Sublingual immunotherapy (SLIT) with monomeric allergoid, given according to the standard scheme, resulted effective and safe. However, the achievement of a clinical benefit requires a long time. We thus performed this study using an administration protocol starting in the co-seasonal period with a 3-day build-up phase and lasting only 6 months, in order to obtain the above benefit in a shorter time.
Methods and resultsThe study, prospective, randomised and controlled versus drug therapy, was conducted on 65 rhinitic and/or asthmatic patients allergic to Parietaria with or without other sensitisations. Twenty-four were allocated to 1,000 AU/week, 21 to 3,000 AU/week and 21 to drug therapy. They were treated from April to September 2006. At baseline, 3 and 6 months a Visual Analogue Scale (VAS) was performed to assess the patients’ well-being. Drug consumption was evaluated by means of monthly diary cards. Bronchial reactivity was investigated at baseline and 6 months by methacholine challenge test. There was a greater VAS improvement in both the SLIT groups than in the controls after 6 months (p < 0.05). In patients taking 3,000 AU/week this was already evident after 3 months. There was a significant reduction in rescue medication consumption between 3 and 6 months (p < 0.05) in all three groups. The bronchial reactivity was reduced only in the SLIT groups (p < 0.001). No adverse events were observed.
ConclusionsAt 6 months the allergoid SLIT showed itself to be effective and safe. In addition the subjective clinical benefit was obtained in a more rapid period, i.e. 3 instead of 6 months, when a higher maintenance dose was administered.
Specific sublingual immunotherapy (SLIT) with monomeric allergoid (allergoid SLIT) has been shown to be clinically effective and safe in many clinical studies.1–7 However, the standard induction build-up phase is rather time consuming, requiring from a minimum of 16days (semi-rush schedule) to a maximum of 14weeks (traditional schedule). In fact the build-up phase of SLIT has been designed according to the same criteria used for injective immunotherapy, where side effects are frequent, local and systemic, and in some rare cases severe and even life-threatening. The safety profile of SLIT and, in particular, of the allergoid SLIT showed itself to be much higher compared to injective immunotherapy, and systemic and anaphylactic reactions are virtually absent, as documented by clinical trials and post-marketing surveillance studies.1–7 We still do not know whether the use of higher dosages of the allergoid SLIT during the maintenance period can lead to faster effects and/or to an increase of its efficacy without compromising the good tolerability of the product.
The aims of the present study were therefore the following: 1) to evaluate the possibility of simplifying the initial build-up phase of the allergoid SLIT by shortening the induction phase to 3days; 2) to verify if this therapy given in the co-seasonal period and lasting no more than 6months, i.e. from April to September, can be effective and safe for Parietaria allergic patients; and finally, 3) to investigate if it is possible to increase the rapidity of effect and/or the efficacy of the allergoid SLIT even further, using a maintenance dosage higher than the standard one of 1,000AU/week, i.e. 3,000AU/week, yet maintaining the safety/tolerability profile of the lower dose.
It is noteworthy that Parietaria in the South of Italy has a very long pollination period, from March to September, with a peak from April to June. In Sicily, where the present study has been performed, Parietaria is practically a perennial allergen, with a pause in August (due to dryness), and another in December.
MATERIALS AND METHODSThe study was prospective, randomized, with three parallel groups receiving either two different dosages of SLIT or the standard chronic pharmacotherapy, taken regularly, for rhinitis and/or mild persistent asthma. All three groups were allowed to receive rescue medication in addition to their assigned therapy in case of urgent need and, in any case, for a very short period (no more than three days).
No run-in period was scheduled. All patients were evaluated at entry to assess their baseline conditions. The parameters considered to evaluate the treatment efficacy were the Visual Analogue Scale (VAS) that was performed at baseline, after 3months, and at the end of the study; the drug consumption that was measured during the whole study; and the assessment of the bronchial hyperreactivity (BHR) by methacholine (MCh) test performed at baseline and at the end of the study.
