Suspected hypersensitivity to betalactam antibiotics in children is a frequent cause of consultation that proves costly in terms of resource utilization – particularly time. Such hypersensitivity is, however, rarely confirmed.
MethodsA short protocol was introduced in which patients at low risk (single episode with mild, non-immediate skin symptoms after the administration of a betalactam antibiotic via the oral route) were subjected to oral provocation (following the obtaining of informed consent) without any other prior evaluations. Patients failing to meet these requirements were studied according to the protocol of the EAACI (specific IgE and skin testing prior to oral provocation).
ResultsA total of 78 patients (56 at low risk) were studied. Five patients had tolerated the medication after the episode, while another six patients failed to complete the study. The study with oral provocation was completed in the remaining 67 patients: according to the protocol of the EAACI in 17 patients, and using the short protocol in 50 patients. Only one patient showed a positive provocation test, of a delayed and mild nature.
ConclusionsDirect oral provocation in low risk patients has been shown to be effective and safe in discarding hypersensitivity to betalactam antibiotics in the majority of the patients studied.
The suspicion of hypersensitivity (HS) to drugs is a very frequent cause of medical consultation. In Spain, such situations account for about 7.5% of all patients seen in paediatric allergology clinics.1 Betalactam antibiotics (BLAs) are the drugs most often implicated in cases of suspected HS, due to their widespread use in the empirical treatment of infections in childhood. However, evaluation of these patients rarely confirms such suspicion.2-4 In our prior series of 503 children under 15 years of age seen for suspected HS to BLAs over a period of seven years, tolerance was confirmed in 68% of the cases, while 25% failed to complete the study (generally due to lack of consent or dropout), and only 6% (32) were diagnosed with allergy to BLA.5 This diagnosis proved significantly more probable in patients with a history of repeated or serious reactions, and in those administered BLAs via the parenteral route.
Based only on the clinical history, it is often difficult to establish whether the suspect reaction was of an immediate type or not. Although skin and laboratory tests are of little help in studying non-immediate reactions6 – which seem to be the reactions most commonly seen in children – they are often performed in order to add supposed safety and reliability to the study of the patients. The study protocols developed by different scientific societies require several patient visits and are targeted at adults.7 Only oral provocation allows the establishment of a definitive diagnosis.8
Based on these considerations, our group developed a protocol for the clinical study of children referred to our clinics with suspected HS to BLAs. The main characteristic of the protocol is its simplicity, justified by the very low frequency of HS to BLAs in childhood, and the mild nature of the symptoms involved. The present study describes the results obtained in the first year of application of the protocol, with a view to establishing its clinical applicability, and proposes a study algorithm inspired by the experience gained.
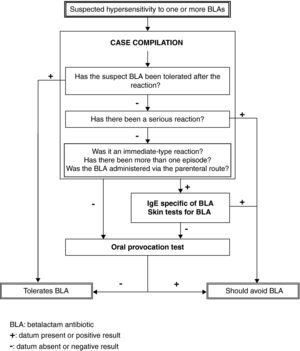
Material and methodsAfter evaluating our own experience and reviewing the recent literature, a protocol was developed for the study of paediatric patients (under 15 years of age) referred for the evaluation of suspected HS to some BLA. The data compiled on the first visit allowed us to evaluate the plausibility of the suspicion and assign the patients to one of the following two groups:
- •
Group A, corresponding to risk subjects, defined by the presence of at least one of the following circumstances:
- *
Typical urticaria within the first hour after administration of a BLA.
- *
Multiple reactions (two or more) with the same or with different BLAs.
- *
Serious reactions: all types of systemic reactions and non-mild skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis, etc).
- *
Reactions related to BLA administration via the parenteral route.
- *
- •
Group B, corresponding to low risk subjects, defined by the absence of all of the above-mentioned circumstances. These are patients with a single non-serious episode and non-immediate skin manifestations (exanthema, urticaria, angio-oedema, etc.) associated with the oral administration of a BLA.
