LRBA deficiency is caused by loss of LRBA protein expression, due to either homozygous or compounds heterozygous mutations in LRBA. LRBA deficiency has been shown to affect vesicular trafficking and autophagy. To date, LRBA has been observed in the cytosol, Golgi apparatus and some lysosomes in LPS-stimulated murine macrophages. The objectives of the present study were to study the LRBA localization in organelles involved in vesicular traffic, phagocytosis, and autophagy in mononuclear phagocytes (MP).
Materials and methodsWe analyzed LRBA colocalization with different endosomes markets using confocal microscopy in MP. We used the autophagy inhibitors to determine the role of LRBA in formation, maturation or degradation of the autophagosome.
ResultsLRBA intracellular trafficking depends on the activity of the GTPase ADP ribosylation factor-1 (ARF) in MP. LRBA was identified in early, late endosomes but did not colocalize strongly with lysosomal markers. Although LRBA appears not to be recruited during the phagocytic cargo uptake, it greatly colocalized with the microtubule-associated protein 1A/1B-light chain 3 (LC3) under a steady state and this decreased after the induction of autophagy flux. Although the use of inhibitors of lysosome fusion did not restore the LRBA/LC3 colocalization, inhibitors of either early to late endosomes trafficking or PI3K pathway did.
ConclusionsTaken together, our results show that LRBA is located in endomembrane system vesicles, mainly in the early and late endosomes. Although LRBA appears not to be involved in the phagocytic uptake, it is recruited in the early steps of the autophagy flux.
Primary immunodeficiency diseases (PID) result from genetic defects that cause quantitative and/or functional alterations in different mechanisms involved in the immune response.1 LRBA deficiency is a PID caused by loss of LRBA protein expression, which can be caused by either homozygous or compound heterozygous mutations in LRBA.2 At a subcellular level, in LPS-stimulated murine macrophages, LRBA has been reported in the cytosol, Golgi apparatus, endoplasmic reticulum, plasma membrane, clathrin-associated vesicles and lysosomes.3 Furthermore, LRBA is necessary for the vesicular trafficking of CTLA-4.4 This suggests that LRBA might be involved in intracellular vesicular trafficking. The intracellular vesicular network is required for multiple functions and control of homeostasis. This multitude of functions can only be ensured by complex and highly dynamic compartments involving early and late endosomes, recycling endosomes, lysosomes, Golgi apparatus and endoplasmic reticulum.5 On the other hand, Autophagy is a mechanism of lysosomal degradation of cytoplasmic components.6 The autophagy process is initiated when a phagophore engulfs part of the cytoplasm, resulting in the autophagosome. Autophagosomes are formed with the aid of several Atg proteins including Atg8 (also called LC3, microtubule-associated protein 1-light chain 3).7 For maturation, the autophagosome must be fused with early or late endosomes forming a structure called amphisome.8,9 Finally, amphisomes are fused with lysosomes to generate an autolysosome and their contents are thereafter digested by lysosomal proteases, and reused by the cell.9 In immortalized B cells from patients with LRBA deficiency, a significant reduction in autophagy in response to starvation is observed, with the accumulation of the autophagosomes and defective fusion of the autophagosome with lysosomes.10 However, to date, the function of LRBA is not known in the autophagy processes.
The aim of this research was to study the localization of LRBA in organelles involved in vesicular traffic, phagocytosis, and autophagy in mononuclear phagocytes (MP).
MethodsReagents and antibodiesHistopaque-1077, 4 beta-phorbol 12-myristate 13-acetate (PMA), ionomycin, brefeldin A (BFA), RPMI 1640, lipopolysaccharide (LPS), Phosphate-Buffered Saline (PBS), Earle's Balanced Salt Solution 1X (EBSS), Rapamycin, chloroquine, bafilomycin, pepstatin/E64d, 4-bromobenzaldehyde N-(2,6-dimethylphenyl) semicarbazone (EGA), wortmannin, LY294002 and paraformaldehyde were obtained from Sigma Aldrich.
