Connective tissue diseases are inflammatory, autoimmune diseases and threaten quality of life. To determine the relationship between staining patterns of antinuclear antibodies and antibodies against extractable nuclear antigens in patients with connective tissue disease.
Materials and methodsObservational, basic, analytical and transversal study. Study conducted in the Immunology Service of the Arzobispo Loayza National Hospital between January 2017 and June 2017. We analyzed 291 samples of patients with CTD and for the detection of anti-nuclear antibody staining patterns, the immunological kit and observation with microscope of at 40X Immunofluorescence and for the detection of the antibodies against extractable nuclear antigens. The Immunoblot method was employed. Statistical analyses were carried out with the statistical package SPSS version 21 for Windows. We used the Pearson Chi-square test for the categorical variables, a value of p<0.05 was considered significant.
ResultsThere was a significant relationship p<0.05 of the homogeneous pattern, the mottled pattern with Anti-histones (p=0.000), Anti-nucleosomes (p=0.000), Anti-Ro 52 (p=0.000), Anti-SSA (p=0.001), Anti-SSB (p=0.003), Anti-dsDNA (p=0.000) with the Pearson Chi-square test. There was a significant relationship of p<0.05 of the centromeric pattern with Anti-Cenp B (p=0.000) with Fisher⿿s exact statistic.
ConclusionsThere was a significant relationship between the anti-nuclear antibody staining patterns and the antibodies to the core extractable antigens in patients with systemic lupus erythematosus, Sjögren⿿s syndrome, Calcinosis, Raynaud⿿s phenomenon, esophageal Dysmotility, sclerodactyly and Telangiectasia (CREST), Scleroderma and Polymyositis.
Connective tissue diseases are inflammatory and autoimmune diseases; the best known are: systemic lupus erythematosus (SLE), progressive systemic sclerosis (ESP), polymyositis (PM), dermatomyositis (DM), rheumatoid arthritis (RA), Sjögren⿿s syndrome (SS) and mixed tissue disease connective (MCT); the most frequent symptoms are: joint pain, joint stiffness, fatigue. They threaten life and quality of life.1,2 The study of antinuclear antibodies (ANA) began with the identification of patients with lupus erythematosus cells.3 These antibodies react against nucleic acids, chromatin and ribo nucleoproteins.4 The technique used for the detection of these antibodies is indirect immunofluorescence; the technique uses cuts from various tissues or the tumor cell line (HEp-2) of the human laryngeal epithelioma as antigen.5,6
In indirect immunofluorescence (IFA), undefined antigens are recognized by antibodies that are present in the patient⿿s serum and offer certain patterns that are interpreted in relation to their association with diseases.7 There are five basic types of fluorescence patterns: homogeneous, mottled, peripheral (ring), centromeric, and nucleolar.8
At the Dos de Mayo National Hospital, 74 cases with diagnostic presumption of connective tissue disease were studied between February 2005 and February 2006: in 48 cases (65%) collagen disease was confirmed; 25 (34%) presented SLE, eight (11%) rheumatoid arthritis, five (7%) MCTD, four (5%) Discoid lupus, three (4%) Scleroderma, two (3%) CREST and one (1%) Sjögren⿿s syndrome. Patients with lupus erythematosus presented 40% mottled pattern, 80% of patients with MCTD had a mottled pattern, 100% of patients with CREST presented a centromeric pattern, 100% of patients with Sjögren⿿s syndrome presented a mottled pattern.9
Autoantibodies directed against extractable nuclear antigens; reactivity in these antigens is more disease specific than the patterns discussed above and can also provide useful clinical prognostic information.10 These antibodies are detected using the immunoblot that has membranes (of nitrocellulose) with the antigens already transferred, the immunocomplexes are identified with specific antibodies marked with enzymes and visualised by bands.11
At the Pablo Tobón Uribe Hospital in Medellin, Colombia, 72 samples were analysed by indirect immunofluorescence, and 37 (51.38%) showed fluorescence for antinuclear antibodies. The predominant fluorescence patterns in the patients studied were the homogeneous pattern in 17 patients (45.94%), the mottled pattern in 11 patients (29.72%) and the cytoplasmic pattern in six patients (16.21%). Antibodies that were detected by linear immunoassay were anti-nucleosomes in 20 (52.6%) samples, anti-dsDNA in 15 (39.47%), anti-Sm in 11 (28.94%) samples; the anti-U1s- nRNP, anti-SSA / Ro60, anti-SSB / La in four (10.52%) and anti-Jo1 in two (5.26%) samples.12