In group A, a study was recommended based on the short algorithm proposed by the European Network for Drug Allergy (ENDA) / European Academy of Allergy and Clinical Immunology (EAACI), except when contraindicated due to the seriousness of the reaction.9 The study sequentially comprised the determination of specific IgE (at least for penicillin G and amoxicillin), skin tests (prick and intradermal with PPL, MDM, penicillin and the implicated antibiotic at the maximum concentrations suggested by ENDA/EAACI) and, if the results proved negative (according to ENDA/EAACI criteria), an open oral provocation test (sequential study). In group B, the recommended study was limited to an open oral provocation test (short study). This test was made in the hospital with the culprit drug at doses of approximately 1/50 the usual single dose, 1/5 the usual single dose, and the usual single dose – administered with a one-hour interval between each dose. In all cases written authorisation was required from at least one of the parents, after having informed them of the available options and of the risks and benefits of each option. In abidance with the legal requirements of the Valencian Community, at least one day was required to elapse between parent authorisation and conduction of the study.10
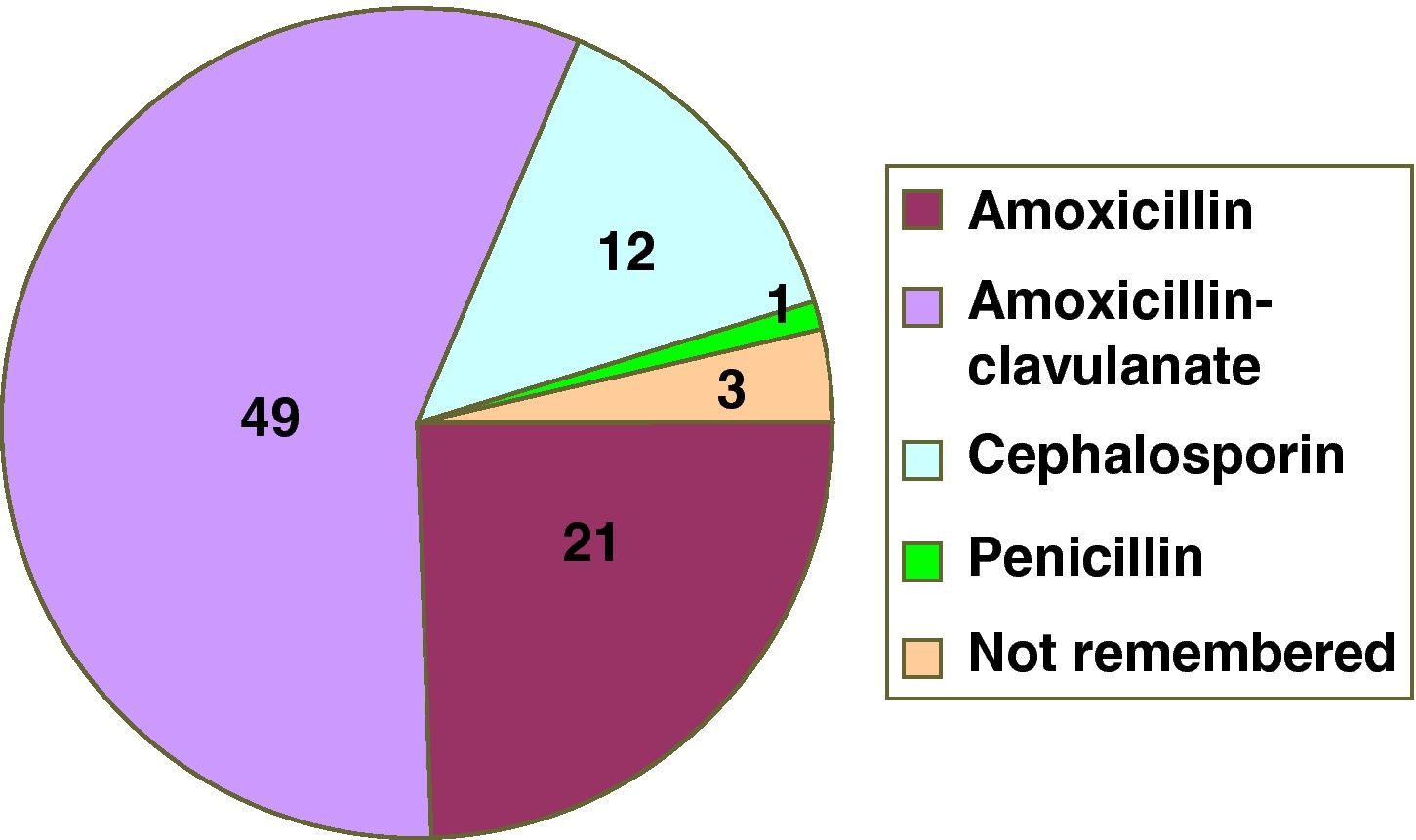
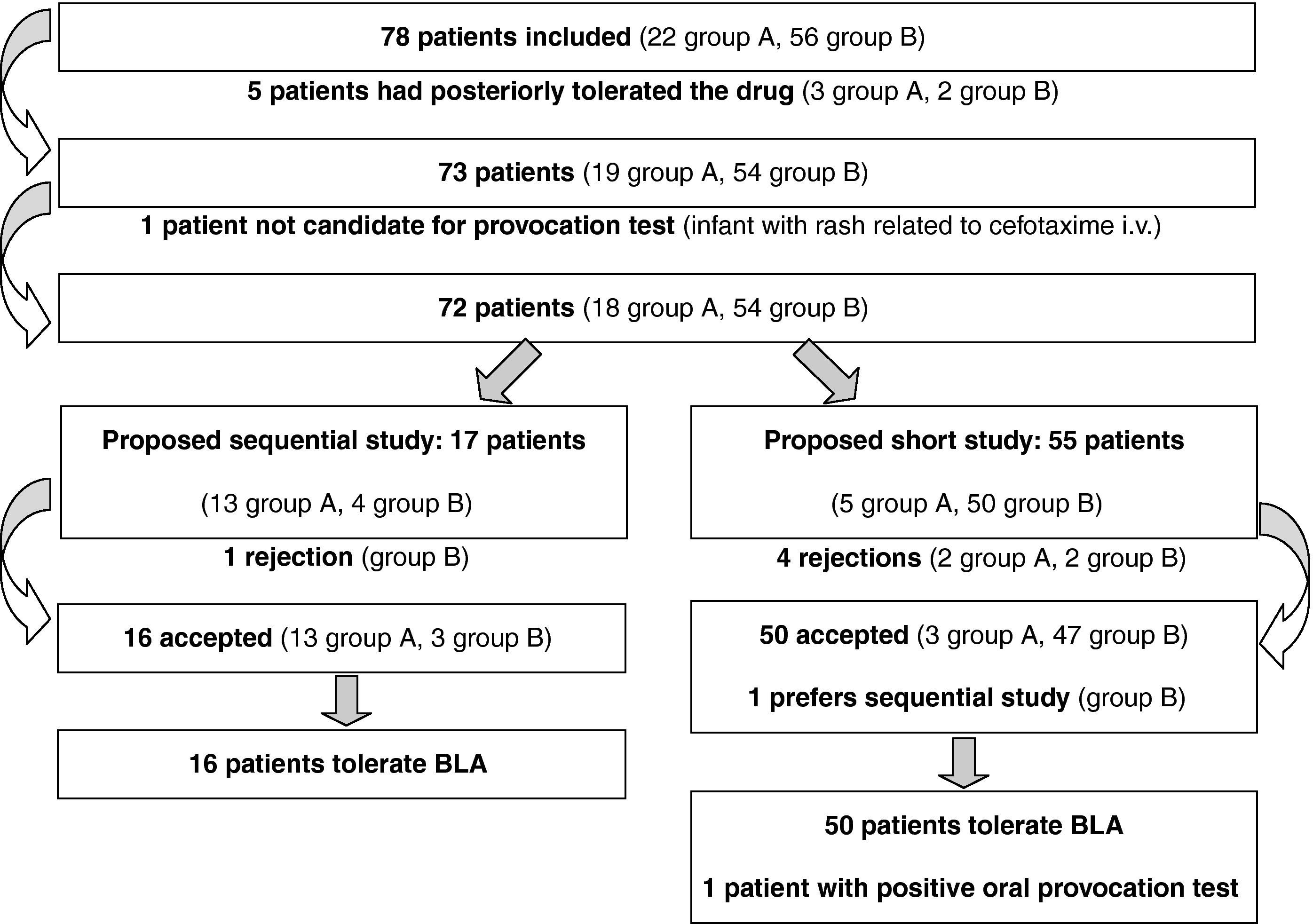
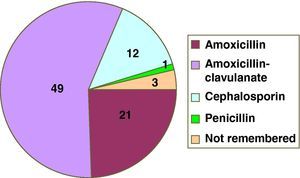
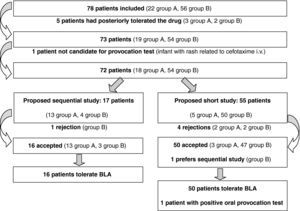