Anti-human CD20 (L27), anti-human CD14 (M5E2), anti-human CD86 (2331), polyclonal anti-human LRBA (HPA019366), polyclonal rabbit anti-human LRBA antibody (HPA023597), anti-EEA-1 (N19), anti-Rab5 (65), anti-Rab7 (117), anti-CD63 (MEN259), polyclonal rabbit anti-human GAPDH, anti-rabbit IgG-peroxidase, anti-Rabbit PE-F(ab)`2 Donkey and polyclonal rabbit anti-human-LC3B antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-human HLA-DR (G46-6) and anti-LAMP1 (MT271) were purchased from Becton Dickinson (BD) Pharmingen (San Diego, CA, USA). Alexa Fluor 488 anti-rabbit IgG (H+L), Alexa Fluor 594 goat anti-mouse IgG (H+L), Hoechst 33,358 and Lysotracker DND-99 were obtained from Molecular Probes (Eugene, OR, USA). Donkey PE anti-Rabbit-F(ab)`2 IgG and anti-human IFN-γ (4SB3) were obtained from eBioscience (Santa Clara, CA, USA). Anti-protein disulfide isomerase, PDI (RL90) was obtained from Abcam, (Cambridge, UK).
Cell differentiation and activationCell cultures were performed with blood from healthy adult donors who were voluntarily recruited. The study was reviewed and approved by the Institutional Review Board from Sede de Investigaci´on Universitaria (SIU), Universidad de Antioquia. Written informed consent was obtained from all participants, according to the Declaration of Helsinki.
Peripheral blood mononuclear cells (PBMC) were obtained from EDTA (Albor Químicos, Bogotá, Colombia) blood samples by centrifugation on Histopaque-1077. To evaluate the intracellular localization of LRBA, 1×106 cells were exposed to PMA/ionomycin (10ng/mL and 1μg/mL, respectively) for 2h, 1mg/mL BFA was added for 4h. Finally, cells were intracellularly stained using the Cytofix/Cytoperm and Perm/Wash buffers (BD), following the manufacturer’s instructions.
To obtain MP, 0.5×106/mL PBMC in RPMI 1640 supplemented with 1 % of autologous serum, were enriched by adherence to plastic plates. Wells were extensively washed to remove non-adherent cells. Adherent cells were cultured in RPMI 1640 supplemented with 10 % autologous serum for 120h to allow differentiation into MP. The purity was evaluated by measuring the percentages of B lymphocytes (CD20+) and monocytes (CD14+) by FACS. The expression of CD86 and HLA-DR and cell morphology were evaluated by FACS or light microscopy, respectively. For activation, MP were incubated w/o 1μg/mL LPS for 16h. To verify cell activation, supernatants of the cultures were collected at different time points and the secretion of TNF-alpha was quantified with the Quantikine® HS ELISA kit (R&D systems MN, USA), following the manufacturer’s instructions.
To inhibit the endocytic pathway in MP, 1mg/mL BFA was added to the LPS-stimulated MP.
Autophagy was induced by amino acid starvation with EBSS or by treatment with rapamycin (50ng/μl) for 1h. We also inhibited the EBSS-induced autophagy by using either 30μg/mL chloroquine, 0,2μg/mL bafilomycin or 10μg/mL pepstatin/E64d. 1mg/mL EGA was also used to prevent transport from early to late endosomes. To inhibit PI3K, we used either 1mg/mL wortmannin or LY294002. Thereafter, cells were washed twice with PBS and fixed with paraformaldehyde 4 % pH7.0.
FACS acquisition was performed using the Fortessa II (BD) and analyzed using the FlowJo V9.9.5 software (Tree Star, Inc. Ashland, OR, USA).
Confocal microscopySequential images from the same focal plane were collected in a confocal scanning laser microscope (Olympus FV1000), (Tokio, Japan) using FluoView image analysis software (FV10-ASW2.1 https://www.olympus-lifescience.com/es/support/downloads/). Images were collected with 60X magnification and 1.45 of numerical aperture objective. The colocalization was evaluated by fluorescence distribution and intensity, between green and red fluorescence using Pearson’s correlation coefficient at a public domain tool named JACoP (http://rsb.info.nih.gov/ij/plugins/track/jacop.html) in ImageJ (https://imagej.nih.gov/ij/).