Bovone et al. published a study with the objective of evaluating the probability of detecting fine reactivity by a commercial linear immunoassay on serum samples that were IFI positive in the screening, and studying their correlation with the patterns and titters observed. They retrospectively analysed 161 patient serum samples between August 1999 and February 2002 that were positive by IFI and were also processed by LIA. Imprints of Hep-2 (Kallestad) and Innogenetic Innolia-ANA commercial equipment were used. It was concluded that the samples with a homogeneous pattern presented positivity by linear immunoassay in 60% with prevalence of anti-histones and Anti-dsDNA. The mottled pattern positivity was 73%. The speckled pattern was more strongly associated with the presence of anti-ENA antibodies.13
In 2014, a study was published with the objective of identifying an association between the immunofluorescence patterns of antinuclear antibodies in the HEp-2 cell and more specific antinuclear reactivities in the serum samples of patients with connective tissue disorders. The 152 samples were subjected to ANA tests by indirect immunofluorescence (IIF) in HEp-2 cells (ALPHADIA) at 1:40 dilution, anti-dsDNA by ELISA and anti-extractable nuclear antigen (anti-ENA) by Dot Immunoblot. The dot strips were analysed for anti-Sm, anti-RNP, anti-SSA / Ro, anti-SSB / La, anti-Scl-70 and anti-Jo-1. Of 152 patients, 110 (72.3%) cases were ANA positive for IIF in HEp-2 cells. The ANA-positive sera exhibited four fluorescence patterns: mottled (50.8%); peripheral (21.6%); homogeneous (18.1%); and nucleolar (9%). The peripheral pattern and homogeneous pattern were predominantly associated with anti-dsDNA (p<0.05). The mottled pattern was significantly associated with anti-ENA (p<0.05). It was concluded that peripheral and homogeneous patterns were strongly associated with anti-dsDNA and the mottled pattern can predict anti-ENA (especially ribo nucleoproteins).14
Studies of this type are practical for the diagnostic help of connective tissue diseases.
The objectives of the present study were to determine the relationship between staining patterns of antinuclear antibodies and antibodies against extractable nuclear antigens in patients with connective tissue disease.
Subjects and methodsAn observational, analytical and cross-sectional study was conducted at the Immunology Service of the Arzobispo Loayza National Hospital between January and June 2017. A total of 291 clinical histories were reviewed and blood samples from patients with connective tissue disease were processed. For the detection of staining patterns of antinuclear antibodies in the patients⿿ sera, the immunological kit and observation with a 40x immunofluorescence microscope were used. For the detection of antibodies against extractable nuclear antigens in patients⿿ sera, the Immunoblot method was used.
This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, was evaluated and approved by the research ethics committee of the Institute of Ethics in Health of the National University of San Marcos Act No. 1811 with Project Code No. 0014; the research work was approved by the Institutional Research Committee and the General Directorate of the Arzobispo Loayza National Hospital with File N ° 007645.
Indirect Immunofluorescence Methodology (IFI- ANA)Preparation of the buffer: Dilute an envelope of PBS in one litre of distilled water and add a vial of tween 20, shake gently. Preparation of the sample (serum): Dilute 990μl of buffer with 10μl of sample (1: 100). Homogenize the tubes in the vortex for approximately five seconds. Place 30μl of the diluted serum immediately, making sure to match the slot in the plate, incubate for 30min. Wash with the buffer, rinsing the sheet previously and then leaving it for five minutes in the Coplin bottle. Load 30μl of conjugate corresponding to the Hep 20-10 Euroimmun AG (GERMANY) kit in each quadrant of the Tray plate. Immediately place the corresponding stamp making sure that the plate slot matches. Incubate for 45min, protecting from exposure to light. Then wash using the buffer, rinsing the sheet previously, leaving it for 6min in the bottle of Coplin. Add one drop of glycerol and place the cover slip. Observe the fluorescence microscope at a 40ÿ magnification. Important: Standardized positive control was placed on each imprint, human serum with antinuclear antibodies (ANA) homogeneous grade IV pattern, sodium azide 0.95g/L. The specificity of the ANA positive Control is verified against the reference serum AF/CDC1 of the Centres for Disease Control (CDC), Atlanta, USA and standardised negative control was placed on each imprint, negative human serum to infectious screening and Antinuclear antibodies negative. The imprints were numbered in case of running more than 10 samples and quickly to avoid desiccation of the imprint.
Immunoblot methodology (anti-ENA)Preparation of the buffer: The concentrated buffer of the work is diluted 1/10 with distilled water.
