Food oral immunotherapy (OIT) involves the administration of the food allergen causing the symptoms, in order to induce tolerance. Primary eosinophilic gastrointestinal disorders (PEGDs) are characterised by an eosinophil-rich inflammation affecting different locations of the digestive tract. We present a series of patients with PEGDs in a group of children following OIT with milk and/or egg.
Material and methodsA prospective study during the period 2006–2014 was performed in paediatric patients subjected to OIT with milk and/or egg. When these children present persistent gastrointestinal symptoms, they are referred to the Paediatric Gastroenterology Unit for evaluation.
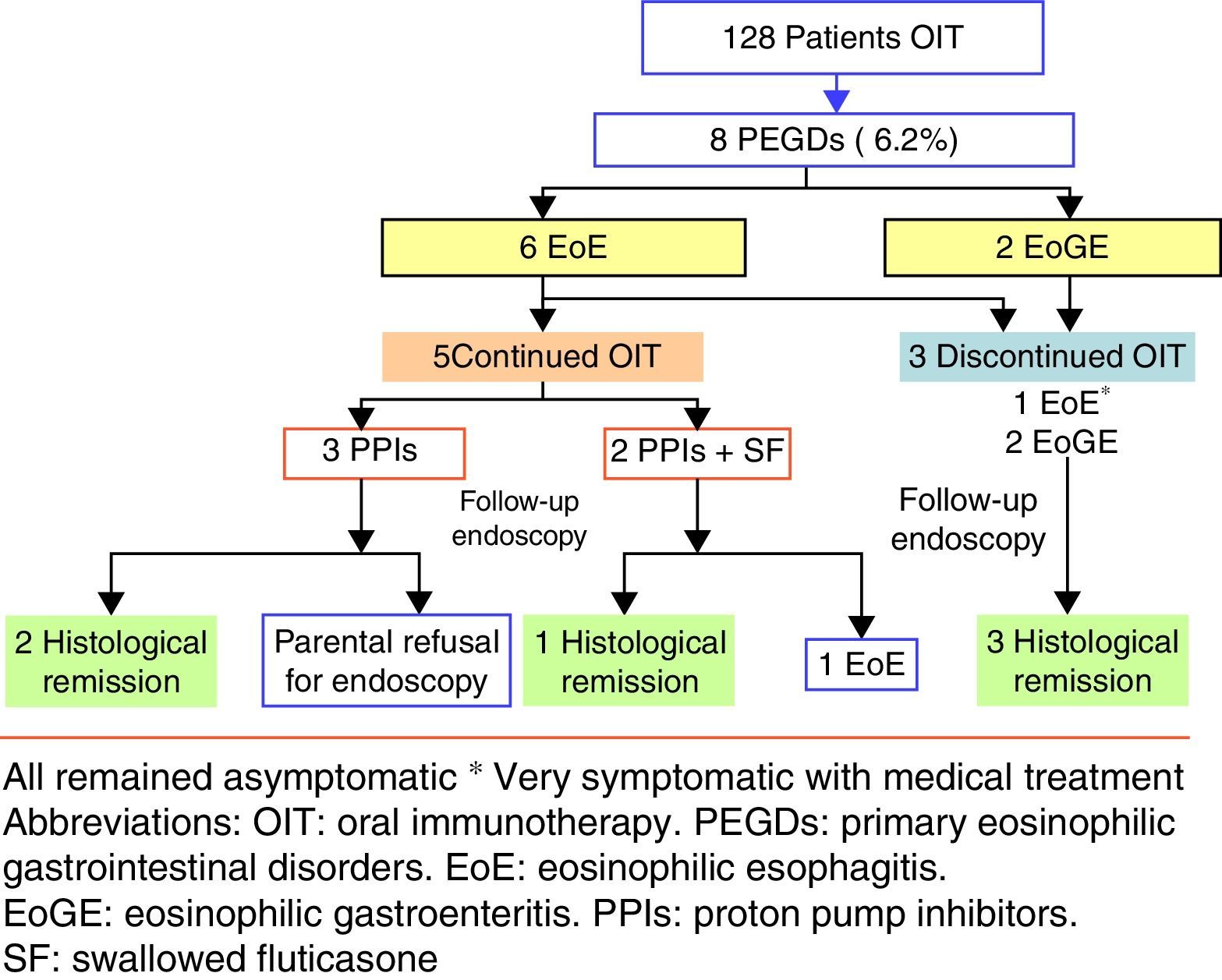
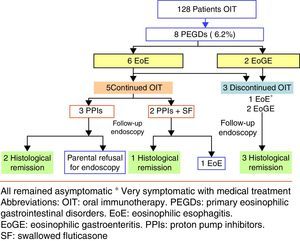
ResultsPrimary eosinophilic gastrointestinal disorders were diagnosed in eight of the 128 cases of OIT (6.25%). The time to PEGDs development was variable: two cases showed symptoms during OIT, and the rest with a median time of 29 months (15–48 months). Food treatment discontinuation was not required in four of the five cases of eosinophilic oesophagitis, although food removal was necessary in patients with eosinophilic gastroenteritis.