ResultsIn the first year of application of the described protocol, we attended 78 children referred due to suspected HS to BLA. There were 37 males and 41 females, with a mean age of 3.4 years at the time of the suspect episode and of 6.7 years at the time of consultation. The implicated drugs are reported in Figure 1. Twenty-two patients were assigned to group A (Table 1) and 56 (72%) to group B. Compilation of the case history in turn showed that five patients had tolerated the suspect BLA at some point in time following the episode (three in group A and two in B) (Figure 2). One patient of one year of age suffered a suspect reaction with cefotaxime via the intravenous route, and a sequential study was made with oral provocation with amoxicillin and cefixime, but testing with cefotaxime was not considered advisable. The short study was proposed to 55 patients, including five patients in group A. Of these, 50 accepted, one preferred the sequential study, and four rejected or failed to report for the study. The sequential study was proposed in 17 patients (13 in group A and 4 in group B). Of these, 16 accepted and one rejected the study. The skin and specific IgE tests were negative in the 17 patients subjected to the sequential study. Of the 67 patients who completed the study, oral provocation proved negative in 66. The only diagnosis of hypersensitivity to amoxicillin corresponded to a two-year-old girl with a history of two episodes of rash during two treatments (with amoxicillin and with amoxicillin - clavulanate). In the oral provocation test she developed a late reaction consisting of mild generalised erythema after eight hours, without itching. As a result, the