Preparation of the sample (serum): Dilute 1500μl of buffer with 15μl of sample (1: 101). Activate the strips by taking them to a plastic support and placing 1600μl of buffer. Homogenize in rotator for six minutes. Remove the buffer and place 1515μl of the diluted sample, leave it in the rotator for 30min. Remove the existing content and proceed to perform the washing. Placing 1500μl of buffer for five minutes in rotation. Repeat this procedure twice more. Remove the contents and place 1500μl of conjugate (ready to use) corresponding to the ANA prolife 3 Euroline Euroimmun (GERMANY) kit. Leave it for 30min on the rotator. Remove the content and proceed to perform the washing. Place 1500μl of buffer for five minutes in rotation. Remove the content and place 1500μl of substrate (ready to use). Leave it for 10min on the rotator in darkness. Remove the existing content and proceed to perform the washing. Placing 1500μl of distilled water for five minutes in rotation. Repeat this procedure twice more. Dry on stove and read the scanner. Important: Standardised positive control was placed on the Immunoblot reaction strip, positive control was human serum with antibodies anti-SSA (Ro), anti-SSB (La), anti-Sm, anti-Sm/RNP, antiJo1 and anti-Scl70, sodium azide 15mmol/L. Calibrated in front of the Corresponding ANA reference serum from the Centres for Disease Control (CDC), Atlanta, USA and standardised negative control, negative human serum to infectious and negative AAN screening were placed.
Statistical methodsAll the statistical analyses were carried out with the statistical package SPSS version 21 for Windows; we used the Pearson Chi-square test for the categorical variables, a value of p<0.05 was considered significant.
ResultsA total of 291 serum samples from patients with connective tissue disease were analysed, clinical histories were reviewed and there was a significant relationship between P<0.05 of the homogeneous pattern and Anti-histones (X2=47.36, p=0.000; OR=7.8), Anti-nucleosomes (X2=45.47, p=0.000, OR=7.76), Anti-dsDNA (X2=32.04, p=0.000, OR=4.66) (See Table 1), Anti-Cenp B (X2=19.33, p=0.000, OR=0.076), Anti-SSA (X2=11.09, p=0.001, OR=0.38), Anti-SSB (X2=9.05, p=0.003, OR=0.28), Anti-SM (X2=4.57, p=0.037, OR=1.79) and Anti-Ro 52 (X2=25.41, p=0.000). ; OR=0.27) (See Table 2) with the Pearson Chi-square test. There was no significant relationship p>0.05 of the homogeneous pattern with the Anti-Rib p. protein (X2=4.11, p=0.064, OR=2.04), Anti- M2 (X2=0.13, p=0.805, OR=0.82), Anti- RNP / SM (X2=0, 42, p=0.526, OR=1178), Anti-Scl (X2=2.41, p=0.126, OR=1.78), Anti-Jo1 (X2=0.27, p=0.792, OR=0, 74) with Pearson⿿s Chi-square test. There was no significant relationship p>0.05 of the homogeneous pattern with the Anti-PCNA (p=0.330, OR=0.40) and Anti-PM100 (p=0.156, OR=2.86) with Fisher⿿s exact statistic.
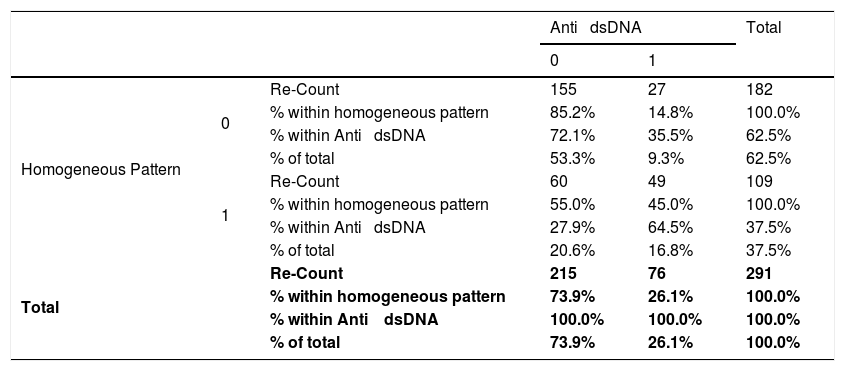
Relationship between the homogeneous pattern and anti-dsDNA in patients with connective tissue disease attended at the national Hospital Archbishop Loayza between January and June 2017.