The colocalization in each experimental point was done in four different fields, each field contained an average of 20 cells, most of the experiments were repeated four times (16 in total) or three times (12 in total). In total 908 were analyzed. All replications were included for analysis.
Phagocytosis assayLyophilized zymosan A from S. cerevisiae (either from Invitrogen, Carlsbad, CA, USA or conjugated with Alexa Fluor 594, Molecular Probes, OR, USA) was diluted in 1X PBS at 5mg/mL. For opsonization, human serum was added to the particles at a 1:1 ratio. Thereafter, zymosan particles were washed twice with 1X PBS, to finally resuspend them at 20mg/ml. MP were then exposed to 300μL of the zymosan particles w/o opsonization. This mixture was incubated first at 4°C and then at 37°C min. Thereafter, cells were washed twice with PBS and fixed with paraformaldehyde 4 % pH 7.0, to undergo a subsequent analysis by confocal microscopy as described above.
Western blot (WB) analysisProtein lysates were size-fractionated by SDS–PAGE (12 gradient gel), and transferred onto PVDF membranes for incubation with the specific antibodies. Signals were detected by the Western Blotting Luminol Reagent (Santa Cruz Biotechnology, TX, USA) using the Syngene G: Box XR5 chemiluminescent and multicolor fluorescent imaging system (Integrated Scientific Solutions, ISS, San Diego, CA, USA).
Statistical analysesThe Shapiro-Wilk test was performed for each variable in which it was desired to evaluate the association. We used a Student’s t-test normal distribution sample and U-Mann Whitney test for non-parametric distribution samples to evaluate two dichotomous quantitative variables. For the quantitative variables, we used a one-way ANOVA and Kruskal Wallis test for the variables with or without normal distribution, respectively. Tukey's or Dunnett's post-hoc tests were performed to evaluate the difference between the groups (ANOVA or Kruskal-Wallis respectively). Analyses were performed in SPSS v24 software at 0.05 significance level (http://www-01.ibm.com/support/docview.wss?uid=swg24041224).
ResultsExpression of LRBA in human mononuclear phagocytes (MP)To demonstrate the purity of the MP monolayer, we evaluated the number of B cells (CD20+) and monocytes (CD14+) before and after adherence (Fig. S1A). Subsequently, as a measurement of the differentiation of monocytes to MP, the increase in the cell surface expression of CD86 and HLA-DR after five days of culture was calculated (Fig. S1B). Our findings were also confirmed by morphological changes in the monolayer, such as the increase in cell size and granularity after differentiation (Fig. S1C). To confirm the effects of LPS in MP, we evaluated the levels of TNF-alpha in the culture supernatants. We observed a higher production of TNF-alpha after 3 and 16h of incubation with LPS (Fig. S1D). We investigated whether the expression of LRBA in MP was modulated by these stimuli. An increase in the intensity of the LRBA band as compared to GAPDH was observed in MP stimulated with LPS/16h by WB (Fig. S1E). These results were confirmed using FACS (Fig. 1F). In addition, we demonstrated that the antibody Ab HPA019366 showed specific binding to intracellular LRBA (Fig. S1G).
