During the last decades there has been an increase in both allergic diseases and allergic sensitisation, probably due to changes in the environment and living habits. ISAAC Phase II was designed to establish the prevalence and associated factors to asthma and allergic disorders in childhood.
AimTo assess the prevalence and factors linked to atopy in 10–11 year-old children from Almería (Spain).
MethodsAs a part of ISAAC II, a survey was conducted among a sample of 1143 schoolchildren using standardised questionnaires and skin-prick testing.
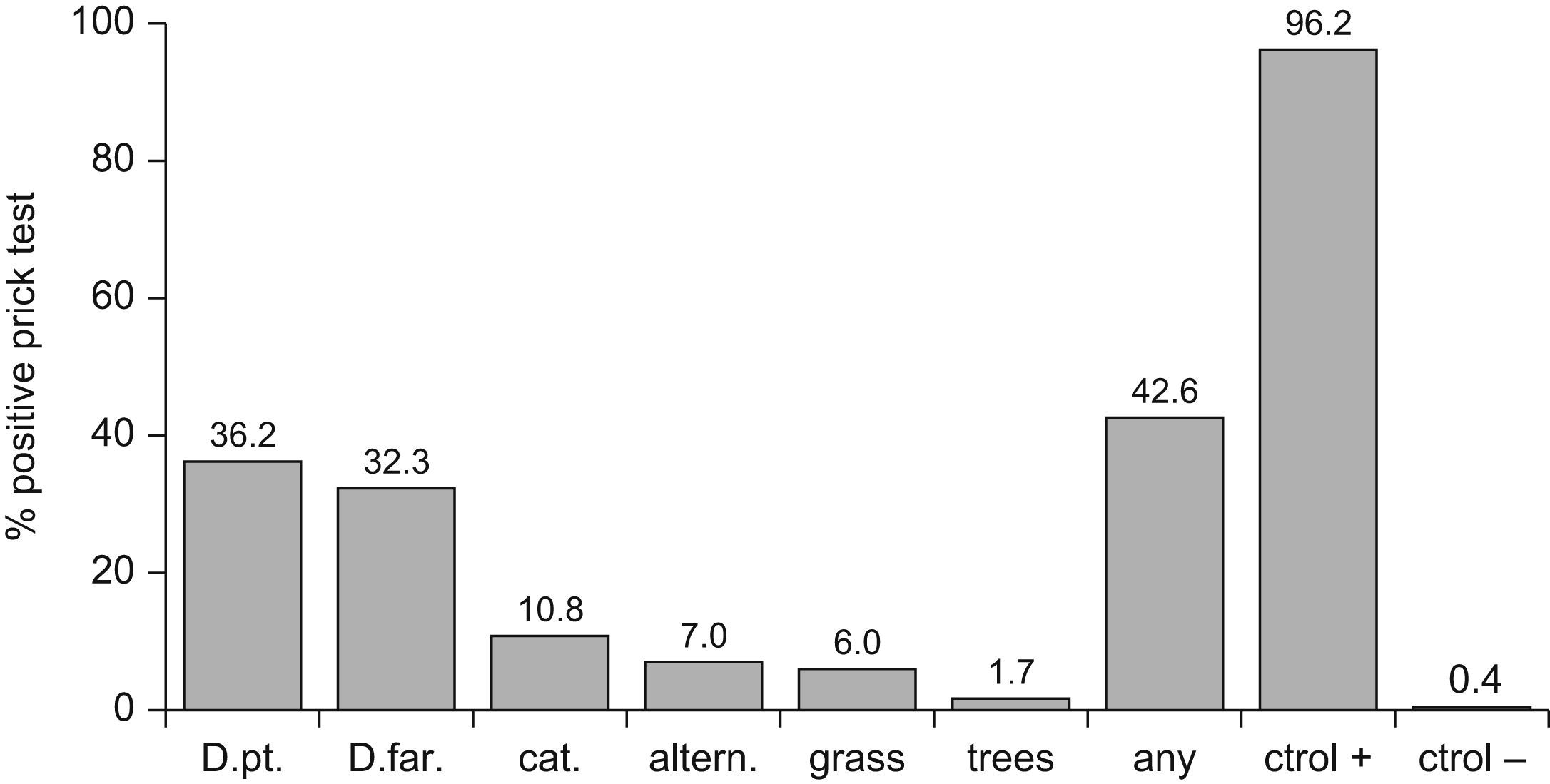
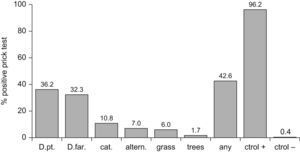
ResultsThe overall prevalence of atopy was 42.5%. Most common sensitisations were to Dermatophagoides pteronyssinus (36.2%), D. farinae (32.3%), cat (10.8%), Alternaria (7%), grass (6%), and tree pollen (1.7%). 34.9% of these sensitisations could be regarded as subclinical sensitisations. The fractions of asthma, rhinitis and eczema attributable to atopy were 49.2%, 40.4% y 18.6%, respectively. After multivariate analysis, the risk of atopy was significantly lower among females (OR 0.62, CI 95% 0.45-0.86); children with older siblings (OR 0.67; CI 95% 0.49-0.92); intestinal parasites (OR 0.68; CI 95% 0.48-0.97); contact with farm animals in the past (OR 0.48 CI 95% 0.23-0.99); or other animals at present (OR 0.53 CI 95% 0.30-0.95). To have an allergic father (OR 2.96 CI 95% 1.77-4.94) was the only significant risk factor.
ConclusionsWe found several independent factors which significantly protect against atopic sensitisation. These protective factors were not the same for asthma, rhinitis or eczema, suggesting that other factors could interact to influence atopy and act against such protective factors.
In 2004, the World Allergy Organization defined atopy as “the personal or familial tendency to produce IgE antibodies in response to low allergen doses, and to develop typical conditions such as asthma, rhinitis or eczema”.1 This definition only describes immune reactivity, but does not imply the presence of clinical symptoms. In this context, while atopy is the most important risk factor for the development of allergic diseases, an atopic patient may yield a positive test in response to a certain allergen against which he or she has never developed symptoms. This may be due to the existence of immunity (natural tolerance) or a subliminal level of IgE antibodies, insufficient to produce symptoms. Likewise, some patients are asymptomatic (spontaneously, or