| Anti ⿿ dsDNA | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | ||||
| Homogeneous Pattern | 0 | Re-Count | 155 | 27 | 182 |
| % within homogeneous pattern | 85.2% | 14.8% | 100.0% | ||
| % within Anti ⿿ dsDNA | 72.1% | 35.5% | 62.5% | ||
| % of total | 53.3% | 9.3% | 62.5% | ||
| 1 | Re-Count | 60 | 49 | 109 | |
| % within homogeneous pattern | 55.0% | 45.0% | 100.0% | ||
| % within Anti ⿿ dsDNA | 27.9% | 64.5% | 37.5% | ||
| % of total | 20.6% | 16.8% | 37.5% | ||
| Total | Re-Count | 215 | 76 | 291 | |
| % within homogeneous pattern | 73.9% | 26.1% | 100.0% | ||
| % within Anti ⿿ dsDNA | 100.0% | 100.0% | 100.0% | ||
| % of total | 73.9% | 26.1% | 100.0% | ||
Absence=0; Presence=1; IC=95%; Chi-squared=32.04; p=0.001; Odds Ratio=4.66; Lower trust limits=2.68; Upper confidence limits=8.2.
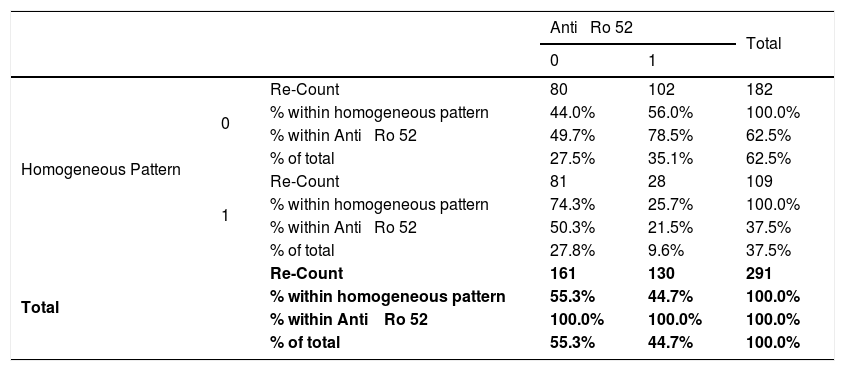
Relationship between the homogeneous pattern and anti-Ro 52 in patients with connective tissue disease attended at the national Hospital Archbishop Loayza between January and June 2017.
| Anti ⿿ Ro 52 | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | ||||
| Homogeneous Pattern | 0 | Re-Count | 80 | 102 | 182 |
| % within homogeneous pattern | 44.0% | 56.0% | 100.0% | ||
| % within Anti ⿿ Ro 52 | 49.7% | 78.5% | 62.5% | ||
| % of total | 27.5% | 35.1% | 62.5% | ||
| 1 | Re-Count | 81 | 28 | 109 | |
| % within homogeneous pattern | 74.3% | 25.7% | 100.0% | ||
| % within Anti ⿿ Ro 52 | 50.3% | 21.5% | 37.5% | ||
| % of total | 27.8% | 9.6% | 37.5% | ||
| Total | Re-Count | 161 | 130 | 291 | |
| % within homogeneous pattern | 55.3% | 44.7% | 100.0% | ||
| % within Anti ⿿ Ro 52 | 100.0% | 100.0% | 100.0% | ||
| % of total | 55.3% | 44.7% | 100.0% | ||
Absence=0; Presence=1; CI=95%; Chi-square=25. 42; p=0. 001; Odds Ratio=0. 27.
Inferior Confidence Limits=0. 16; Upper Confidence Limits=0. 45.
There was a significant relationship of p<0.05 of the mottled pattern with Anti-histones (X2=10.26, p=0.002, OR=0.379), Anti-nucleosomes (X2=112.82, p=0.001, OR=0.342), Anti-Ro 52 (X2=52.65, p=0.000, OR=6.20) (See Table 3), Anti-Cenp B (X2=11.28, p=0.001, OR=0.26), Anti -SSA (X2=25.42, p=0.000, OR=3.82), Anti-dsDNA (X2=6.13, p=0.016, OR=0.50), Anti-RNP / SM (X2=5, 65, p=0.019, OR=1.80) and Anti-SSB (X2=23.57, p=0.000, OR=6.22) (See Table 4) with the Chi-square Pearson test. There was no significant relationship p>0.05 of the mottled pattern with the Anti-Rib p protein (X2=0.67, p=0.476, OR=1.33), Anti-M2 (X2=1.31, p=0.331 ; OR=0.55), Anti-Scl (X2=0.10, p=0.852, OR=0.88), Anti-Jo1 (X2=3.11, p=0.120, OR=0.36), Anti-SM (X2=0.55, p=0.892, OR=0.93) with the Chi-square Pearson test. There was no significant relationship p>0.05 of the mottled pattern with the Anti-PCNA (p=0.348, OR=0.48) and Anti-PM100 (p=0.072, OR=0.15) with Fisher⿿s exact statistic.