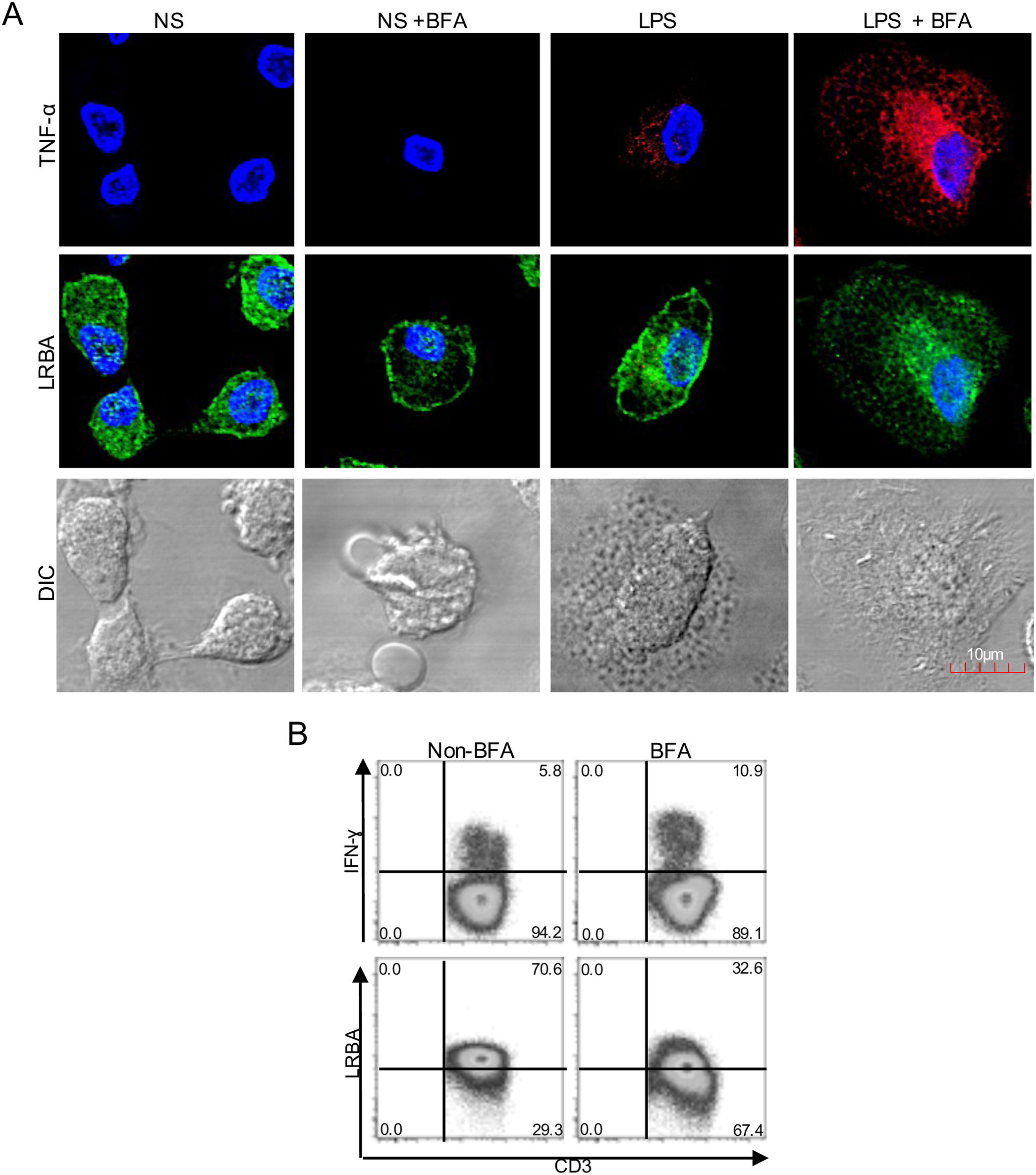
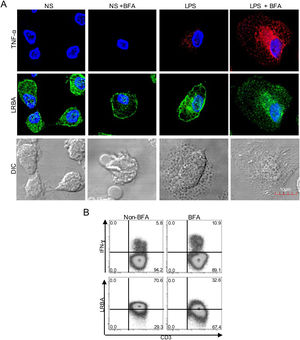
Intracellular distribution of LRBA after exposure to Brefeldin A (BFA). Mononuclear phagocytes (MP) from a healthy donor (A) were obtained, stimulated with LPS (1μg/mL, 16h) w/o BFA (1mg/mL, last 4h) and intracellularly stained on cover glasses to evaluate the expression of LRBA by confocal microscopy. Intracellular TNF-alpha staining was used as positive control for the BFA exposure in MP. On the other hand, PBMC from HD with PMA/Ionomycin stimulation exposed or not to BFA (1mg/mL, 4h) were obtained and intracellularly stained for LRBA. This staining pattern was compared with that for IFN-ɣ. The LRBA and IFN-ɣ staining gating is shown only for the CD3+ cells (B). Results are representative from two experiments (n=2). Magnification: 63×.