after receiving immunotherapy) and continue to yield positive allergen test results – thus indicating the development of immunity (acquired tolerance) against the allergen. In both situations we would be speaking of atopy without allergy. In the industrialised world it is estimated that 30–50%2,3 of the paediatric population presents allergic sensitisation, but only some of them will show allergic symptoms2,4, indicating that atopy is not the only factor responsible for the development of these disorders.
In the last decades there has been a parallel increase in the frequency of atopic diseases and in allergic sensitisation, suggesting that both increments are related. Since important variations in population genetics cannot have taken place in such a short period of time, the underlying cause may correspond to changes in environmental factors.5 In this context, the International Study of Asthma and Allergies in Childhood (ISAAC), in its Phase II, offers the ideal instruments for trying to identify the factors associated with these increments, making it possible to establish national and international comparisons.
In this study, we aim to describe the prevalence of allergic sensitisation (atopy) and the distribution of the most relevant allergens in 10- and 11-year-old children from Almería (South-east Spain), and to investigate the factors associated with the presence of atopy in this patient population.
Materials and methodsA cross-sectional study was made to analyse the relationship between environmental conditions, personal and familial factors (independent variables) and the frequency of atopy (dependent variable). The methodology used was that of the ISAAC Phase II, which has been described previously in detail,6 based on an investigational survey in which the parents or tutors completed homologated questionnaires on respiratory, nasal and skin symptoms manifesting in the previous year and at other times in the past. Questions were also included relating to personal and family antecedents, the domestic environment, living and eating habits, socioeconomic level and the use of treatments and healthcare services. The study population consisted of 10- and 11-year-old schoolchildren from 29 public schools in the city of Almería (Spain), randomised according to option B of the ISAAC II sampling methods.6 Ethical approval of the study was obtained from the Clinical Research Ethics Committee of Torrecárdenas Hospital, as well as from the regional health authorities.