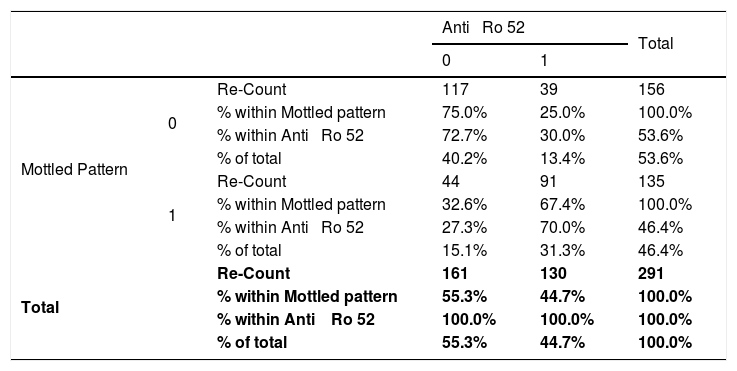
Relationship between the mottled pattern and anti-Ro 52 in patients with connective tissue disease attended at the national Hospital Archbishop Loayza between January and June 2017.
| Anti ⿿ Ro 52 | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | ||||
| Mottled Pattern | 0 | Re-Count | 117 | 39 | 156 |
| % within Mottled pattern | 75.0% | 25.0% | 100.0% | ||
| % within Anti ⿿ Ro 52 | 72.7% | 30.0% | 53.6% | ||
| % of total | 40.2% | 13.4% | 53.6% | ||
| 1 | Re-Count | 44 | 91 | 135 | |
| % within Mottled pattern | 32.6% | 67.4% | 100.0% | ||
| % within Anti ⿿ Ro 52 | 27.3% | 70.0% | 46.4% | ||
| % of total | 15.1% | 31.3% | 46.4% | ||
| Total | Re-Count | 161 | 130 | 291 | |
| % within Mottled pattern | 55.3% | 44.7% | 100.0% | ||
| % within Anti ⿿ Ro 52 | 100.0% | 100.0% | 100.0% | ||
| % of total | 55.3% | 44.7% | 100.0% | ||
Absence=0; Presence=1; IC=95%; Chi-squared=52.65; p<0.001; Odds Ratio=6.20; Lower trust limits=3.72; Superior Confidence limits=10.34.
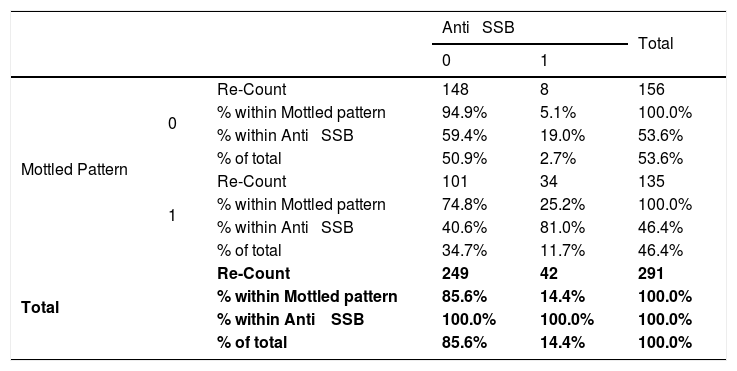
Relationship between the mottled pattern and the anti-SSB in patients with connective tissue disease attended at the national Hospital Archbishop Loayza between January and June 2017.
| Anti ⿿ SSB | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | ||||
| Mottled Pattern | 0 | Re-Count | 148 | 8 | 156 |
| % within Mottled pattern | 94.9% | 5.1% | 100.0% | ||
| % within Anti ⿿ SSB | 59.4% | 19.0% | 53.6% | ||
| % of total | 50.9% | 2.7% | 53.6% | ||
| 1 | Re-Count | 101 | 34 | 135 | |
| % within Mottled pattern | 74.8% | 25.2% | 100.0% | ||
| % within Anti ⿿ SSB | 40.6% | 81.0% | 46.4% | ||
| % of total | 34.7% | 11.7% | 46.4% | ||
| Total | Re-Count | 249 | 42 | 291 | |
| % within Mottled pattern | 85.6% | 14.4% | 100.0% | ||
| % within Anti ⿿ SSB | 100.0% | 100.0% | 100.0% | ||
| % of total | 85.6% | 14.4% | 100.0% | ||
Absence=0; Presence=1; IC=95%; Chi-squared=23.57; p<0.001; Odds Ratio=6.22; Lower trust limits=2.76; Upper Trust limits=14.00.