LRBA has been previously detected in the endoplasmic reticulum, trans-Golgi network, endocytic vesicles, lysosomes and plasma membrane in immune cells.3,4 Typically, this transport of molecules throughout the cell is controlled by the recruitment of proteins into coated vesicles that are transported from the trans-Golgi network toward the endosomes.11 Thus, we aimed to study the intracellular localization of LRBA upon inhibition of the endocytic pathway, by using BFA. BFA prevents the assembly of coat proteins onto the membrane by inhibiting the GTP-dependent interaction of ADP ribosylation factor (ARF) GTPases with the Golgi membrane, impairing the formation of transport vesicles.12
We treated MP with BFA on the steady state or after LPS stimulation. To verify the effect of the exposure with BFA, we evaluated the intracellular production of TNF-alpha. BFA prevents the secretion of cytokines by inducing intracellular accumulation of secretory vesicles. Non-stimulated MP did not produce detectable levels of TNF-α, however, this increased upon LPS stimulation. After BFA exposure, the accumulation of TNF-alpha-positive vesicles in LPS-stimulated MP was observed (Fig. 1A). Strikingly, the exposure of MP to BFA resulted in an altered intracellular distribution of LRBA. Cells exposed to BFA showed less LRBA staining in the cytoplasm, an effect independent on the LPS exposure (Fig. 1A). To confirm the decrease in LRBA staining after exposure to BFA, we evaluated the expression of this molecule in PMA/Ionomycin-stimulated peripheral blood mononuclear cells (PBMC) w/o BFA by FACS. These results were compared to those from IFN-gamma intracellular staining in the same cells. The intracellular accumulation of IFN-gamma was evident after BFA exposure. On the contrary, LRBA expression decreased after BFA (Fig. 1B). Taken together, these results demonstrated that disruption of ARF GTPases is sufficient to prevent trafficking of LRBA from the Golgi apparatus to endosomes vesicles, and consequently we observe an intracellular decrease in the LRBA expression.