ConclusionsWe report the highest prevalence of PEGDs in children subjected to OIT, and the first cases of eosinophilic gastroenteritis following food OIT.
The monitoring of new digestive signs and symptoms after OIT is crucial for the diagnosis of these disorders, and prolonged follow-up is required. The management of such patients and the need or not to eliminate the food should be assessed on an individualised basis, according to the severity of the condition, its evolution and response to different treatment alternatives.
The prevalence of food allergy in childhood is high, with a negative impact upon general perceived health, family and social activities, and quality of life of both patients and their families. Current treatment strategy in such cases is to strictly avoid the offending food, provide rescue medication to control the adverse reactions caused by transgressions, and afford adequate patient education. Despite such measures, however, food allergy remains one of the most frequent causes of anaphylactic reactions in children and young adults.1–3
Food oral immunotherapy (OIT) involves the administration of increasing doses of the food to which the patient is allergic, with the aim of reducing the symptoms associated to natural exposure (desensitisation) and, if possible, achieve permanent tolerance. Food oral immunotherapy may be regarded as an alternative to elimination diets in patients with IgE-mediated allergy that maintain clinical reactivity beyond the age when natural tolerance is usually reached.3–5 The technique is fundamentally used with cow's milk and egg, since in our setting it is difficult to successfully avoid these foods, and transgressions are frequent and can be serious.6,7 Although death as a result of anaphylaxis is infrequent in such cases, the risk must not be underestimated – particularly in adolescents and asthmatic individuals.
Published studies to date indicate that OIT is able to achieve desensitisation to cow's milk and egg in 84% and 81% of the patients, respectively.8–10 However, the ability of OIT to induce permanent or sustained tolerance has not, to date, been extensively evaluated. The procedure is not without risks, and prolonged maintenance treatment is required. It therefore must be accepted by the patient and/or family, after receiving adequate information about the treatment.
Primary eosinophilic gastrointestinal disorders (PEGDs) are a heterogeneous group of diseases characterised by an eosinophil-rich inflammation affecting different locations of the digestive tract: eosinophilic oesophagitis (EoE), eosinophilic gastroenteritis (EoGE) (gastritis/enteritis) and eosinophilic colitis (EoC). Eosinophilic oesophagitis is the most common form, and is a chronic oesophageal disorder of immune origin, mediated by antigens and clinically and histopathologically characterised by oesophageal dysfunction and a predominantly eosinophilic infiltration (≥15 eosinophils per high-power microscopy field (eos/hpf)). Eosinophilic oesophagitis mainly affects males and is typically associated to other allergic disorders such as asthma and atopic dermatitis. Although the underlying aetiopathogenesis is not clear, mainly food allergens and inhaled aeroallergens to a lesser extent are known to play an important role in the development of the disease.11–13
Although the development of PEGD after OIT has been described in the recent literature, the relationship between them remains controversial. All available publications refer to sporadic cases of EoE.14–19 Recently, Lucendo et al. have published a systematic review and meta-analysis demonstrating a prevalence of EoE after OIT of 2.7%.20 There are no published cases of EoGE and EoC after food OIT.
ObjectiveThe present study aims to describe the incidence and characteristics of PEGDs manifested in a prospective group of children with persistent IgE-mediated food allergy undergoing to OIT with milk and/or egg.
