To compare with a control group the frequency of psychiatric disorders and severity of psychiatric symptoms in preschool children with atopic eczema.
MethodsThe study included children between the ages of 3–5 who were diagnosed to have atopic eczema. The parents of the children with atopic eczema were interviewed in person and were asked to fill in “The Early Childhood Inventory-4” form. This form assesses the psychiatric disorders and symptoms severity in children between the ages of 3–5.
ResultsThe atopic eczema group included 80 patients (38 male, 42 female) with a mean age of 48.4±15.7 months and the control group included 74 patients (41 male, 33 female) with a mean age of 49.9±15.19 months. It was established that 68.8% of the group with atopic eczema received at least one psychiatric diagnosis. Between the psychiatric disorders, ADHD (Odds ratio: 2.57, 95% CI: 1.049–6.298, p=0.035), enuresis and encopresis (Odds ratio: 2.39, 95% CI: 1.121–5.097, p=0.022) and attachment disorder (Odds ratio: 2.03, 95% CI: 1.046–3.953, p=0.035) were found to be significantly higher when compared with the healthy control group. When the groups were compared in terms of psychiatric symptom severity scores calculated by using ECI-4, ADHD severity (p=0.043), conduct disorder severity (p=0.001), anxiety disorders severity (p<0.001), eating disorders severity (p=0.011) and tic disorder severity (p=0.01) were found to be higher in the atopic eczema group.
ConclusionPsychiatric illnesses are frequent in preschool children with atopic eczema.
Atopic eczema (AE) is a chronic, relapsing, and highly pruritic dermatitis that generally develops in early childhood, and has a characteristic age-dependent distribution. Atopic eczema is relatively common, affecting 10–20% of children in developed countries.1,2 The chronic process in atopic eczema affects the quality of life, sleep pattern, and behaviour development.3–5 Few studies on this subject have shown that Attention Deficit Hyperactivity Disorder (ADHD) is more frequent in children with atopic eczema.6–8 Children with chronic skin diseases such as atopic eczema were found to have more psychiatric disorders such as depression and anxiety.9 Although studies have so far reported that in allergic illnesses such as atopic eczema, psychiatric disorders such as ADHD and tic disorder are frequent, no studies have assessed the psychiatric illness symptoms in preschool children with atopic eczema extensively.
The purpose of this study is to find out the frequency of psychiatric disorders in children between the ages 3–5 with atopic eczema.
Materials and methodsThis multi-centred study was conducted between June 2013 and January 2014. Children between the ages of 3–5 who were diagnosed with atopic eczema or who were being monitored for atopic eczema were included in the study. The parents of patients with atopic eczema who were admitted to the Pediatric Allergy and Asthma Clinics of Inonu University, Ondokuz Mayıs University, Adnan Menderes University and Kanuni Sultan Süleyman Training and Research Hospital were interviewed and asked to fill out a sociodemographic data form. This form included the patients’ information on demographic, sociodemographic characteristics, education status, family characteristics, education level of the parents, and socioeconomic level of family. After this form was completed, Early Childhood Inventory 4 (ECI-4) was completed in about 30min by the parents who provided care to the patients. The volunteer control group underwent the same procedure after being informed of the study and giving their consent. All scales were evaluated by a child psychiatry consultant. Those patients whose forms were not completely and correctly filled out were excluded from the study.
“The early childhood inventory-4”, developed by Sprafkin and Gadow,10,11 is a scale designed to evaluate the behavioural, emotional, and cognitive symptoms of children between the ages of three and five according to DSM-IV diagnostic criteria. Disorders such as schizophrenia, which rarely occur between the ages of three and five, are not investigated in the ECI-4. However, diagnoses such as eating, sleep, and attachment disorders, which occur more frequently during these ages, are included. The ECI-4 is composed of 108 items that are rated as “never”, “sometimes”, “often” and “nearly always”. Sprafkin and Gadow graded the ECI-4 in two different ways: symptom score points and symptom severity points. In the number of symptoms scoring, “never” and “sometimes” are scored as 0 and “often” and “almost always” as 1. Scores obtained for each disorder in ECI-4 are added. If this overall score is equal to or higher than the number of symptoms required for DSM-IV diagnosis, symptom criteria score for that disorder is evaluated as “yes”. In scoring the severity of symptoms, “never” is scored as 0, “sometimes” as 1, “often” as 2 and “almost always” as 3. Scores obtained from questions are added and the severity score of the involved disorder is found.10,11 The score's reliability/validity study in Turkey was carried out by Başgul et al.12 in children between the ages of 3 and 5. There are two different forms of the scale, one of which is completed by the parents and the other by teacher. In this study, the parent form was used.