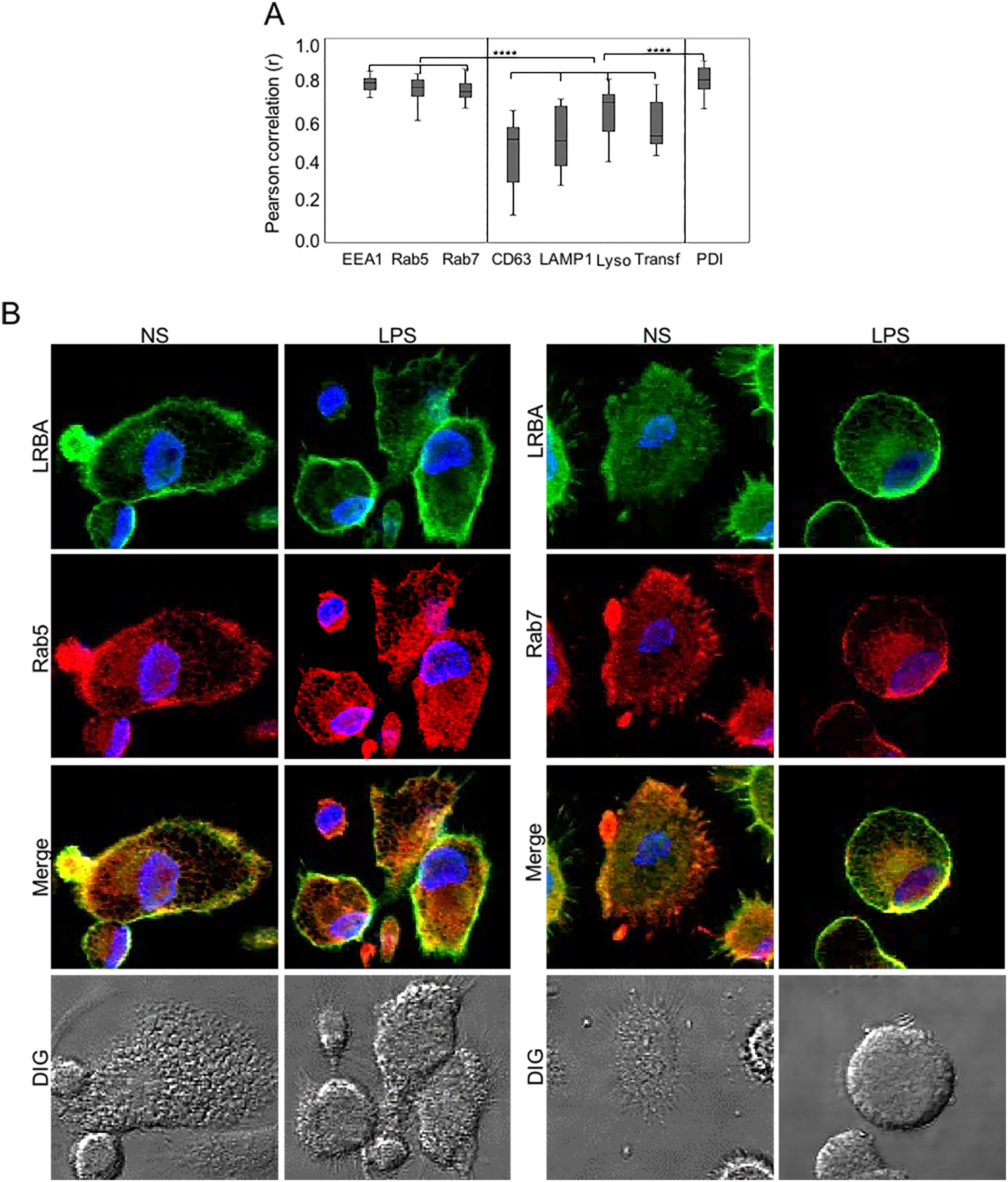
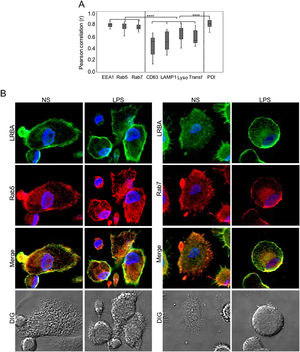
Colocalization of LRBA with vesicular markersThe results above demonstrate that blocking vesicular trafficking alters the intracellular distribution of LRBA. To understand this better, we dissected the intracellular localization of LRBA, targeting either early (EEA1+ or Rab5+) or late endosomes (Rab7+), lysosomes (LAMP1+, CD63+), lysotracker (acidic organelles such as peroxisomes, some endosomes, phagosomes, autophagosomes and Lysosomes) or endoplasmic reticulum (PDI+) in MP cells w/o LPS stimulation. We also included transferrin to trace vesicles involved in the trafficking of early and recycling endosomes.13
We did not observe significant differences in the colocalization of LRBA with the different markers in MP unexposed or exposed to LPS. Percentages of LRBA colocalization with EEA-1, Rab5 and Rab7 were 76.4±12, 83.4±4 and 76.9±6 without stimuli and 81.3±4, 78.9±6 and 76.6±6 with LPS, respectively and the colocalization pattern with these markers was very homogeneous among cells (Fig. 2A). To a lesser extent, LRBA colocalized with Lysotracker, transferrin, and PDI (70.7±14, 52.5±13, and 83.1±7 % without stimuli and 68.9±10, 50±15 and 77.3±2 with LPS, respectively); however, the colocalization percentages with these markers were very heterogeneous among cells (Fig. 2A). In a lesser extent, LRBA colocalized with CD63 and LAMP1 (50.7±18 % and 49.9±16 without stimuli and 50.5±19 and 40.3±9 with LPS, respectively) (Fig. 2A). We observed no significant differences between the colocalization of LRBA with early and late endosomal markers (EEA1, Rab5, Rab7, and PDI) in MP. However, this was greater than the colocalization observed with lysosomal markers and transferrin. (Fig. 2A). Fig. 2B shows a representative image of the LRBA colocalization with Rab5 and Rab7 in MP w/o LPS. The LRBA colocalization with the other markers is shown in Fig. S2.