For the present study, atopy was taken to be synonymous of allergic sensitisation, the latter being understood as representing positivity to at least one allergen, as determined by prick testing. The battery of test allergens included the following extracts (ALK-Abelló®, Madrid, Spain): Dermatophagoides pteronyssinus; Dermatophagoides farina; cat dander; Alternaria; grass pollen mixture (Dactylis glomerata, Lolium perenne, Festuca pratensis, Poa pratensis, Phleum pratense and Avena eliator); tree pollen mixture (Betula verrucosa, Alnus glutinosa and Corylus avellana); positive control (histamine); and negative control (saline solution). A reaction was regarded as positive if the wheal size was 3mm or greater.
The risk of each allergic disease attributable to atopy was calculated by the formula [P(R−1)/R], where R is the odds ratio (OR) for the allergic disease and P is the proportion of atopy in the children with the mentioned allergic disease.
The SPSS® version 12.0 statistical package for Microsoft Windows® was used for data analysis. Calculations were made of the prevalence of symptoms and the frequency of the rest of the variables based on the ratio between the number of positive responses and the number of questionnaires completed for each question. The chi-squared test (χ2) was used to evaluate the association between atopy and dichotomic qualitative variables, while logistic regression analysis was used to assess the association to polychotomic variables, to define the parameters that are correlated to atopy in the past year (assigning risk or protective character). Statistical significance of associations was accepted for p<0.05. Subsequently, a multiple logistic regression analysis was made with all the variables yielding p<0.20.
ResultsA total of 1143 schoolchildren participated in the study (49.8% participation rate): 98.7% were Spanish children, with a mean and median age of 10.7 years, and a slight male predominance (52%). Ninety percent weighed over 2500g at birth, and 71.8% started breastfeeding, maintained for over 6 months in 28.2% of the cases. One-half of the children had siblings (median 1; 5% twins), and 77% attended nursery school. The families were seen to acquire pets over the years: at the time of the study, 26% had birds, 23% dogs, and 9.8% had cats in the home, while 43% and 18% of the children came into contact with dogs and cats, respectively, at least once a week.
At the time of the response, 60% of the children were exposed to tobacco smoke in the home (46.6% of the mothers are smokers, 39% smoked during the first year of life of the child, and 19% did so during pregnancy). Gas was the most commonly used cooking fuel (94.4% in the first year vs 68.9% at present). This percentage has presently decreased as a result of the introduction of other energy sources, specifically electricity (8.2% during the first year of life vs 36 at present), which is currently the main energy source used for home heating purposes (63.2%). The use of air conditioning has increased five-fold during the last 10 years (6.6% vs 30.3%), and the great majority of bedrooms have single-glazed windows (81.9% vs 68.8%). On the other hand, 5% of the homes presented damp spots, and 1.5% had visible fungal presence currently (10.5% and 4.5% respectively during the first year of life of the children) on the walls or ceiling.
