than one reaction was observed, involving one or more systems; or when any signs or symptoms were observed in children under one-year of age.20
When isolated erythema broke out as a result of skin contact with CM or whether subjective symptoms were observed in children older than one year old, challenge was not interrupted.20 The reactions were classified according to the severity into mild (cutaneous and/or upper respiratory tract symptoms only), moderate (gastrointestinal tract symptoms or multiple systems involvement) and severe (laryngeal symptoms, lower respiratory tract and/or cardiovascular symptoms).18
Parents were instructed to notify the researcher if any delayed reactions occurred after discharge.18
The data collected were analysed using the SPSS statistical program, v.13.0. The qualitative and dichotomous variables were compared using the chi-square test. For comparison of parametric and nonparametric variables, Student t-test and Mann–Whitney test were used, respectively. The Kappa coefficient was used to compare the symptoms reported by relatives and those shown by children during the challenge. For all tests an alpha≤0.05 was adopted for determination of statistical significance.
ResultsAll children studied had positive SPT for CM (total milk or any protein fraction tested). OFC was positive in 41.3% (19/46) of children. Among the 27 children who had negative outcomes, six children reacted on skin contact with CM, with spontaneous improvement after about 20min, and milk ingestion elicited no reaction.
Cutaneous symptoms were the most common (73.7%), followed by respiratory (57.9%) and gastrointestinal (36.8%). Cardiovascular symptoms were not observed in the studied group. According to the severity of clinical manifestations, we found that 57.9% (11/19), 36.8% (7/19) and 5.3% (1/19) of the children had mild, moderate and severe reactions, respectively (Table 1).
Severity of clinical manifestations observed during oral food challenge, according to organ system involved.
| Severity | Organ system involved | n (%) |
| Mild | Cutaneous and respiratory (upper respiratory tract) | 6 (31.6) |
| Cutaneous | 3 (15.8) | |
| Respiratory (upper respiratory tract) | 2 (10.5) | |
| Moderate | Gastrointestinal | 3 (15.8) |
| Cutaneous and gastrointestinal | 2 (10.5) | |
| Cutaneous, gastrointestinal and respiratory (upper respiratory tract) | 2 (10.5) | |
| Severe | Cutaneous and respiratory (lower respiratory tract) | 1 (5.3) |
No patients needed to take medication by venous access, epinephrine injection, systemic corticosteroids or bronchodilator agent. Treatment for all positive challenges consisted only of oral antihistamines and they remained under medical observation until resolution of symptoms (at least 1h). All patients were prescribed with antihistamines and oral corticosteroids and were instructed to attend the emergency department and contact the main researcher in the event of any reaction.
The median interval between CM ingestion and the onset of symptoms was 15min, varying between 30s and 105min. Most of the reactions (68.4%) initiated in the first 20min after the first ingestion, 21.0% between 20 and 40min, 5.3% in 65min, and 5.3% in 105min.
The median of amount of CM required for the onset of symptoms was 1mL, varying between 1 and 75mL. For most children (63.2%), intake of 1mL (0.03g of CM protein) was enough to trigger the symptoms, for 26.3%, 5.3% and 5.3% of them, they required an intake of a cumulative dose of 5mL (0.17g of CM protein), 30mL (1.02g of CM protein) and 75mL of CM (2.55g of CM protein), respectively, to elicit the symptoms. Fig. 1 shows the correlation between time and amount of food that triggered the observed symptoms.