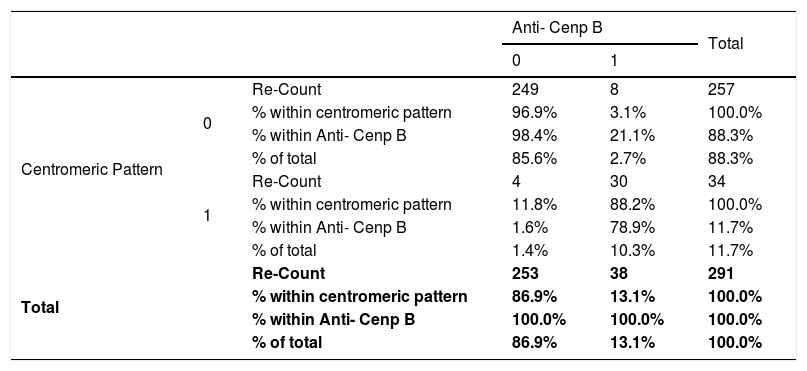
There was a significant relationship of p<0.05 of the centromeric pattern with the Anti-nucleosomes (X2=5.10, p=0.023, OR=0.21), Anti-Ro 52 (X2=9.03, p=0.003;=0.28), Anti-SSA (X2 = 4.23, p=0.046, OR=0.36), Anti-RNP / SM (X2=8.71, p=0.003, OR=0.22), Anti-dsDNA (X2=5.96, p=0.013, OR=0.24) with the Chi-square Pearson test. There is a significant relationship between p<0.05 of the centromeric pattern with Anti- Cenp B (p=0.000, OR=233.43) (See Table 5), Anti- M2 (p=0.045, OR=3.23) with the statistic exact from Fisher. There was no significant relationship p>0.05 of the centromeric pattern with Anti- histones (X2 = 3.73, p=0.074, OR=0.31), Anti-Sm (X2=2.20, p=0.205;=0.479) with the Chi-square test of Pearson and there was no relationship with the Anti-Rib p protein (p=0.095, OR=0.19), Anti SSB (p=0.192, OR=0.33), Anti- Scl (p=0.776, OR=1.09), Anti Jo1 (p=0.704, OR=0.48), Anti PCNA (p=1000, OR=0.83), Anti-PM100 (p=1000; OR=1.08) with Fisher⿿s exact statistic.
Relationship between the centromeric pattern and the anti-CENP B in patients with connective tissue disease attended at the national Hospital Archbishop Loayza between January and June 2017.
| Anti- Cenp B | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | ||||
| Centromeric Pattern | 0 | Re-Count | 249 | 8 | 257 |
| % within centromeric pattern | 96.9% | 3.1% | 100.0% | ||
| % within Anti- Cenp B | 98.4% | 21.1% | 88.3% | ||
| % of total | 85.6% | 2.7% | 88.3% | ||
| 1 | Re-Count | 4 | 30 | 34 | |
| % within centromeric pattern | 11.8% | 88.2% | 100.0% | ||
| % within Anti- Cenp B | 1.6% | 78.9% | 11.7% | ||
| % of total | 1.4% | 10.3% | 11.7% | ||
| Total | Re-Count | 253 | 38 | 291 | |
| % within centromeric pattern | 86.9% | 13.1% | 100.0% | ||
| % within Anti- Cenp B | 100.0% | 100.0% | 100.0% | ||
| % of total | 86.9% | 13.1% | 100.0% | ||
Absence=0; Presence=1; IC=95%; Fisher⿿s exact test p<0.001; Odds Ratio=233.43; Lower trust limits=66.31; Superior Confidence limits=821.79.
There was a significant relationship between p<0.01 of the cytoplasmic pattern with Anti-M2 (p=0.002, OR=6.68) and Anti-Jo1 (p=0.000, OR=20.81) with Fisher⿿s exact statistic. There was no significant relationship between p>0.05 of the cytoplasmic pattern with Anti-histones (X2=0.65, p=0.447, OR=1.45), Anti- nucleosomes (X2 = 2.16, p=0.192, OR).=1.93), Anti Ro 52 (X2=0.005, p=1000, OR=0.97), Anti-SSA (X2=0.04, p=1000, OR=0.90), Anti-RNP / SM (X2=0.49, p=0.660, OR=0.72), Anti-dsDNA (X2=0.49, p=0.481, OR=1.37), Anti-Sm (X2=0.017, p=1000; OR=0.93) with the Pearson Chi-square test.
There was no significant relationship p>0.05 of the cytoplasmic pattern with Anti-Cenp B (p=1000, OR=0.90), Anti-Rib p protein (p=0.213, OR=1.89), Anti-SSB (p=0.551, OR=0.49), Anti-Scl (p=0.333, OR=0.31), Anti-PCNA (p=0.599, OR=1.19), Anti-PM100 (p=0.517; OR=1.54) with Fisher⿿s exact statistic.