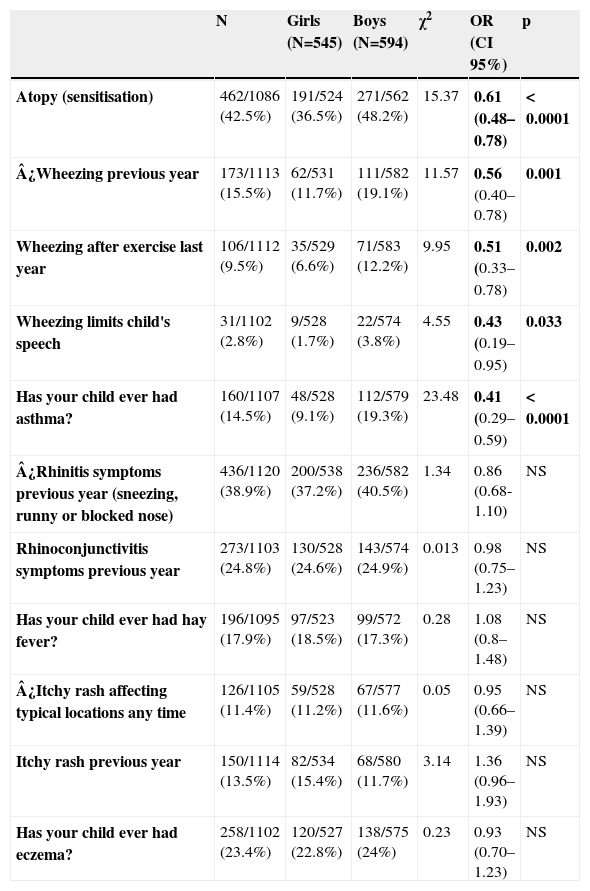
Prevalence of allergic sensitisation was 42.5% (464/1086), and was 1.3 times less common in girls (36.5%-191/524) than in boys (48.2%-271/562) -OR 0.62; 95%CI 0.45-0.86; p<0.0001- (Table 1). The distribution of positive allergens was as follows (Figure 1): Dermatophagoides pteronyssinus (36.2%), Dermatophagoides farinae (32.3%), cat dander (10.8%), Alternaria (7%), grass pollen (6%) and tree pollen (1.7%). Likewise, the prevalence of wheezing in the previous year was 1.6 times less common in girls than in boys (11.7% versus 19.1% -OR 0.56; 95%CI 0.40-0.78; p 0.001), although no gender-linked differences were found in relation to symptoms of rhinitis and eczema (Table 1) according to the definitions exposed in the ISAAC methods.6
Relationship between sex and asthma, Rhinitis and atopic eczema symptoms assessed by univariate
analysis using Chi2 test.
| N | Girls (N=545) | Boys (N=594) | χ2 | OR (CI 95%) | p | |
| Atopy (sensitisation) | 462/1086 (42.5%) | 191/524 (36.5%) | 271/562 (48.2%) | 15.37 | 0.61 (0.48–0.78) | < 0.0001 |
| ¿Wheezing previous year | 173/1113 (15.5%) | 62/531 (11.7%) | 111/582 (19.1%) | 11.57 | 0.56 (0.40–0.78) | 0.001 |
| Wheezing after exercise last year | 106/1112 (9.5%) | 35/529 (6.6%) | 71/583 (12.2%) | 9.95 | 0.51 (0.33–0.78) | 0.002 |
| Wheezing limits child's speech | 31/1102 (2.8%) | 9/528 (1.7%) | 22/574 (3.8%) | 4.55 | 0.43 (0.19–0.95) | 0.033 |
| Has your child ever had asthma? | 160/1107 (14.5%) | 48/528 (9.1%) | 112/579 (19.3%) | 23.48 | 0.41 (0.29–0.59) | < 0.0001 |
| ¿Rhinitis symptoms previous year (sneezing, runny or blocked nose) | 436/1120 (38.9%) | 200/538 (37.2%) | 236/582 (40.5%) | 1.34 | 0.86 (0.68-1.10) | NS |
| Rhinoconjunctivitis symptoms previous year | 273/1103 (24.8%) | 130/528 (24.6%) | 143/574 (24.9%) | 0.013 | 0.98 (0.75–1.23) | NS |
| Has your child ever had hay fever? | 196/1095 (17.9%) | 97/523 (18.5%) | 99/572 (17.3%) | 0.28 | 1.08 (0.8–1.48) | NS |
| ¿Itchy rash affecting typical locations any time | 126/1105 (11.4%) | 59/528 (11.2%) | 67/577 (11.6%) | 0.05 | 0.95 (0.66–1.39) | NS |
| Itchy rash previous year | 150/1114 (13.5%) | 82/534 (15.4%) | 68/580 (11.7%) | 3.14 | 1.36 (0.96–1.93) | NS |
| Has your child ever had eczema? | 258/1102 (23.4%) | 120/527 (22.8%) | 138/575 (24%) | 0.23 | 0.93 (0.70–1.23) | NS |
The variables marked with ¿ have been considered representative of asthma, rhinitis and eczema in this study.