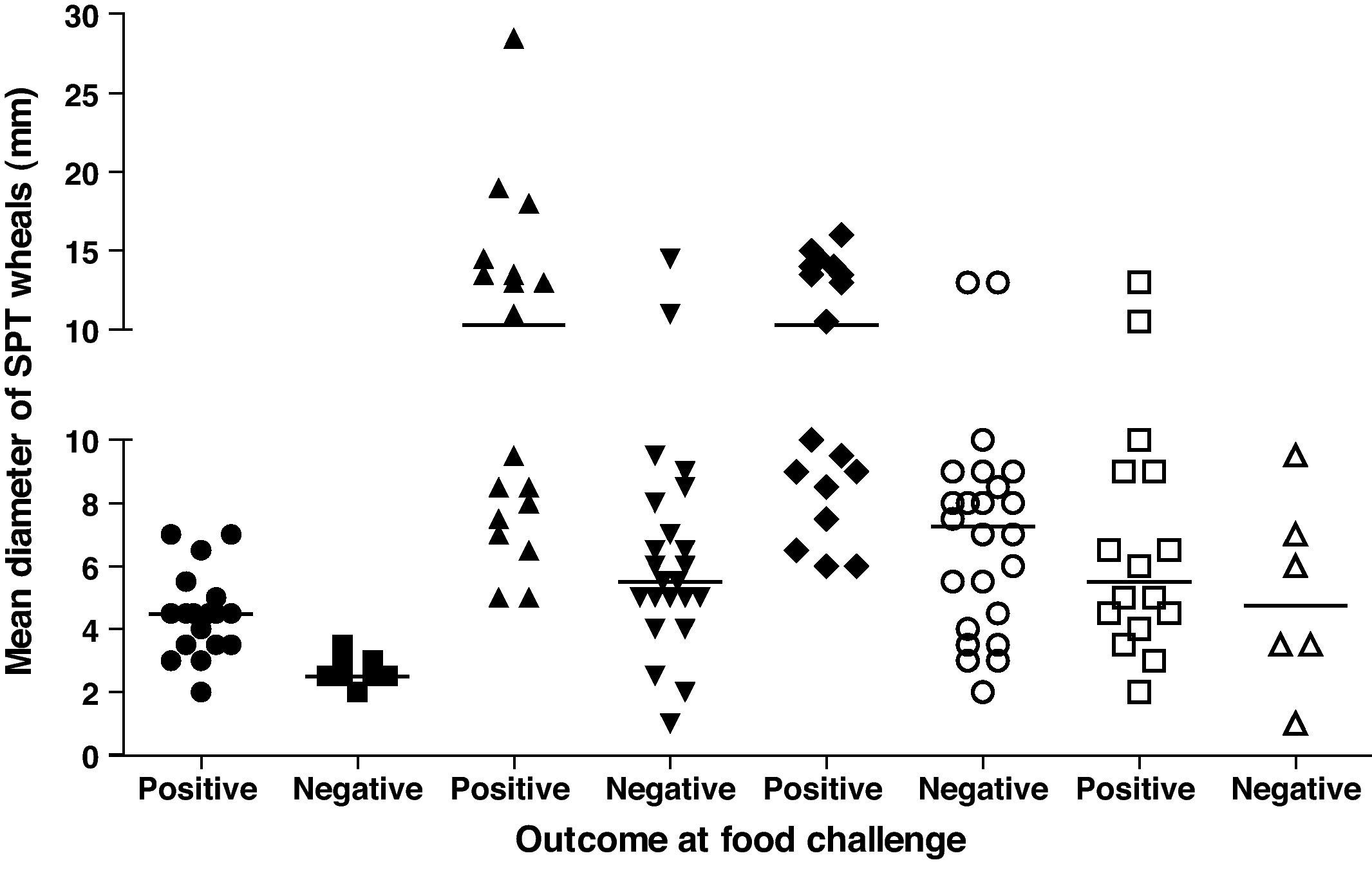
Among children with positive challenge, 89.5% had positive SPT for whole CM, 78.9% for casein, 94.7% for alpha-lactalbumin and 94.7% for beta-lactoglobulin (Table 2).
Positivity in skin prick tests and oral food challenges results.