Atopic eczema diagnosis was conducted according to the Hanifin and Rajka criteria based on patient history and clinical characteristics.13
Data analysisStatistical analysis was performed using Statistical Package for Social Sciences (SPSS) 15.0 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as frequency and percentage for categorical variables, whereas quantitative data were expressed as median for non-normally distributed data and as mean for normally distributed data. We used the Mann–Whitney U test and Student's t-test to compare two groups (atopic eczema group and healthy control group), and the Chi-square test to compare the categorical variable. We considered a two-sided p<0.05 as statistically significant.
Ethical considerationsThe study was approved by the ethics committee of Inonu University, Faculty of Medicine. The written informed consent was obtained from the parents of all the participants.
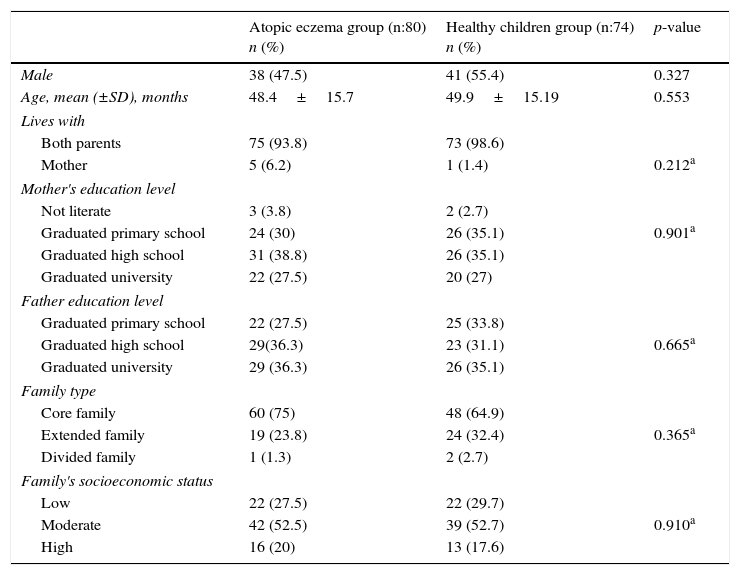
ResultsThe atopic eczema group included 80 patients (38 male, 42 female) with a mean age of 48.4±15.7 months and the control group included 74 patients (41 male, 33 female) with a mean age of 49.9±15.19 months. There were no statistically significant differences between the groups in terms of age and gender (p>0.05). The sociodemographic characteristics of both groups are presented in Table 1. No statistically significant differences were found between the two groups with respect to sociodemographic characteristics (p>0.05).
Sociodemographic features of patients.
| Atopic eczema group (n:80) n (%) | Healthy children group (n:74) n (%) | p-value | |
|---|---|---|---|
| Male | 38 (47.5) | 41 (55.4) | 0.327 |
| Age, mean (±SD), months | 48.4±15.7 | 49.9±15.19 | 0.553 |
| Lives with | |||
| Both parents | 75 (93.8) | 73 (98.6) | |
| Mother | 5 (6.2) | 1 (1.4) | 0.212a |
| Mother's education level | |||
| Not literate | 3 (3.8) | 2 (2.7) | |
| Graduated primary school | 24 (30) | 26 (35.1) | 0.901a |
| Graduated high school | 31 (38.8) | 26 (35.1) | |
| Graduated university | 22 (27.5) | 20 (27) | |
| Father education level | |||
| Graduated primary school | 22 (27.5) | 25 (33.8) | |
| Graduated high school | 29(36.3) | 23 (31.1) | 0.665a |
| Graduated university | 29 (36.3) | 26 (35.1) | |
| Family type | |||
| Core family | 60 (75) | 48 (64.9) | |
| Extended family | 19 (23.8) | 24 (32.4) | 0.365a |
| Divided family | 1 (1.3) | 2 (2.7) | |
| Family's socioeconomic status | |||
| Low | 22 (27.5) | 22 (29.7) | |
| Moderate | 42 (52.5) | 39 (52.7) | 0.910a |
| High | 16 (20) | 13 (17.6) | |
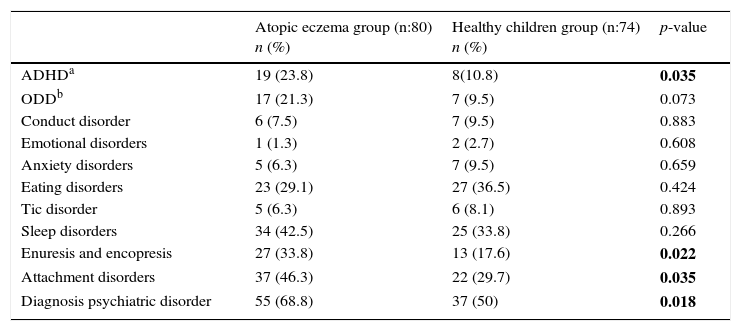
When groups were compared with respect to the psychiatric diagnoses they received measured by ECI-4, it was established that 68.8% of the group with atopic eczema received at least one psychiatric diagnosis, while 50% of the control group had received at least one psychiatric diagnosis, with a statistically significant difference (p=0.018). The psychiatric diagnoses made with the data obtained from ECI-4 are presented in Table 2. As shown in the table, ADHD (Odds ratio: 2.57, 95% CI: 1.049–6.298, p=0.035), enuresis and encopresis (Odds ratio: 2.39, 95% CI: 1.121–5.097, p=0.022) and attachment disorder (Odds ratio: 2.03, 95% CI: 1.046–3.953, p=0.035), were found to be significantly higher when compared with the healthy control group. Oppositional defiant disorder (ODD) was found higher in atopic eczema group but the difference was not statistically significant (p=0.073). In the atopic eczema group, the rate of psychiatric disorders was not statistically significant according to atopic eczema severity while SCORAD index was used (p=0.66).