The main factors significantly associated to atopy found in the univariate analysis, using the chi-squared test, were the following: female gender (OR 0.61; 95%CI 0.48-0.78 p< 0.0001); the existence of siblings (OR 0.63; 95% CI 0.41-0.96 p 0.034); breastfeeding for more than two months (OR 0.74; 95%CI 0.58-0.94 p 0.015); intestinal parasitisation (OR 0.75; 95%CI 0.57-0.99 p<0.047); parent allergic disease (OR 1.48; 95%CI 1.16-1.9 p 0.001) – specifically asthma and rhinitis in the father (OR 2.1; 95%CI 1.26-3.51 p 0.004 and OR 1.96; 95%CI 1.31-2.92 p 0.001, respectively) – contact in the first year of life with farm animals (OR 0.46; 95%CI 0.25-0.87 p 0.014), current sporadic contact with dogs (OR 0.68; 95%CI 0.53-0.87 p 0.002) and other animals (OR 0.50; 95%CI 0.32-0.78 p 0.002), current use of synthetic material bedclothes (OR 1.38; 95%CI 1.06-1.78 p 0.014), current bare flooring (OR 1.44; 95%CI 1.05-1.98 p 0.021), current passive smoking (OR 0.71; 95%CI 0.55-0.91 p 0.007), antecedents of chimney heating (OR 0.33; 95%CI 0.12-0.89 p 0.022), and single glazing (OR 1.63; 95%CI 1.15-1.23 p 0.005).
After dividing the series by subjects weighing less and more than 2500g, no statistically significant differences were found in the prevalence of allergic diseases and sensitisation.
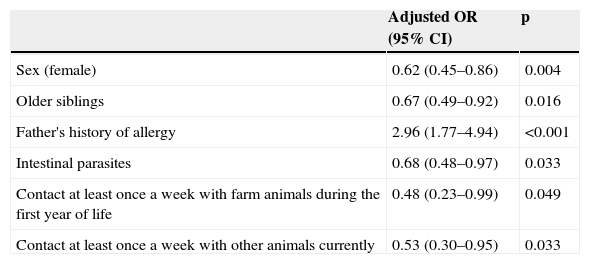
Following univariate analysis, multiple logistic regression analysis was made (Table 2) with all the variables exhibiting statistical significance (p<0.20). The factors (mostly of a protective nature) found to be associated to the presence of atopy in the children from Almería were the following: female gender (OR 0.62, CI 95% 0.45-0.86 p 0.004), older siblings (OR 0.67; CI 95% 0.49-0.92 p 0.016), a history of intestinal parasitisation (OR 0.68; CI 95% 0.48-0.97 p 0.033), contact with farm animals during the child's first year of life (OR 0.48 CI 95% 0.23-0.99), or other animals at present (OR 0.53 CI 95% 0.30-0.95 p 0.033), and father with some allergic disorder (OR 2.96; CI95% 1.77-4.94 p<0.001). These protective factors for sensitisation were not protective for asthma, rhinitis or eczema (data not shown).
Factors linked to atopy assessed by multivariate logistic regresión.
| Adjusted OR (95% CI) | p | |
| Sex (female) | 0.62 (0.45–0.86) | 0.004 |
| Older siblings | 0.67 (0.49–0.92) | 0.016 |
| Father's history of allergy | 2.96 (1.77–4.94) | <0.001 |
| Intestinal parasites | 0.68 (0.48–0.97) | 0.033 |
| Contact at least once a week with farm animals during the first year of life | 0.48 (0.23–0.99) | 0.049 |
| Contact at least once a week with other animals currently | 0.53 (0.30–0.95) | 0.033 |
OR indicates odds ratio; CI confidence interval; RAA: risk attributable to atopy (see text).