| Oral food challenge | ||
| Positive (n=19)n (%) | Negative (n=27)n (%) | |
| Number of positive food extractsa | ||
| One | 0 (0.0) | 3(11.1) |
| Two | 2 (10.0) | 17 (63.0) |
| Three | 4 (20.0) | 7 (26.0) |
| Four | 13 (68.4) | 0 (0.0) |
| Positive food extractsa | ||
| Whole milk | 17 (89.4)c | 3(11.1) |
| α-Lactoalbumin | 18 (94.7) | 22(81.5) |
| β-Lactoglobulin | 18 (94.7) | 24 (88.9) |
| Casein | 15 (78.9)c | 5 (18.5) |
| Mean diameter of wheals (mm)b [range] | ||
| Whole milk | 4.5 [2.0; 7.0]c | 2.5 [2.0; 3.5] |
| α-Lactoalbumin | 10.2 [5.0; 28.5]c | 5.2[1.0; 14.5] |
| β-Lactoglobulin | 10.2 [6.0; 16.0]c | 7.5 [2.0; 13.0] |
| Casein | 5.5 [2.0; 13.0] | 4.7 [1.0; 9.5] |
Comparing children with positive and negative OFC, we observed a significantly greater frequency of positive SPT for whole CM and casein in the first group (Table 2). Children with positive OFC also had more frequent positive outcomes for all food extracts tested (68.4% vs. 0%; p<0.05) (Table 2).
The mean diameter of SPT wheals in children with positive OFC was significantly higher than in children with negative OFC for whole CM, alfa-lactoalbumin, and beta-lactoglobulin (Table 2, Fig. 2).
There was a significant correlation between the symptoms reported by relatives in the clinical history and those observed during the OFC in 68.4% of positive challenges (Kappa=0.728; p<0.001).
DiscussionThe DBPC food challenge is considered the “gold standard” in the diagnosis of food allergies.10 However, because it is time consuming, laborious and expensive; its use has been limited. Therefore, the diagnosis of food allergies is usually established based on clinical history, physical examination, presence of specific IgE and restricted diets, in order to verify possible improvement of symptoms.
Once the importance of OFC in clinical practice is established, the open challenge may be an alternative to DBPC test, since it can be considered more cost-efficient, faster and simpler.21
Although more rigorous than the open test for foods, some authors believe that DBPC would be restricted to scientific research and selected cases in clinical practice, for example, patients presenting subjective symptoms only.11
A retrospective study comparing open with DBPC food challenges included 137 children, aged from 1 to 15 years, with positive SPT for food (milk, eggs, wheat, peanuts, sesame and cod) or suggestive history of food hypersensitivity (immediate or delayed). The authors confirmed that 73% of positive open OFC were confirmed by the DBPC, when reactions were observed for up to 2h after ingestion of food. Furthermore, despite the methodological limitations of the study (retrospective, involving children without a clinical history suggestive of food hypersensitivity and small sample size) the authors considered the use of open OFC enough for the diagnosis of food allergy in cases where the reactions are immediate and objective.22
According to Nowak-Wegrzyn et al.11 the open OFC should be the first choice to evaluate an adverse reaction in patients with a high risk of negative outcomes, such as children on exclusion diet of CM with reports of accidental ingestions without symptoms, or children suspected of CMA IgE mediated without laboratory confirmation. In other words, open OFC is especially useful to refute the diagnosis of food allergy.
A retrospective study conducted to evaluate the safety of open OFC included 39 children and teenagers (median age 2.2 years) who underwent open food challenges, selected based on clinical history, results of SPT at the initial and subsequent evaluations, and food specific IgE values lower than those proposed by Sampson23 as predictive levels for positive OFC.21 The objectives were frequency of positive challenges, severity of reactions and treatment needed. There were only 10% of positive challenges in patients who underwent challenge with CM, no patient had severe reaction and no patient received epinephrine or required hospitalisation. Despite the limited sample size, inclusion of patients with a lower risk of severe reactions and the heterogeneity of the sample (diagnostic evaluation and confirmation of tolerance), the authors concluded that open food challenges are safe for patients selected based on history and food specific IgE approaching negative predictive values. It is important to point out that the test should always be performed by trained professionals in an appropriate place where all the medication and equipment for emergency treatment are available.
In the present study, 41.3% of children had positive open OFC. The frequency was higher than in the aforementioned study, but the fact should be considered that the present sample included only children with positive SPT for CM and/or its protein fractions associated with a suggestive history of IgE-mediated CMA. This means that, for the selected sample, we already expected a higher frequency of positive outcomes. In all positive cases, the remission of signs and symptoms occurred only with the use of oral antihistamines, with no need for further interventions. In one case, two organ systems were involved (urticaria, sneezing and mild wheezing), which would be considered a mild anaphylactic reaction according to Muraro et al.3 Although there was formal indication for epinephrine, symptoms were mild and the doctor in charge decided to start treatment with antihistamines with good results.