patient was re-evaluated with a sequential study. Although the specific IgE and skin tests were negative (including the late reading after 48hours), the repeat oral provocation test elicited a reaction similar to the previous one – the patient therefore being diagnosed as hypersensitive. The final results of the study of the 78 patients are reported in Table 2.
Reasons for the assignment of 22 patients to group A (risk subjects) for true HS to BLA.
| • Multiple (more than one) episodes: 12 |
| ∘ With the same BLA: 7 |
| ∘ With different BLAs: 5 |
| • Immediate-type reaction: 9 |
| • Parenteral administration: 3 |
| • Severe reaction: 0 |
Two patients presented two risk factors: multiple reactions and immediate-type reaction in one case, and immediate-type reaction and parenteral administration in the other.
Results obtained in the 78 patients studied for suspected hypersensitivity to betalactam antibiotics.
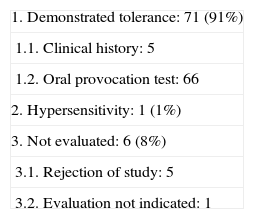
| 1. Demonstrated tolerance: 71 (91%) |
| 1.1. Clinical history: 5 |
| 1.2. Oral provocation test: 66 |
| 2. Hypersensitivity: 1 (1%) |
| 3. Not evaluated: 6 (8%) |
| 3.1. Rejection of study: 5 |
| 3.2. Evaluation not indicated: 1 |
As in many other aspects of medical and allergological practice, children are not small adults. The study protocols proposed by the main expert groups and warranted by the scientific societies are based on experiences and studies from adult populations.11 Studies in children are fewer, and have not allowed the development of specific protocols for securing a correct diagnosis.