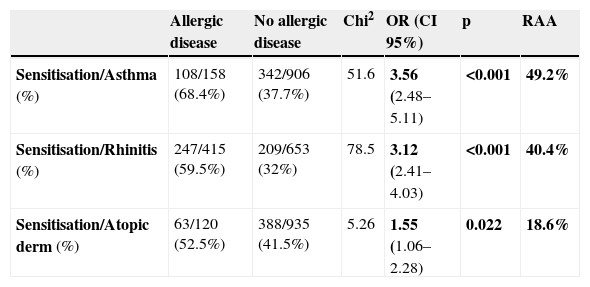
In the last year, the prevalence of wheezing, symptoms of rhinitis and atopic dermatitis were 15.5%, 38.9% and 11.4%, respectively. Table 3 reports the percentages of children with asthma, rhinitis and atopic dermatitis presenting sensitisation, and the population risk attributable to atopy, which were 49.2%, 40.4% and 18.6% for asthma, rhinitis and atopic dermatitis, respectively. On the other hand, of the 464 schoolchildren with at least one positive prick test, 162 (34.9%) presently showed no asthma, rhinitis or eczema; therefore, these cases could be considered to correspond to subclinical sensitisations.
Allergic sensitisation in 10- and 11-year-old schoolchildren from Almería with allergic diseases, and corresponding risk attributable to atopy.
| Allergic disease | No allergic disease | Chi2 | OR (CI 95%) | p | RAA | |
| Sensitisation/Asthma (%) | 108/158 (68.4%) | 342/906 (37.7%) | 51.6 | 3.56 (2.48–5.11) | <0.001 | 49.2% |
| Sensitisation/Rhinitis (%) | 247/415 (59.5%) | 209/653 (32%) | 78.5 | 3.12 (2.41–4.03) | <0.001 | 40.4% |
| Sensitisation/Atopic derm (%) | 63/120 (52.5%) | 388/935 (41.5%) | 5.26 | 1.55 (1.06–2.28) | 0.022 | 18.6% |
OR: odds ratio. RAA: risk attributable to atopy (see text).
The overall participation rate was 49.8% (1143/2293). The main reasons that parents argued for not participating in the survey were doubts about the aim of the study, rejection to skin prick or blood test or questionnaire completion (90%), children out of age range (8%); and refusal of the pupils (2%). Surveys conducted in a school frame run the risk of participation bias, with those parents with family or personal history of allergic diseases being more prone to sign a consent, justifying a larger prevalence of atopy and atopic diseases. Another factor affecting selection bias is that repeated use of the schools to perform socio-sanitary programmes (Almería had already participated in ISAAC I) could predispose the parents to a non-collaborative attitude. However, the response rate was similar to the other three Spanish centres participating in ISAAC II: Cartagena (58.9%), Valencia (43.7%), and Madrid (53.2%).7
The reported prevalence of allergic sensitisation in the non-selected paediatric population of western countries is 30–50%.2,3,8 In our series the prevalence was found to be 42.5%, out of which 162 cases (34.9%-or 15% of the sample) were considered as subclinical as they showed no symptoms. This value is much greater than the values reported by other studies within ISAAC Phase II.9,10 To the best of our knowledge, there is no available data regarding prevalence of sensitisation in other Spanish ISAAC centres, so we cannot make comparisons. On the other hand the Spanish ISAAC III group found considerable geographic variation in the prevalence of asthma and rhinitis symptoms,11,12 suggesting that factors other than atopy could be responsible for the geographic distribution of asthma. Dust mites were the most frequent sensitising allergens. This can be expected in cities such as Almería, located on the Mediterranean coast, with a warm and humid climate ideally suited to dust mite proliferation.