Material and methodsA prospective study during the period 2006–2014 was performed in paediatric patients subjected to OIT with milk and/or egg due to persistent allergy to these foods as demonstrated by clinical findings, prick testing, specific-IgE quantification and a positive open oral food challenge. During both phases, an initial dose escalation and maintenance phase, strict monitoring of new gastrointestinal signs and symptoms was carried out (eating problems, vomiting, weight loss, abdominal pain, chest pain, burning sensation, persistent diarrhoea, dysphagia of oesophageal food impaction). None of the patients presented digestive symptoms before starting the OIT. When such children presented persistent gastrointestinal symptoms, they were referred to the Paediatric Gastroenterology Unit for evaluation and they were first clinically evaluated, and if PEGDs was suspected then digestive endoscopy was performed and biopsy specimens obtained.
Eosinophilic gastroenteritis involves selective eosinophilic infiltration of the stomach and small and/or large intestine, in the absence of other possible causes of eosinophilia of the digestive tract. It is characterised by variable involvement of the layers of the gastrointestinal wall, with different degrees of infiltration and spread which will change the associated symptoms (eosinophilic gastroenteritis is defined by eosinophil-predominant inflammation in one or more of the following locations: ≥10–30eos/hpf in the stomach and small intestine and ≥70eos/hpf in the large intestine). On the other hand, eosinophilic colitis is defined as eosinophilic infiltration limited to the colon, in the absence of other possible causes of eosinophilia. The aetiopathogenesis of both, EoGE and EoC is even less well known than that of EoE, although an underlying allergic mechanism has also been suggested, and the condition sometimes improves when an elimination diet is introduced.21–23
Statistical analysisProportions were expressed as percentages and 95% confidence intervals (CI). Continuous variables were presented as mean±standard deviation.
ResultsIn the period 2006–2014, our Paediatric Allergy Unit applied the OIT protocol in 97 cases with cow's milk and 31 cases with egg. In that period, PEGDs were diagnosed in eight of the global 128 cases of OIT representing 6.25% of the sample (95% CI: 2.05–10.4%). Most of the affected patients were males between 3 and 14 years of age. Eosinophilic oesophagitis was diagnosed in six patients with exclusively oesophageal involvement, while the other two patients were diagnosed with EoGE (one with oesophageal and duodenal disease and the other with oesophageal and colon involvement). Six of the affected patients had undergone OIT with cow's milk, one with egg, and one with both foods.