Colocalization of LRBA with vesicular markers in mononuclear phagocytes (MP).
MP from healthy donors were obtained. LRBA and the different vesicular markers were intracellularly stained on cover glasses and colocalization was visualized by confocal microscopy and analyzed using the Pearson correlation. (A) Colocalization analysis from LRBA among the different markers in LPS-stimulated cells. A representative example of the colocalization from LRBA (green) with either Rab5 or Rab 7 (red) in MP w/o LPS is also shown. Magnification: 63×. Lyso: Lysotracker, Transf: Transferrin NS: Unstimulated cells, LPS: Lypopolissacharide. Results are representative from four experiments with MP obtained from different healthy controls (n=4). *p-value <0.05. Magnification: 63×.
Phagocytosis is an essential component of the innate immune response. Phagocytic vacuoles acquire degradative and microbicide properties through a complex series of interactions with early and late endosomes and culminate in the fusion of phagosomes with lysosomes.14 We evaluated the colocalization of LRBA and LAMP1 during phagocytic uptake of zymosan particles. However, similar colocalization of LRBA/LAMP1 in cells w/o exposure to either zymosan or opsonized zymosan was observed (Data not shown). We demonstrated that LRBA not only colocalizes with lysosomes, also with early and late endosomes in MP. Therefore, we evaluated the colocalization of LRBA with zymosan particles labeled with Alexa Fluor 594w/o opsonization (Fig. S3). Zymosan particles were readily detected inside the cells after phagocytosis and the number of particles per cell notably increased after opsonization (Fig. S3). However, a modest colocalization of LRBA with the zymosan particles w/o opsonization was found (Fig. S3). This is also reflected by the percentages of colocalization between LRBA and Zymosan, which were very low (Fig. S3). These results suggest that although LRBA is located in the endosomal compartment, it is not translocated to the phagosomal membrane during the phagocytic uptake.
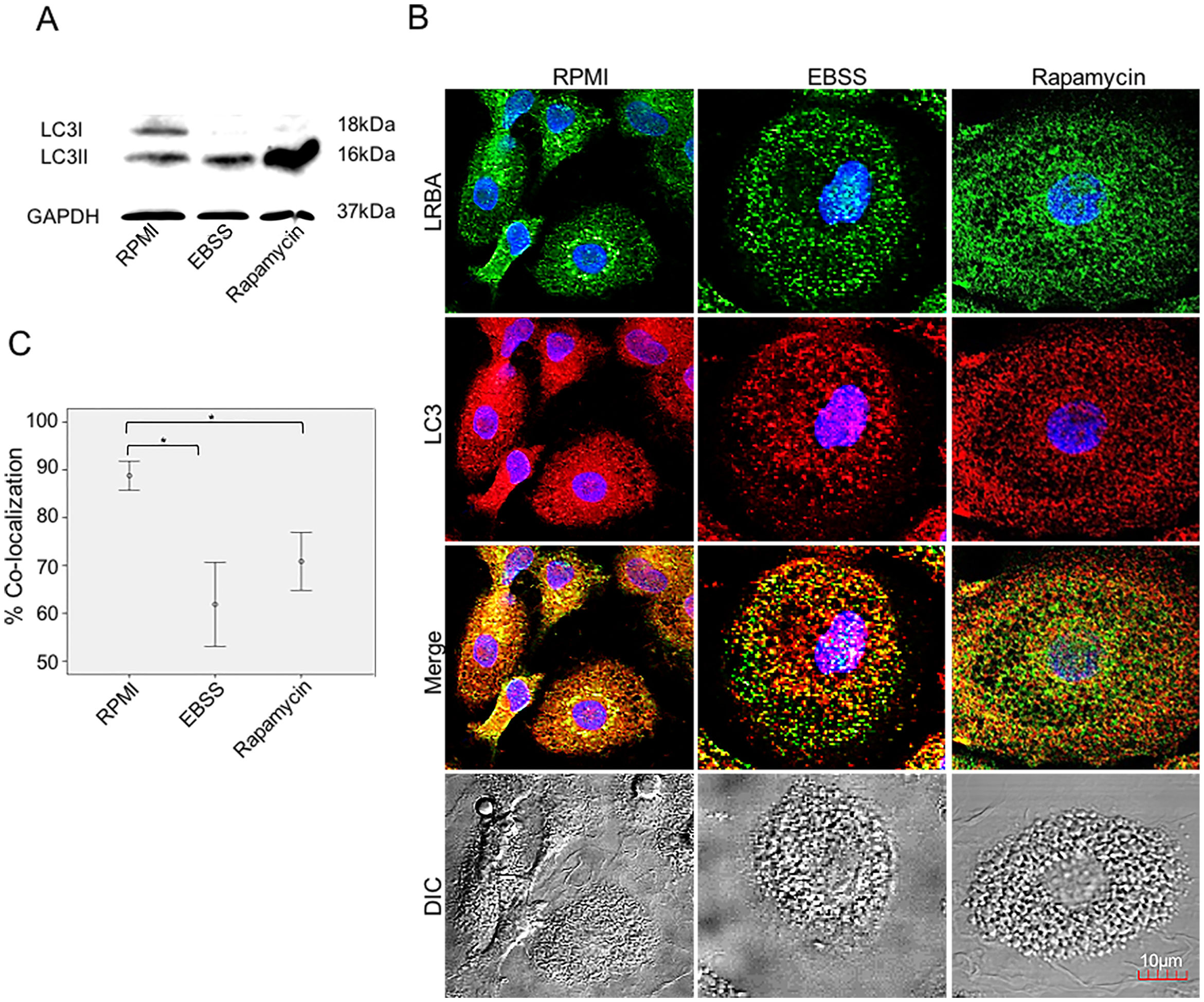
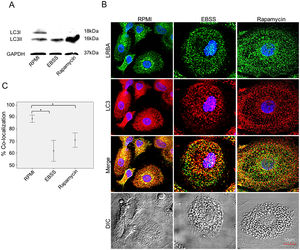