Similar results were obtained in the study by Ito et al.24 which evaluated 133 Japanese children (mean age 2.7±1.9 years) with a suggestive history of IgE-mediated CMA and/or presence of specific IgE, confirmed by open OFC with CM in a hospital setting. Positive challenges were observed in 35.8% of the children and in 9.3% of them, epinephrine injection was necessary.
According to the European Academy of Allergy and Clinical Immunology (EAACI), children under three years of age may be submitted to open OFC with the same reliability as the DBPC.13 In contrast, Niggemann and Beyer25 indicate the open OFC only for children under one year of age, since they consider that older children present a greater risk of subjective symptoms. In the Japanese guidelines, open challenges are considered appropriate for infants and small children, but there is no specification about age.9,26 Venter et al. did not find significant differences in the percentage of agreement between open and DBCP OFC performed in children younger and older than two years of age.22 In the present study, isolated gastrointestinal symptoms (nausea and vomiting) were found only in two children. In such cases, OFC was considered positive, since children were less than one year old. In this sample, open challenge was suitable for the age group of up to three years.
We should recognize that the interpretation of results in an OFC is very delicate, and there is no exact definition of which symptoms reflect a positive outcome and how to classify the severity of reactions. For example, symptoms such as abdominal pain, nausea, complaints of throat tightness and itching lips, reported by patients, can be considered subjective in some cases, while in others it may be the beginning of an anaphylactic reaction.3,24 Therefore, Nowak-Wegrzyn et al.11 report that in young children, especially those who still cannot speak, attitudes such as putting a hand in the mouth, tongue rubbing, neck scratching or behavioural changes may be signs of a severe reaction and, depending on the level of the patient's discomfort and doctor's judgment, the OFC should be discontinued and appropriate treatment administered.
In this study we observed that 89.5% of children with CMA had symptoms in the first 40min of provocation, similar to data reported by Ito et al.24 where 83.7% of children had symptoms within the first 30min of testing. This finding supports the determination of the intervals between the doses used in this study (20min between each dose until the fourth and 15min for the remaining doses). It is important to comment that in the presence of subjective symptoms, it is recommended that the intervals between the doses be extended to permit the remission of symptoms prior to administration of the next dose.10,11
Regarding the relationship between the results of the SPT and OFC, there were no relationships between the positivity of tests with alpha-lactoalbumin and beta-lactoglobulin and positivity in the OFC. Compared with casein, this combination was present. However, considerations should be made about the use of this extract, since this protein fraction represents 70% of the total protein of the CM. Therefore, it is suggested that the SPT with whole CM extracts would be sufficient to assist the diagnosis investigation. It is noteworthy that among the 27 children with negative challenges, 81.5% and 88.9% had positive SPT for alpha-lactoalbumin and beta-lactoglobulin, respectively. This data reinforces the idea that exclusion diets should not be instituted or maintained based on the SPT outcomes only, which is associated with a sensitisation to a food but not necessarily translatable into clinical reaction.27,28
For the group with positive OFC there was a significant correlation between the symptoms previously reported by the parents in the clinical history and those observed during the challenge. This suggests that clinical history can help doctors predict which reactions may be expected during the test and identify patients at higher risk for severe reactions. However, the small sample size in our study prevents extrapolating the results. It is always important to perform OFC in proper conditions with trained staff prepared for possible severe reactions.
This study had some limitations, such as a lack of control in the recording of delayed reactions, heterogeneity of the sample, including children who were being subjected to the OFC either for the diagnosis of CMA or its follow up.
Despite these limitations, the open OFC with CM was adequate to confirm or to exclude the diagnosis of CMA. The method employed in this study was simple, easy to apply and can to be followed in any institution with qualified professionals and safe conditions for its performance.
Conflict of interestThe authors have no conflict of interest to declare.