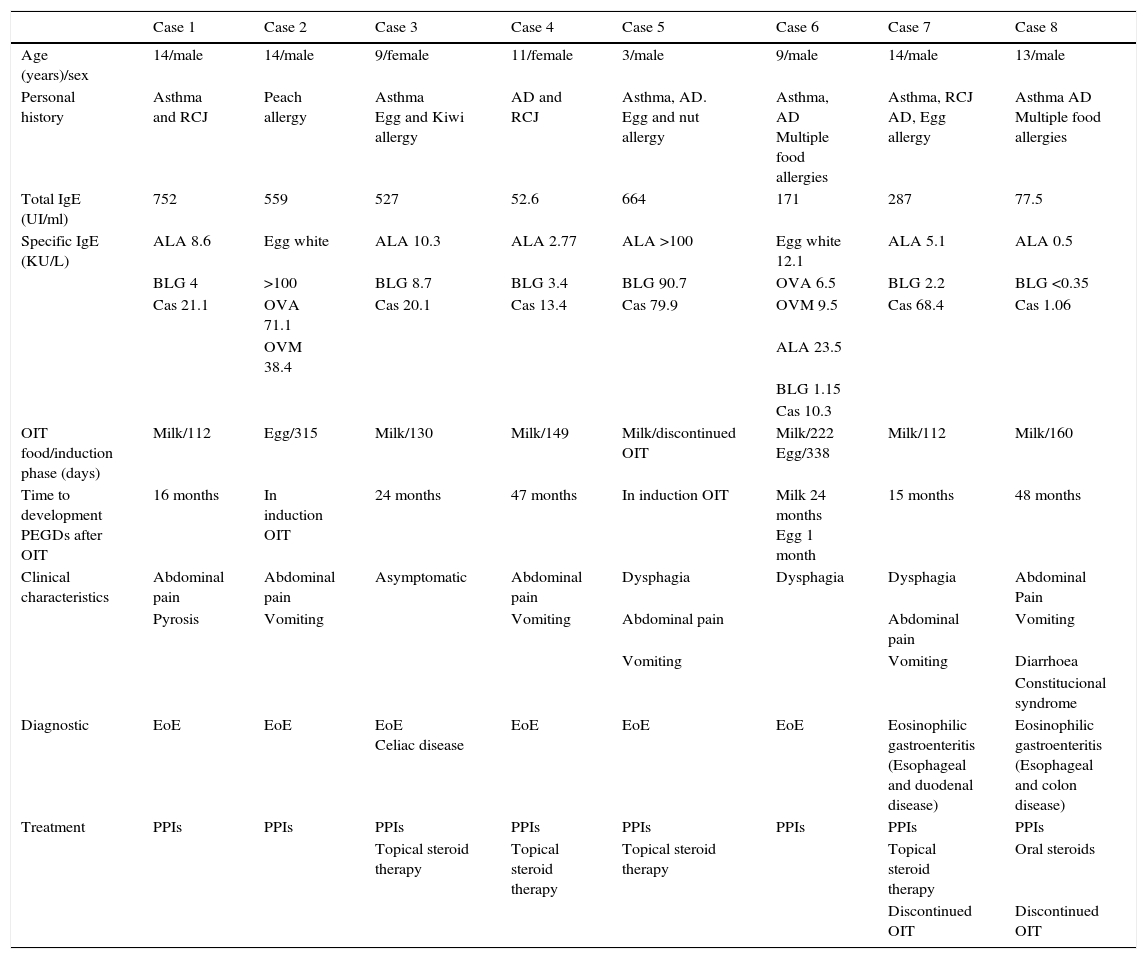
All the patients had a history of allergic disorders (asthma, rhinoconjunctivitis, atopic dermatitis or allergy to different foods). The previous total IgE and specific IgE (ImmunoCAP) titres corresponding to each of the implicated foods are shown in Table 1.
Case summary.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| Age (years)/sex | 14/male | 14/male | 9/female | 11/female | 3/male | 9/male | 14/male | 13/male |
| Personal history | Asthma and RCJ | Peach allergy | Asthma Egg and Kiwi allergy | AD and RCJ | Asthma, AD. Egg and nut allergy | Asthma, AD Multiple food allergies | Asthma, RCJ AD, Egg allergy | Asthma AD Multiple food allergies |
| Total IgE (UI/ml) | 752 | 559 | 527 | 52.6 | 664 | 171 | 287 | 77.5 |
| Specific IgE (KU/L) | ALA 8.6 | Egg white | ALA 10.3 | ALA 2.77 | ALA >100 | Egg white 12.1 | ALA 5.1 | ALA 0.5 |
| BLG 4 | >100 | BLG 8.7 | BLG 3.4 | BLG 90.7 | OVA 6.5 | BLG 2.2 | BLG <0.35 | |
| Cas 21.1 | OVA 71.1 | Cas 20.1 | Cas 13.4 | Cas 79.9 | OVM 9.5 | Cas 68.4 | Cas 1.06 | |
| OVM 38.4 | ALA 23.5 | |||||||
| BLG 1.15 | ||||||||
| Cas 10.3 | ||||||||
| OIT food/induction phase (days) | Milk/112 | Egg/315 | Milk/130 | Milk/149 | Milk/discontinued OIT | Milk/222 Egg/338 | Milk/112 | Milk/160 |
| Time to development PEGDs after OIT | 16 months | In induction OIT | 24 months | 47 months | In induction OIT | Milk 24 months Egg 1 month | 15 months | 48 months |
| Clinical characteristics | Abdominal pain | Abdominal pain | Asymptomatic | Abdominal pain | Dysphagia | Dysphagia | Dysphagia | Abdominal Pain |
| Pyrosis | Vomiting | Vomiting | Abdominal pain | Abdominal pain | Vomiting | |||
| Vomiting | Vomiting | Diarrhoea | ||||||
| Constitucional syndrome | ||||||||
| Diagnostic | EoE | EoE | EoE Celiac disease | EoE | EoE | EoE | Eosinophilic gastroenteritis (Esophageal and duodenal disease) | Eosinophilic gastroenteritis (Esophageal and colon disease) |
| Treatment | PPIs | PPIs | PPIs | PPIs | PPIs | PPIs | PPIs | PPIs |
| Topical steroid therapy | Topical steroid therapy | Topical steroid therapy | Topical steroid therapy | Oral steroids | ||||
| Discontinued OIT | Discontinued OIT |
Abbreviations
1 RCJ: rhinoconjunctivitis. 2 AD: atopic dermatitis. 3 IgE: immunoglobulin E.
4 ALA: alpha-lactalbumin, BLG: beta lactoglobulin, CAS: casein.
OVA: ovalbumin; OVM: ovomucoid, 6 OIT: oral immunotheraphy.