Diagnoses of psychiatric disorders determined by ECI-4.
| Atopic eczema group (n:80) n (%) | Healthy children group (n:74) n (%) | p-value | |
|---|---|---|---|
| ADHDa | 19 (23.8) | 8(10.8) | 0.035 |
| ODDb | 17 (21.3) | 7 (9.5) | 0.073 |
| Conduct disorder | 6 (7.5) | 7 (9.5) | 0.883 |
| Emotional disorders | 1 (1.3) | 2 (2.7) | 0.608 |
| Anxiety disorders | 5 (6.3) | 7 (9.5) | 0.659 |
| Eating disorders | 23 (29.1) | 27 (36.5) | 0.424 |
| Tic disorder | 5 (6.3) | 6 (8.1) | 0.893 |
| Sleep disorders | 34 (42.5) | 25 (33.8) | 0.266 |
| Enuresis and encopresis | 27 (33.8) | 13 (17.6) | 0.022 |
| Attachment disorders | 37 (46.3) | 22 (29.7) | 0.035 |
| Diagnosis psychiatric disorder | 55 (68.8) | 37 (50) | 0.018 |
Bolded p-values indicate statistical significance.
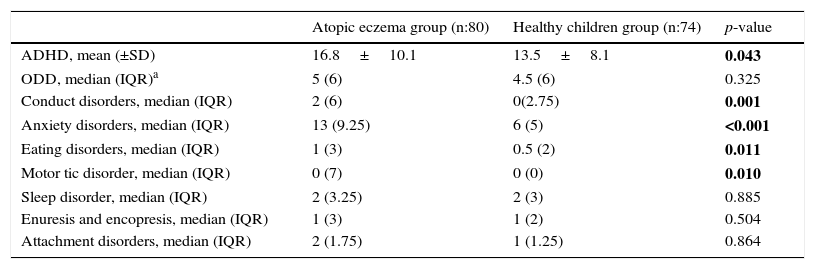
When the groups were compared in terms of psychiatric symptom severity scores calculated by using ECI-4, symptom severity of ADHD (p=0.043), conduct disorder (p=0.001), anxiety disorders (p<0.001), eating disorders (p=0.011) and tic disorder (p=0.01) were found to be higher in the atopic eczema group. Psychiatric symptom severity scores obtained with ECI-4 are presented in Table 3.
Psychiatric symptom severity determined by ECI-4.
| Atopic eczema group (n:80) | Healthy children group (n:74) | p-value | |
|---|---|---|---|
| ADHD, mean (±SD) | 16.8±10.1 | 13.5±8.1 | 0.043 |
| ODD, median (IQR)a | 5 (6) | 4.5 (6) | 0.325 |
| Conduct disorders, median (IQR) | 2 (6) | 0(2.75) | 0.001 |
| Anxiety disorders, median (IQR) | 13 (9.25) | 6 (5) | <0.001 |
| Eating disorders, median (IQR) | 1 (3) | 0.5 (2) | 0.011 |
| Motor tic disorder, median (IQR) | 0 (7) | 0 (0) | 0.010 |
| Sleep disorder, median (IQR) | 2 (3.25) | 2 (3) | 0.885 |
| Enuresis and encopresis, median (IQR) | 1 (3) | 1 (2) | 0.504 |
| Attachment disorders, median (IQR) | 2 (1.75) | 1 (1.25) | 0.864 |
Bolded p-values indicate statistical significance.