There was a significant relationship between p<0.05 of the nucleolar pattern with the Anti-RNP / SM (p=0.030, OR=0.64), Anti-Scl (p=0.010, OR=7.2), Anti-PM100 (p=0.001, OR=27.70) with Fisher⿿s exact statistic. There was no significant relationship p>0.05 of the nucleolar pattern with Anti-histones (p=0.213, OR=0.77), Anti-nucleosomes (p=0.691, OR=0.47), Anti Ro-52 (p=0.519, OR=1.57), Anti-Cenp B (p=0.611, OR=0.86), Anti-Rib p protein (p=0.60, OR=0.87), Anti-SSA (p=0.459, OR=1.91), Anti-M2 (p=0.442, OR=1.94), Anti-SSB (p=1000, OR=0.73), Anti-dsDNA (p=0.454, OR=0.34), Anti-Jo1 (p=1000, OR=0.94), Anti-Sm (p=1000, OR=0.84), Anti-PCNA (p=1000, OR=0.96) with Fisher⿿s exact statistic.
There was no significant relationship p>0.05 of the NUMA-1 standard with Anti-histones (p=1000, OR=0.78), Anti-nucleosomes (p=1000, OR=0.79), Anti Ro-52 (p=0.588, OR=2.50), Anti-Cenp B (p=1000, OR=0.86), Anti-Rib p protein (p=0.328, OR=3.61), Anti-SSA (p=0.214, OR=4.77), Anti-M2 (p=1000, OR=0.93), Anti-SSB (p=1000, OR=0.85), Anti-RNP / SM (p=0.554; OR=0.65), Anti-dsDNA (p=0.570, OR=0.73), Anti-Scl (p=1000, OR=0.88), Anti-Jo1 (p=1000, OR=0.94).), Anti-Sm (p=0.575, OR=0.74), Anti-PCNA (p=1000, OR=0.96), Anti PM 100 (p=1000, OR=0.97) with Fisher⿿s exact statistic.
There was no significant relationship p>0.05 of the lysosome pattern with Anti-histones (p=1000, OR=0.78), Anti-nucleosomes (p=1000, OR=0.79), Anti Ro-52 (p=1000, OR=0.55), Anti-Cenp B (p=1000, OR=0.86), Anti-Rib p protein (p=1000, OR=0.87), Anti-SSA (p=1000) OR=0.70), Anti-M2 (p=1000, OR=0.066), Anti-SSB (p=1000, OR=0.85), Anti-RNP / SM (p=0.344, OR=0, 34), Anti-dsDNA (p=1000, OR=0.73), Anti-Scl (p=1000, OR=0.89), Anti-Jo1 (p=1000, OR=0.94), Anti- Sm (p=0.251; OR=0.248), Anti-PCNA (p=1000, OR=0.96), Anti PM 100 (p=1000, OR=0.972) with Fisher⿿s exact statistic.
DiscussionAutoimmune diseases are a group of disorders of high clinical complexity, unknown etiology and difficult diagnosis, caused by the loss of immunological tolerance mechanisms, with the capacity to produce autoantibodies that act against their own cellular structures, causing local or systemic damage that lasts in time. The estimated worldwide incidence for this type of diseases is 90 / 100,000 people per year with prevalences ranging from 3 to 5% of the general population.15
The detection of antinuclear antibodies with Indirect Immunofluorescence in the laboratory in this group of patients with connective tissue disease provides a useful element, since in many cases it allows to shorten the time of diagnosis and the start of treatment. In our investigation, the cut-off titration, established for the initial diagnosis of patients with clinical suspicion of autoimmune disease, was 1: 100 present in all the patients analysed, followed by 1: 160, where all patients were also positive. This cut-off point is currently recommended by the Clinical and Laboratory Standards Institute and by the International Consensus on Antinuclear Antibody Patterns.16⿿18
In our investigation, it was shown that 114 (39.2%), 149 (51.2%), 23 (7.9%) and five (1.7%) patients presented antinuclear antibodies by Indirect Immunofluorescence with the titration 1: 320, 1: 1000.1: 3200 and 1: 10,000 respectively; those that exceed the 1: 160 titration value increase the probability that this will be considered as a criterion of autoimmunity.19,20
In one study conducted of 72 patients with suspected autoimmune disease, 58 were women (80.5%) and 14 were men (19.5%). The patients were between the ages of 21 and 85, and the mean age was 37.2 years; in our research, the determined frequency showed women predominated 217 (74.6%) to men 74 (25.4%), patients had a greater frequency of ages between 38 and 56 years and an average age of 47 years, very similar to that obtained in Colombia by Benitez, 2011.21
The pattern of antinuclear antibodies in our most frequent investigation in the studied population was the mottled pattern, present in 41.9% of the total of patients, similar to the study carried out in Ecuador, where the frequency of the fine mottled pattern was 37.1%22; with a predominance of the female sex with 30.9%, unlike in males, which was 17.24%. In our research, sociodemographic characteristics showed 74.6% women and 25.4% men, because women have greater hormonal variability, related to the production of sex hormones (estrogens and androgens) that can cause immunological sensitisation at a fertile age (prior to menopause),15,23,24 activating the production of self-reactive tolerant lymphocytes, mostly before the age of 50.25,26
The frequency of expressivity of antibodies against extractable nuclear antigens found by Immunoblot was 48 (31.8%) of the 151 positive ANA-IFI patients with clinical suspicion of autoimmune disease,22 this being a minor result, compared with a similar study where 38 (52.7%) of antibody frequency was obtained by Immunoblot for the 72 cases with positive ANA-IFI21; In our study, a relative frequency was determined in 100% of the patients with positive ANA-IFI presented antibodies against removable nuclear antigens by Immunoblot.