However, paediatric allergology clinics receive an important number of patients requiring evaluation for suspected hypersensitivity (HS) to drugs – very particularly including betalactam antibiotics (BLAs). Our own experience and that of other authors published in the medical literature indicate that most suspected reactions are not confirmed. However, drawing this conclusion is costly for both the families of the patients and for the healthcare system, particularly because of the time consumed and the visits required. Our protocol aims to reduce this cost without compromising patient safety.
The protocols recommended by the expert groups of the different allergological societies imply several stages with the aim of detecting sensitised patients in which oral provocation is not considered indicated. These protocols are designed to reduce the risks for the patient, but also hide deleterious effects that are more patent in the case of children. In effect, given the extremely low true incidence of the problem in childhood, exhaustive patient study poses two significant inconveniences: the excessive cost of the study, which affect the families and the healthcare system, and the risk of false-positive results.
The excess cost of a study based on the official recommendations has not been quantified, but it is easy to understand on analysing the number of visits, the healthcare professionals involved, and the material resources required. Our protocol has allowed 49 patients (73% of all those who completed the study) to avoid blood and skin tests. Apart from the direct cost savings and patient discomfort avoided, the families have had to visit the hospital less than half as often, and the associated time saved has been made available for the care of other patients. All this has afforded non-quantified satisfaction for both the families of the children, and the professionals implicated.
On the other hand, all the patient studies culminated in oral provocation testing, which proved negative in 66 of the 67 patients. In the event the sequential study had been made and some patients were sensitised to BLAs, an erroneous diagnosis of allergy would have been established. We do not know the sensitivity and specificity of the skin tests and of the determination of specific IgE in children, and thus of the corresponding positive and negative predictive values – these parameters moreover being conditioned by the true prevalence of BLA allergy, which is undoubtedly very low. In one study, up to 10% of the children were sensitised to BLAs without a prior history of allergy.12 In fact, most subjects diagnosed with allergy to BLA are diagnosed on the basis of the results of the mentioned tests, rather than on provocation testing.13,14
Our previous and current experiences suggest that patient safety is not compromised by this short protocol. Even the patients in group A were seen to tolerate provocation with the drug, thus indicating the low specificity of the criteria considered to define risk (immediate or multiple reactions) in detecting subjects with HS to BLAs.
The main limitation of our study is the small number of patients studied. However, our pilot experience offers support for continuing this line of work, and adds to similar experience gained in predominantly adult populations.15 Although it is not possible to quantify the risk associated with this study procedure, our data suggest that the risk is reasonable and no greater than that involved in other more laborious protocols.
Our prior experience and the studies made in children show positive oral provocation reactions to be mild in all cases,8,16 with a risk possibly lower than that assumed in provocation testing with foods. The contradictory fact that some patients in group A were subjected to the short protocol may be due to specific factors in their clinical histories that led the clinician and family to choose this option. Our study does not allow the detection of patients with late-type HS to BLAs, which is both infrequent and mild.8,16 On the other hand, the procedure which allows us to guarantee the absence of late-type HS, particularly as regards the duration of exposure, has not been established. Thus, considering the above, we do not feel it justified to prolong exposure of the child to the suspect BLA in order to complete the study. By discarding immediate or serious HS to the BLA, the patient is allowed to use the medication under real life conditions (with medical indication), with sufficient safety. If exposure of this kind does not cause symptoms, use of the medication is completely safe. In contrast, if new suspect symptoms develop, the patient should be seen again in the specialised clinic to evaluate their importance and programme whatever study is considered opportune.