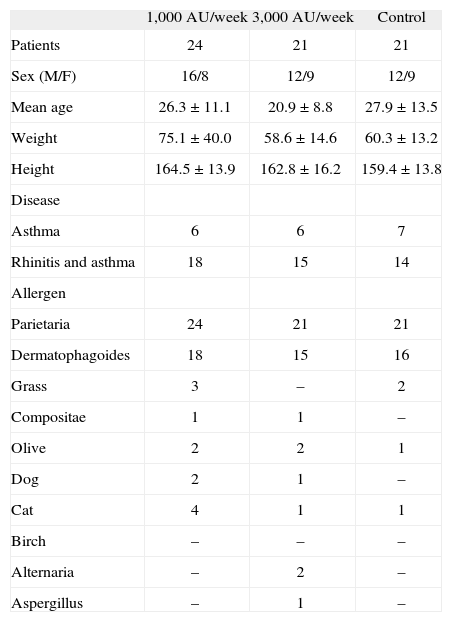
PatientsSixty-five patients suffering from rhinitis and/or mild persistent asthma and having never received any form of specific immunotherapy previously, have been enrolled. Patients' characteristics at baseline are described in Table I. Twenty-four patients (16M, 8F, mean age 26 ± 11years) received the lower dose of the allergoid SLIT, 21 patients (12M, 9F, mean age 21 ± 8years) the higher one and the remaining 21 (12M, 9F, mean age 28 ± 13years) the standard chronic pharmacotherapy. All 65 patients were mainly sensitized to Parietaria as confirmed by a positive (> 3mm) skin prick test response (extracts Lofarma S.p.A., Milan, Italy) and positive CAP assay results (class II or greater) (CAP System EIA, Pharmacia, Uppsala, Sweden), and also, although to a lesser extent, to another allergen: House dust mite (n = 49), Grass (n = 5), Compositae (n = 2), Olive (n = 5), Dog epithelia (n = 3), Cat epithelia (n = 5), Birch pollen (n = 2), Alternaria (n = 2), Aspergillus (n = 1). Subjects suffering from systemic or immunological diseases, major anatomical alterations of the upper airways, renal insufficiency, coronary heart disease, neurologic or psychiatric diseases, receiving chronic corticosteroid or beta-blocking treatments were not admitted, nor were pregnant women. Finally, patients with mild BHR (defined as MCh PD20 > 800μg at baseline out of pollen season) were excluded from the study. All patients signed an informed consent before entering the study.
Patients' characteristics at baseline
| 1,000AU/week 3,000AU/week | Control | ||
| Patients | 24 | 21 | 21 |
| Sex (M/F) | 16/8 | 12/9 | 12/9 |
| Mean age | 26.3 ± 11.1 | 20.9 ± 8.8 | 27.9 ± 13.5 |
| Weight | 75.1 ± 40.0 | 58.6 ± 14.6 | 60.3 ± 13.2 |
| Height | 164.5 ± 13.9 | 162.8 ± 16.2 | 159.4 ± 13.8 |
| Disease | |||
| Asthma | 6 | 6 | 7 |
| Rhinitis and asthma | 18 | 15 | 14 |
| Allergen | |||
| Parietaria | 24 | 21 | 21 |
| Dermatophagoides | 18 | 15 | 16 |
| Grass | 3 | – | 2 |
| Compositae | 1 | 1 | – |
| Olive | 2 | 2 | 1 |
| Dog | 2 | 1 | – |
| Cat | 4 | 1 | 1 |
| Birch | – | – | – |
| Alternaria | – | 2 | – |
| Aspergillus | – | 1 | – |
SLIT is a monomeric carbamylated allergoid (Lais®, Lofarma S.p.A. Milan, Italy)8 biologically standardised in allergenic units (AU) and prepared as orosoluble tablets (allergoid SLIT). The tablets had to be taken in the morning on an empty stomach and kept under the tongue for 1–2 minutes until dissolution before swallowing. During the 3-day build-up phase, only the 1,000AU tablets were used. The scheme was the following: 1 tablet the first day, 2 tablets the second day and 3 tablets the third day, for a total amount of 6,000AU in three days. Subsequently, the patients have been treated either with a maintenance dosage of 1,000AU/week (i.e. 1 tablet once a week) or 3,000AU/week (i.e. 1 tablet 3 times a week) according to a computer-generated randomisation list. SLIT was administered continuously for 6months. All the 65 SLIT patients were treated for Parietaria and for the other most relevant allergen (mainly house dust mite), whenever possible, using two different tablets. However, they were never treated for more than two allergens in any case.