This study is the first one to evaluate extensively psychiatric disorder and psychiatric symptom severity in preschool children with atopic eczema by using ECI-4. Our results show that rates of psychiatric disorder and psychiatric symptom severity are higher than those of the control group.
Few studies carried out thus far bring to mind an association between allergic diseases such as atopic eczema and frequency of ADHD. In his study, Chen et al.7 reported atopic eczema in 8.4% of the patients diagnosed as ADHD. Although the association between these two diseases cannot be explained fully, atopic eczema with a chronic course brings to mind that it may be a risk for ADHD. In patients with atopic eczema, cytokines are released as a result of chronic inflammation. These inflammatory cytokines (such as IL-1, IL-2, IL-4, IL-5, IL-10, IL-13 and INF-gamma) affect brain development in preschool age group, especially infants. These cytokines affect the developing areas of the brain (anterior cingulate cortex, prefrontal cortex, corpus callosum and neurotransmitter system) directly. These areas control especially attention, motivation, motor and cognitive functions. Changes in maturation in these areas are thought to increase the risk of ADHD. Epidemiological data show that children with AE have an approximately 1.5-fold increased risk for ADHD-symptoms and that the population attributable risk for ADHD explained by AE is about 10%.14,15 In parallel with the literature, the frequency of ADHD according to ECI-4 in the patients with atopic eczema was 2.5 times higher when compared with the healthy control group. ADHD symptom severity was also found to be higher when compared with the control group.
There are some studies which have discovered pathophysiological links between stress and allergic disease.16,17 Many studies have shown that patients with AE also have a history of chronic stress and experience severe impairment in their quality of life (QoL), resulting in significant emotional distress.18,19 Similarly, anxiety symptom severities of the AE cases in our study were found to be higher than that of the control group. In a study by Slattery et al.,20 anxiety was found to be associated with asthma and allergic rhinitis while it was not found to be associated with AE. Psychological stress has been reported to cause neuroendocrin response and it has also been reported that this response can affect the skin physiology in many different aspects.21,22 At the same time, psychological stress was reported to cause abnormal skin barrier function.23,24 Taken together, AE causes stress to the patient and AE may be triggered by stress.
Enuresis and encopresis are elimination disorders which are frequent in preschool children. 12–16% of five-year-old children have enuresis while 4.1% of five to six-year-old children have encopresis.25 In our study, enuresis and encopresis were seen 2.4 times more in patients with atopic eczema. Enuresis and encopresis are reported to be associated with psycho stressors and they are reported to occur after drastic life experiences.26 In these cases, chronic illness is a psycho stressor for frequent admissions to hospital and thus, this is thought to be the reason for frequent occurrence of enuresis and encopresis in AE cases. In addition, developmental problems are common in both enuresis and encopresis.27,28 It was mentioned above that the inflammatory cytokines released in AE affect neurodevelopment. The presence of ADHD which was found to be higher in the AE group with enuresis nocturnal supports the presence of developmental problems in these cases.
Atopic eczema has been accepted as an expression of defects in the relationship between mother and child by early period psychoanalytic writers.29 The mothers of these babies have been reported to have an infantile personality and they have been reported to have an ambivalent attitude towards their children.30 In their study with adults, Dieris-Hirche et al.31 found that in patients with atopic eczema, there were significant correlations between the patients’ attachment characteristics on the one hand and the detriment to skin-specific quality of life on the other. The patients with AE have at least tendentially significantly less secure attachment attitudes than the control group. In our study, the finding that the patients with atopic eczema had twice more attachment disorder when compared with the control group supports these findings.
Important limitations of the study are absence of face-to-face psychiatric interviews with the cases and the use of only ECI-4 in the diagnosis of psychiatric disorders. However, it is a fact that this is the first study that assessed psychiatric disorders and severity of psychiatric symptoms in preschool children extensively. Moreover, the study is multicentred and involved a relatively large number of cases. Our findings need to be supported with studies which diagnose psychiatric disorders with face-to-face psychiatric assessments.
ConclusionAs a result, in our study, two thirds of AE patients were found to have at least one psychiatric disorder and this rate was found to be higher than that in the control group. ADHD, elimination disorders (enuresis and encopresis) and attachment disorders determined with ECI-4 were found to be higher in the AE patients when compared with the control group. In addition, ADHD, conduct disorder, anxiety disorders, eating disorders and tic disorder symptom severities determined with ECI-4 were found to be higher in patients with atopic eczema.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial supportNo financial support was provided.
Conflict of interestThe authors have no conflict of interest to declare.