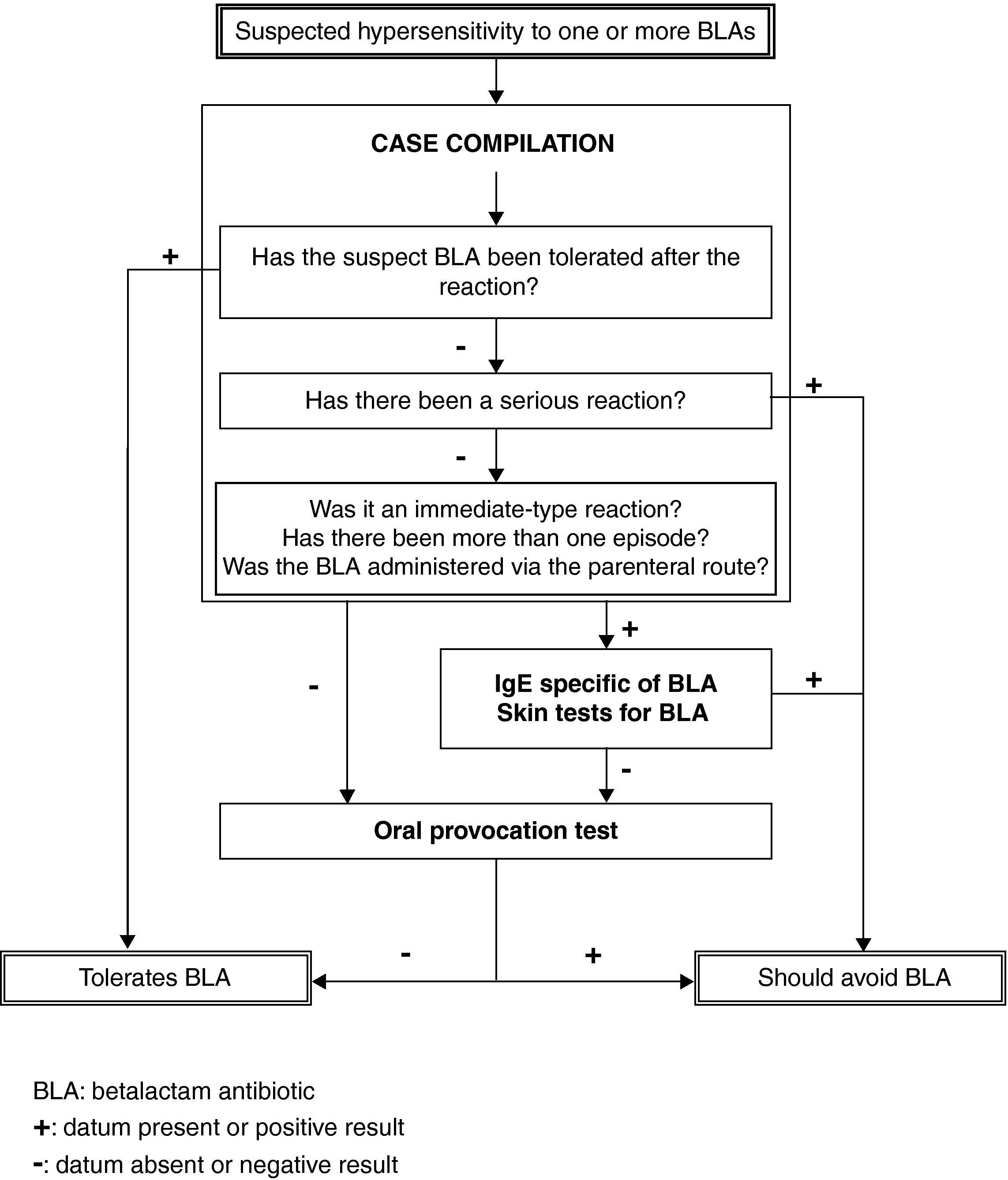
Based on our prior experience, enriched by the present study and backed by the articles published by other authors, we propose an algorithm for the study of paediatric patients with suspected HS to BLA, as shown in Figure 3. This algorithm allows application of a short protocol to a large proportion of patients, with a reasonable level of safety, sparing families and the healthcare system from excessive dedication to a problem which in any case is generally shown to be non-existent.
Financial supportThis study has received no external funding, but has been awarded the second prize for the best oral communication at the XXXIV Congress of the Spanish Society of Pediatric Allergy and Clinical Immunology.
Conflicts of interestNo specific conflict of interest can be detected.