The fluorescence of the fine speckling by Indirect Immunofluorescence is characterised by the presence of small fine specks, throughout the nucleoplasm, where its nucleoli may not be stained, as well as the chromatin of mitotic cells and is related to the production of antibodies anti-SS-A / Ro 60KD, anti-SSB / La, anti-Mi-2 and anti-Ku, in diseases such as Sjögren⿿s syndrome, systemic lupus erythematosus, cutaneous lupus, systemic sclerosis and dermatomyositis or polymyositis,18,27 in our investigation showed the significant relationship of the mottled pattern with Anti-histones, Anti-nucleosomes, Anti-Ro 52, Anti-Cenp B, Anti-SSA, Anti-SSB, Anti-dsDNA, Anti-RNP / SM and the mottled pattern was significantly related to Sjögren⿿s syndrome and systemic lupus erythematosus.
The detection of antibodies anti-SS-A / Ro60 KD individually, is associated with the diagnosis of Sjögren⿿s syndrome in patients with photosensitivity, cutaneous lupus, vasculitis and of greater importance in neonatal lupus, because there is trans-placental transfer of these antibodies (maternal IgG), which can cause transient eruptions in the baby, with photosensitivity and congenital heart block, compromising the life of the new-born in 100% of cases.28,29
Followed by the anti-SS-A / Ro52 KD present in 17 cases considering that the difference of this with the SS-A / Ro 60KD is only the protein fraction to which it is directed, but its conceptualisation is the same,30 in our research a relation was found of 91 (31.3%) cases that presented mottled pattern and anti Ro 52; 17 (5.8%) presented mottled pattern and anti-SSA, anti-Ro 52.
In a study conducted in Ecuador by Arevalo, 2018,22 showed that six cases of patients with connective tissue disease with homogeneous pattern and with 66.7% of expressiveness corresponded to the dsDNA antigens, histones, nucleosome. In our research we show the significant relationship of the homogeneous pattern with Anti-histones 47 (16.2%), Anti-nucleosomes 45 (15.5%), Anti-Ro 52 28 (9.6%), Anti-Cenp B 2 (0.7%), Anti-SSA 20 (6.9%), Anti-SSB 7 (2.4%), Anti-dsDNA 49 (16.8%) and Anti-SM 35 (12.0) %) cases with the Pearson Chi-square statistical tests, and the homogeneous pattern was significantly related to systemic lupus erythematosus.
It was shown that there is a significant relationship of homogeneous pattern, mottled pattern, centromeric pattern and cytoplasmic pattern with antibodies against extractable nuclear antigens in patients with connective tissue disease.
In conclusion, antinuclear antibodies can reduce the cost of detailed immunological work with minimal loss in diagnostic accuracy of the disease. The autoantibodies directed against extractable nuclear antigens; the reactivity in these antigens is more specific to the disease than the previously indicated patterns and it was shown that there is a significant relationship of the homogeneous pattern, mottled pattern, centromeric pattern, cytoplasmic pattern with antibodies against removable nuclear antigens in patients with connective tissue disease.
Authorship contributionsJOM participated in the conception, study design, data collection, analysis and interpretation of data, drafting of the article, JOM, JAA, JOC, MGH, LDH performed the critical review of the article. They all approved the final version of the article.
Source(s) of supportThe study was funded by the authors.
Conflicting interest (If present, give more details)None.