7 PEGDs: primary eosinophilic gastrointestinal disorders.
8 EoE: eosinophilic oesophagitis.
9 PPI: protom pump inhibitor.
The time of onset of digestive disorders after OIT varied according to the different cases. Six of eight patients developed PEGDs after OIT (all with cow's milk), with a median time of 29 months (15 months–4 years). In one of these patients, OIT with egg was performed after finishing OIT with cow's milk (case 6). In the remaining two patients the diagnosis of PEGDs was established in the course of OIT.
The most common initial symptom was abdominal pain (in six of the eight cases), followed by vomiting (in five cases) and dysphagia (in three cases). The two patients with EoGE experienced more intense symptoms, with greater clinical repercussion (diarrhoea and constitutional syndrome in the patient with colon involvement). One of the patients (case 3) was asymptomatic, and EoE was detected on occasion of the endoscopic study carried out due to the suspicion of celiac disease.
The five patients with EoE received treatment with proton pump inhibitors with or without swallowed corticosteroids (fluticasone 500–750μg/daily), and OIT was maintained. The two patients who were diagnosed recently received a double dose of proton pump inhibitors [PPIs] (1mg/kg per dose, twice daily for 8–12 weeks), with histological response. These patients were diagnosed as PPI-responsive oesophageal eosinophilia. Four patients had a good clinical response, and only one (case 5) required OIT discontinuation in order to control the symptoms. In the patient with celiac disease, medical treatment was prescribed in view of the persistence of eosinophilic infiltration after eliminating gluten from the diet. Endoscopic controls after treatment were performed in four of the five patients, with endoscopic improvement or normalisation in three of them. Due to the greater digestive tract involvement in the two patients with EoGE, the food used in OIT was eliminated and treatment was started with proton pump inhibitors and corticosteroids. Clinical and histological remission was achieved in both cases (Fig. 1).
DiscussionIn our experience, the prevalence of primary eosinophilic gastrointestinal disorders (PEGDs) in patients who have received food OIT is 6.25% (95% CI: 2.05–10.4%). Very few studies are available on the development of EoE or other eosinophilic digestive disorders in patients who undergo food OIT, and most of the existing publications correspond to retrospective studies14–16 and case reports.17,18 The series described in our study is the largest published to date, and reports a higher frequency of PEGD than other studies, where the prevalence ranges between 2.5%6,9 and 3.5%.3 On the other hand, a recent systematic review on the evidence of an association between newly manifesting EoE and OIT published by Lucendo et al.20 found that the prevalence of this disorder after OIT (egg, milk, peanut or wheat) was 2.7% (95% CI: 1.7–4%). This is considerably lower than our own figure, which may be explained because the systematic review only included EoE patients and not other PEGDs.
There may be a number of explanations for the higher prevalence found in our study. In our opinion, the most important factor is the close clinical monitoring performed during follow-up. In this regard, we compiled a detailed clinical history and conducted a physical examination on each visit in order to detect signs and symptoms suggestive of gastrointestinal disease. When such disease was suspected, the patients were referred to the Paediatric Gastroenterology Unit for evaluation. On the other hand, there is no sign, symptom or laboratory test parameter pathognomonic of PEGDs. Furthermore, in the specific case of EoE, there is a known lack of correlation between clinical and histological findings.24
Gastrointestinal symptoms are common in the context of food OIT. However, while the symptoms associated to IgE-dependent mechanisms tend to manifest immediately after food exposure (in under 2h), the manifestations of PEGDs in relation to OIT usually exhibit no such sequential relationship – although they characteristically persist over time. In the case of younger patients, vomiting is usually the most prevalent symptom in both scenarios – this sometimes makes it difficult to establish a correct differential diagnosis.25 However, in the case of dysphagia or food impaction–more characteristic of EoE12 – a high degree of suspicion must be established, since such manifestations are not commonly reported by the patients during OIT.26 In our series, the most frequent symptom was abdominal pain, followed by vomiting, dysphagia or food impaction (present in only three of our patients, specifically cases 5–7).
Oral immunotherapy is a novel form of treatment, and long-term safety of OIT is nowadays poorly reported, so it remains unknown if long-term adverse effects can be induced. Consequently, patient monitoring must be prolonged over time. This may also be one of the reasons why the prevalence of PEGDs in our series was higher than in other studies, since follow-up in our case covered periods of up to nine years.