The prevalence of allergic sensitisation, asthma symptoms, and medical diagnosis of asthma found was greater in boys than in girls, coinciding with the observations of Arshad et al.13 and Sears et al.14, while no gender-linked differences were found in relation to rhinitis and dermatitis. Although the different percentages of sensitisation between males and females could explain in part the differences in asthma prevalence at these ages, the fact that no influence was observed upon the atopic dermatitis and rhinitis rates suggests that other factors in addition to atopy intervene in these disorders, forming part of complex interactions. Other authors have reported a greater male prevalence of sensitisation, rhinitis and asthma in the prepubertal stage,14,15 a situation which reverses in adolescence, with greater frequencies in females.15 This tendency subsequently persists up to menopause,16 suggesting the implication of hormonal factors. Specifically, the male predominance of asthma before puberty and female predominance after puberty has been explained in terms of structural and functional differences in the airways,17,18 and in relation to differences in self-perception of the symptoms,19 although such bias is lessened in surveys of the parents, or under a medical diagnosis of asthma.
No statistically significant differences were found in the prevalence of allergic diseases and sensitisation between subjects weighing less and more than 2500g at birth. Some authors have observed an increased incidence of allergic sensitisation and total IgE levels directly proportional to gestational age and weight at birth.20 This may be explained in part by the fact that caesarean delivery, a situation which some authors associate with increased allergy risk,21,22 is more likely in infants of greater weight and first borns. In contrast, other investigators point to prematureness and low weight at birth as risk factors for allergic sensitisation and asthma in the schooling period.23,24
We have found that schoolchildren who were breastfed for more than two months had a lesser risk of sensitisation, although this effect has not been seen to offer protection against allergic diseases (data not shown). Other authors25 have found breastfeeding to be associated with a greater risk of atopic dermatitis. A metaanalysis concluded that the early introduction of solid foods increases the risk of eczema, although there are few data supporting its correlation to the rest of allergic disorders.26 Therefore, caution is required in interpreting studies which report a greater prevalence of allergy in children who have received prolonged breastfeeding, since such data can be interpreted in the opposite sense, i.e., such children could correspond to subjects with an important familial burden of atopy or an early start of allergy, with mothers who are more aware of the beneficial effects of breastfeeding, and who therefore tend to prolong it as much as possible (reverse causality).
The presence of siblings was correlated to a lower risk of atopy in our schoolchildren. In general, most authors associate exposure to other children with lesser allergic disease rates27–29 – this being explained by the stimulation of Th1-mediated responses. However, when evaluating these studies, other factors must be taken into account, such as the number of people living at home, socioeconomic status, family antecedents of atopy, or registered infections during infancy. We found intestinal parasitosis to be a protective factor against atopy, although this was paradoxically correlated to a greater rather than to a lesser risk of rhinitis or atopic dermatitis with an OR of 1.5 and 1.6, respectively (to be published elsewhere). These observations are in conflict with those of a study where parasitic infections were seen to protect against allergic disorders in developing countries.30 The explanation could be that the association between allergy and infections, particularly those caused by helminths, is complex, since each population has its own genetic and environmental characteristics, limiting the possibility of offering a valid explanation for the different parts of the world. On the other hand, a recent metaanalysis has concluded that parasitoses in general do not protect against asthma, and that while some species may reduce asthma risk, infection caused by Ascaris lumbricoides (more frequent in our setting) would increase such risk.31
We have found that schoolchildren exposed to dogs and farm animals have a lesser prevalence of allergic sensitisation. The effects of exposure to other children and to domestic animals upon allergic diseases would not be linked to any concrete infection, and exposure to germs (pathogens and saprophytes) in general would be more decisive.32 An environment with a strong bacterial endotoxin presence during maturation of the immune system could favour the predominance of Th1 type responses, though once the predominance of Th2-mediated responses becomes consolidated in allergic subjects, exposure to endotoxin could act as an allergic response triggering factor.33 Some controversy exists regarding the correlation between the type of animal and allergic disease, since apart from the occasional study,34 exposure to cat dander is taken to be a risk factor, while exposure to dogs acts as a protective factor.35,36 After discarding a reverse causality bias a metaanalysis concluded that pets at home do not influence the risk of allergic diseases in children.37
In agreement with our own observations, other investigators have found an inverse relationship between smoking and sensitisation8 or allergic diseases38 (with the exception of asthma, where smoking exerts a dual effect by limiting intrauterine lung development and causing bronchial irritation at a later stage). This protective effect of smoking could be attributed in part to its association with a lower socioeconomic level (serving as a possible confounding factor), or to reverse causal bias whereby families with a history of atopy deliberately adopt measures such as the avoidance of smoking and pets. Synthetic materials used in the bedding have been related to increased sensitisation and allergic disease, since they retain a larger number of allergens, while chimney heating in the first year of life appears to exert a protective effect although this could also reflect interaction with the rural setting, where exposure to farm animals (among other protective factors) is more common.39
The existence of parental allergy, specifically asthma or rhinitis, is a sensitisation risk factor. Although we have no information on the percentage of allergic sensitisation among the parents of our children series, this study shows that atopy is inherited independently of the type of allergic disease found in the parents, confirming the important genetic component of both atopy and allergic disorders.