The chronic standard pharmacological therapy consisted of antihistamines (cetirizine or loratadine tablets 10mg, once daily) and long term intranasal steroids (fluticasone propionate, 125μg, 2 sprays per nostril/die) in association with long acting bronchodilators (salmeterol, 100μg/die) for patients with asthmatic symptoms as well.
The rescue medication, to be administered for symptom control, only in the event of urgent need and for no more than three days, was the following in all three groups: cetirizine or loratadine tablets 10mg, two or more tablets/day, inhaled salbutamol 100μg, 2–3 puffs or more/die, intranasal fluticasone propionate 250μg, 2 or more sprays per nostril/die and beclometasone tablets 1mg, 1 tablet once or twice daily.
Clinical evaluationPatients were required to fill in a specific graduated scale called Visual Analogue Scale (VAS) which explores the degree of patient well-being and thus, indirectly, the severity of his/her symptoms during the last six or three months. In our study the maximum level of well-being was 10 and the minimum was 0. The VAS had to be filled in at entry, and after 3 and 6months. The consumption of the rescue medications was scored 1 point if no drug was consumed in that month, 2 points if the consumption was scarce (i.e. no more than 5days with the need of a rescue therapy in that month), 3 if this was in the average (i.e. no more than 10days with the need of a rescue therapy in that month) and 4 if this was elevated, regardless of the kind of drug (i.e. more than 10days with the need of a rescue therapy in that month). Then, at 3 and 6months, a cumulative drug intake score was calculated, each kind of drug being scored separately and differently from the others, with systemic steroids having the highest score.
All patients were also required to record on a separate diary any untoward effect. As far as the allergoid SLIT is concerned, adverse events (AE) were subdivided into local AE (oral itching, swelling of tongue) and systemic (asthma, rhinitis, urticaria, abdominal pain/diarrhoea, anaphylaxis).
Methacholine challengeMCh bronchial provocation test was performed using an ampoule-dosimeter (Mefar Elettromedicali, Brescia, Italy). An inspiratory effort for 0.5s activated a solenoid valve, delivering 5 μL of solution. Lyophilized MCh chloride (Lofarma S.p.A, Milan, Italy) was reconstituted, to obtain 0.2% and 1.0% concentrations. After saline control, MCh was administered in double increasing amounts: each subject inhaled 1 and 2 breaths of 0.2 % MCh solution (each breath corresponding to 10μg of MCh), followed by 1, 2, 4, 8, 16, and again 16 breaths of 1.0% solution (each corresponding to 50μg of MCh). fev1 was recorded about 2 minutes after each MCh dose. All bronchial challenges were performed at the same time of the day under the same environmental conditions. The MCh test was done out of the pollen season both at entry and at the study end. The cumulative administered dose of MCh causing a reduction of 20% of the baseline FEV1 (PD20) was computed by interpolating the MCh cumulative doses immediately preceding and following the 20% fall of FEV1.
Degree of BHRThree arbitrary classes of BHR were considered: mild = PD20 > 800μg/ml (in this case the patient was not included in the study), mild-moderate = PD20 ranging from 400 to 800μg/ml and moderate-severe = PD20 < 400μg/ml (9).
Statistical analysisTo evaluate the changes of VAS, MCh and symptom score in comparison to the baseline values the Wilcoxon Signed Ranks Test was used. To determine whether the values of a particular variable differ among the three populations (1,000AU vs 3,000AU vs control), the Mann–Whitney test for intergroup comparison was used. P values less than 0.05 were considered significant.
RESULTSBuild-up phase, drop-outs and safetyAll the patients tolerated both the 3-day induction build-up phase and the 6-month maintenance therapy very well. Furthermore no patient interrupted the study because of adverse events.
VASThe VAS results are described in Figure 1. At baseline there were no significant differences among the three groups. A significant increase of VAS values has been observed in all the three study groups in comparison to baseline (p < 0.001). Considering the three different groups, it is worth noting that, after 6months, the scores obtained with both the SLIT dosages are statistically better than those observed in the controls (p < 0.05) while, at the 3rd month, only that obtained with the higher dose was statistically superior to the control (p < 0.05).
During the last 3months there was a statistically significant reduction in the consumption of the rescue medication (mainly antihistamines and bronchodilators) in comparison to the first 3months, in both the 1,000AU and the control group (p < 0.05). However, in those patients treated with the higher SLIT dose that did not happen, probably because the rescue drug consumption was already very low during the first three months, i.e. during the peak of the pollen season, probably because of the very rapid action of this dosage. Besides, comparing the 3 groups at the 3rd month of therapy, the 3,000AU dosage was the only one that showed a reduction in rescue medication consumption in comparison to the control group (p < 0.05) while there was not any statistical difference between the results of the two SLIT groups (Fig. 2).