All our patients reported other concomitant allergic conditions such as asthma, rhinoconjunctivitis or atopic dermatitis. The relationship between PEGDs (particularly EoE) and these allergic diseases is known.11,13 The prevalence of EoE in the general population is estimated at 1.1%,27 although the prevalence of PEGD in high risk allergic populations is not clear. A recent study recorded a prevalence of EoE of 8.3% in allergic children28 – a figure which comes closer to that found in our series. Consequently, since all our patients suffered such allergic disorders, they appear to conform a high risk group for PEGD – and this may also help explain our recorded higher prevalence of 6.25%.
Our series also describes the first published cases of EoGE following food OIT. Eosinophilic gastroenteritis is less common than EoE, and few references of the disease can be found in the literature. As a result, establishing the diagnosis may be more complicated.14 The symptoms are usually confusing, with a broad variety of signs and symptoms, depending both on the affected zone of the digestive tract and on the intestinal wall layers infiltrated by eosinophils. In our series, case 7 was characterised by vomiting, abdominal pain and intermittent dysphagia approximately 15 months after OIT with cow's milk, while case 8 involved intense abdominal pain, diarrhoea and important anorexia, with weight loss altering normal life and even preventing the child from attending school, four years after the administration of OIT, also with cow's milk. Thus, our two cases were not characterised by a common clinical picture, and the diagnosis could only be established after performing endoscopic studies.
In view of the relationship between PEGDs (fundamentally EoE) and allergic disease, it could be postulated that introduction of the food in our patients was possibly not the only cause involved in the development of gastrointestinal pathology. Since endoscopic study before OIT is not indicated in asymptomatic patients, digestive manifestations coinciding with introduction of the food, suggest the onset of PEGDs, although without being able to confirm its previous existence. We were only able to confirm the absence of previous eosinophilic digestive disease in case 3, since in that patient an endoscopic study had been made before administering OIT, due to the presence of celiac disease.
In three of our cases, EoE was diagnosed in the course of OIT, which may point to the introduction of the food in the diet as the underlying causal factor. In the rest of the series, the time between OIT and the diagnosis exceeded one year, with a range of 15 months to over four years. Thus, in these cases a possible causal relationship between OIT and PEGDs is more difficult to establish. Such a long time interval once again underscores the need for prolonged monitoring of these patients.
Regarding treatment, exclusion of the cow's milk introduced in the context of OIT was decided in the two patients with EoGE, followed by a favourable clinical and histological course that remains long term.
In the six patients with EoE, food elimination was decided as the first treatment option only in one patient (case 5) in which the clinical manifestations appeared in the dose scaling phase of OIT, since the parents rejected medical treatment. After removal of food, symptoms disappeared and normal histological findings were shown. In the other five patients the food was not eliminated, establishing drug treatment, followed by symptoms resolution in all cases. Three of these five children had normal histological findings, despite not having eliminated the food. Regarding the other two cases (also with disappearance of the symptoms following medical treatment), one rejected control endoscopy (case 1), and the other showed persistent endoscopic abnormalities (case 3). Morais et al.18 recommend to discontinue OIT when EoE is diagnosed in the dose-scaling phase, and other authors advise the removal of the food in patients who have developed EoE after OIT.16,17 In our series we found both, endoscopic healing and disappearance of the symptoms to be possible despite maintenance of the food introduced in OIT. In our opinion, in the case of allergic patients with EoE, the important improvement in their quality of life represented by the possibility of consuming a food without the risk of serious reactions,29 and the fact that the removal of the food does not guarantee the resolution of EoE, implies that the decision to eliminate it or not should be made on an individualised basis, depending on the diagnosis, symptoms, evolution and response to other available treatments. However, in the case of EoGE, we consider that the food introduced in OIT should be removed, due to the severity of the clinical condition.
In conclusion, the series of primary eosinophilic gastrointestinal disorders described in our study is the largest published to date in children who have undergone food oral immunotherapy. We underscore the need for correct and prolonged patient gastrointestinal follow-up, maintaining a high degree of suspicion in individuals with persistent digestive tract symptoms. The decision to eliminate the food from OIT should be agreed with the patients and their families on an individualised basis, depending on the severity of the condition, the symptoms, evolution and response to other possible treatment alternatives.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.