In the same way that some patients with asthma, rhinitis and atopic dermatitis are not atopic (i.e., they show no sensitisation), other subjects are atopic but do not suffer disease of any kind. In order to calculate the proportion of allergic disease attributable to atopy, Pearce et al.40 carried out a metaanalysis describing the relationship between asthma and atopy. The authors concluded that the percentage of asthma cases (in children and adults) attributable to atopy is in the range of 30–40%. Later, Arshad et al.11 confirmed these results not only for asthma but also for rhinitis and eczema – offering an explanatory model of allergic diseases at four years of age in which 30–40% of such disorders are attributable to atopy and the remaining 60–70% to other factors, including particularly those associated with the affected target organ.
In the present study, with asthma, rhinitis and atopic dermatitis prevalence of 15.5%, 38.9% and 11.4%, respectively, the population risk attributable to atopy for these diseases was 49.2%, 40.4% and 18.6%. Therefore, the latter could be regarded as the least atopic of the allergic diseases, at least within the age range of our patient series. An aspect to be taken into account is that the coexistence of sensitisation and symptoms is not always of a causal nature (it would be necessary to demonstrate that the allergen is the cause of the clinical picture); as a result, the term “attributable” risk may not be the best choice. Furthermore, it must be considered that the prevalence of allergic sensitisation is dependent upon the amount and quality of the allergen extracts used, and that both –sensitisation and the clinical manifestations of atopy- may vary over time.
Our results suggested that the protective factors for sensitisation are not the same for asthma, rhinitis or eczema (to be published), and that other factors may interact to influence atopy and act against such protective factors.
One of the limitations of the study is the high rate of sensitisation reported. This result can be explained by the low response rate, contributing to a `cooperation′ bias by which families with atopic antecedents are more willing to enrol on the survey and to complete the questionnaires more carefully. Moreover, this study was performed exclusively in public schools, where children and their families are less prone to participate in surveys.7 Nevertheless, the strength of this study is to be framed within ISAAC Phase II, which provides very valuable information about the prevalence of atopy, asthma and allergic disease worldwide, as well as their linked factors.
ConclusionsFemale gender, a history of intestinal parasitisation, older siblings and contact with animals exert a protective effect, while the father with some allergic disorder is a risk factor associated to atopy according to the results of the logistic regression analysis. The atopy protective factors found in our series do not imply protection against asthma, rhinitis or atopic dermatitis. This, and the fact that the factors associated to each of these individual disease conditions do not coincide, point out to the complexity of the interaction between the genetic and environmental factors influencing atopy and allergic diseases independently.
The authors thank Cristina Méndez-Vidal, Antonio Miranda-Vizuete, and Fernando Fernández-Gutierrez, for critical reading of the article.
Conflict of interest
The authors have no conflict of interest to declare.