Methacholine challengeA significant increase of MCh PD20 was observed, at the end of the study, in comparison to the baseline values, in both the patients treated with 1,000AU (p < 0.05) and in those treated with 3,000AU (p < 0.001). It has to be noted that, in the patients assigned to the higher dosage, the PD20 values were a bit lower both at baseline and after 6months, without reaching statistical significance. Neither clinical nor statistical differences were observed in the patients treated only with drugs (Fig. 3).
DISCUSSIONThe schedule used for the induction build-up phase in the present study has two peculiarities: the shortness of the up-dosing phase, 3days, and the fact that the initial dose was quite high i.e. a tablet containing 1,000AU, corresponding to the lower maintenance dose per week. That allows the handling of a unique type of tablet titrated at 1,000AU, then consistently simplifying the treatment and preventing mistakes in dosages. Moreover, with the present schedule the maintenance phase can begin much earlier, with a possible consistent advantage as regards the quickness in reaching a clinical benefit. The schedule employed in the present study consists in administering a cumulative dose of 6,000AU in 3days, slightly higher than that (4,000AU) employed by Rossi & Monasterolo in their ultra-rush up-dosing study, where the administraron of all the dosages lasted only 20 minutes.4 In both studies the administration of such high dosages in a short time did not determine the appearance of relevant adverse reactions. Similar results were obtained in the study of Gammeri et al.10 On the whole, these data confirm the good tolerability and safety of the allergoid SLIT, even if administered in a very short time. That is probably ascribable to the low IgE-binding activity of the active principle8 which prevents the IgE-mediated allergen presentation by dendritic cells to TH2 cells, which is the key-mechanism to explain the strong increase of allergen-specific IgE observed in the course of SLIT with native grass allergens.11 On the contrary, in the course of SLIT with Dermatophagoides carbamylated allergoid a progressive decrease of allergen-specific IgE was observed.12
As regards the efficacy, a correlation can be noted between the SLIT dose, the clinical effects and time, i.e. both the SLIT doses were shown to be more effective than the controls at the 6th month as far as the VAS and the MCh challenge were concerned, without any difference between them but, on the other hand, when the 3rd month is considered, a significantly greater improvement of the VAS score and a significant reduction of drug consumption in comparison to the controls was observed only with the 3,000AU dose. This, to our opinion, could be a possible demonstration of the greater rapidity of effect of the higher dosage in comparison to the lower one.
A dose–response effect concerning SLIT effectiveness has in fact been previously described also by other authors.13 Yet, in this case, more than the absolute efficacy, it is the rapidity of effect obtainable by increasing the frequency of administration in a certain period of time that emerges from our data. This time-response effect was also observed by Di Gioacchino et al. in an immunological study comparing the effects on IL-10 and other cytokines (INF-γ, IL-4, IL-6, IL-2, TNF-α) of two different SLIT induction schemes, one lasting 14weeks and the other 16days. It emerged that the faster way of administration, with the consumption of the tablets closer one to the other, was associated with a greater movement of the above parameters regardless of the total amount of allergen administered.
In our study the speed of administration during the build-up phase was the same in the two groups, so this was not able to influence the results between the two SLIT groups, while the total dosage of allergen and the frequency of administration were different. In the light of these study limitations, at the moment we can state that: 1) to obtain a clinical benefit in a more rapid period, i.e. 3months instead of, for example, 6months, we can act on two different factors: the total dosage of allergen administered and the frequency of its administration; 2) after a longer period of time, 6months or more, no significant difference between the clinical effects of the two administration schemes (1 tablet once a week vs 1 tablet three times a week) was observed.
Considering that the maximum pollination period for Parietaria in Southern Italy ranges from April to June14 (Fig. 4) which corresponds with the worsening of the allergic symptoms in most patients, we can speculate that a maintenance dosage based on the administration of 3,000AU started immediately before the beginning of the maximum pollination period, followed by a further three months of treatment with 1,000AU once a week, could be useful to obtain a more rapid and prolonged clinical benefit without any remarkable adverse reaction in neither the short nor the long term.