LRBA-LC3 colocalization under autophagic fluxWe evaluated the colocalization of LRBA and LC3 (an autophagosomal marker) in MP w/o exposure to autophagy inducers by confocal microscopy. First, we evaluated the expression of LC3-I and LC3-II in MP upon exposure to autophagic flux by WB (Fig. 3A). The conversion of cytoplasmic LC3-I to lipid-bound LC3-II is associated with the formation of autophagosomes7. The increase in the LC3-II band was evident in both EBSS and rapamycin-exposed MP (Fig. 3A). After the autophagy flux, morphological changes were also observed in MP: cells were much larger and vacuolated (Fig. 3B). Also, the distribution of the LRBA markedly changed after autophagy flux. In basal conditions, LRBA is condensed around the nuclei whereas upon induction of autophagy is more dispersed in the cytoplasm and becomes punctuated (Fig. 3B). Importantly, we observed that the colocalization LRBA/LC3 was approximately 90 % in MP under basal conditions (Fig. 3C). This suggests that LRBA and LC3 are located in similar cell compartments before the autophagy flux. Strikingly, the LRBA/LC3 colocalization decreased to approximately 60 % upon induction of autophagy (Fig. 3C). No differences in the LRBA/LC3 colocalization were observed when the autophagy inducers were compared (Fig. 3C).
Colocalization of LRBA with LC3 in mononuclear phagocytes (MP) upon exposure to autophagy inducers.
MP were obtained and cultured in EBSS (1X) or exposed to Rapamycin (50μg/ml) for 1h. Autophagy influx was confirmed by LC3-I/LC3-II Western blot using GAPDH as a housekeeping protein. The lipidated form of LC3 (LC3-II) is induced after exposure to the autophagy inducers (A). The intracellular staining of LRBA and LC3 by confocal microscopy (B) and the colocalization analysis LRBA/LC3 using the Pearson correlation (C) are shown. Results are representative from four experiments with MP obtained from different healthy controls (n=4). *p-value <0.05. Magnification: 63×.
Our data suggest that LRBA is not recruited together with LC3 to the autophagosome upon triggering the autophagic flux. Another possibility would be a rapid degradation of LRBA upon recruitment to the autophagolysosomes.
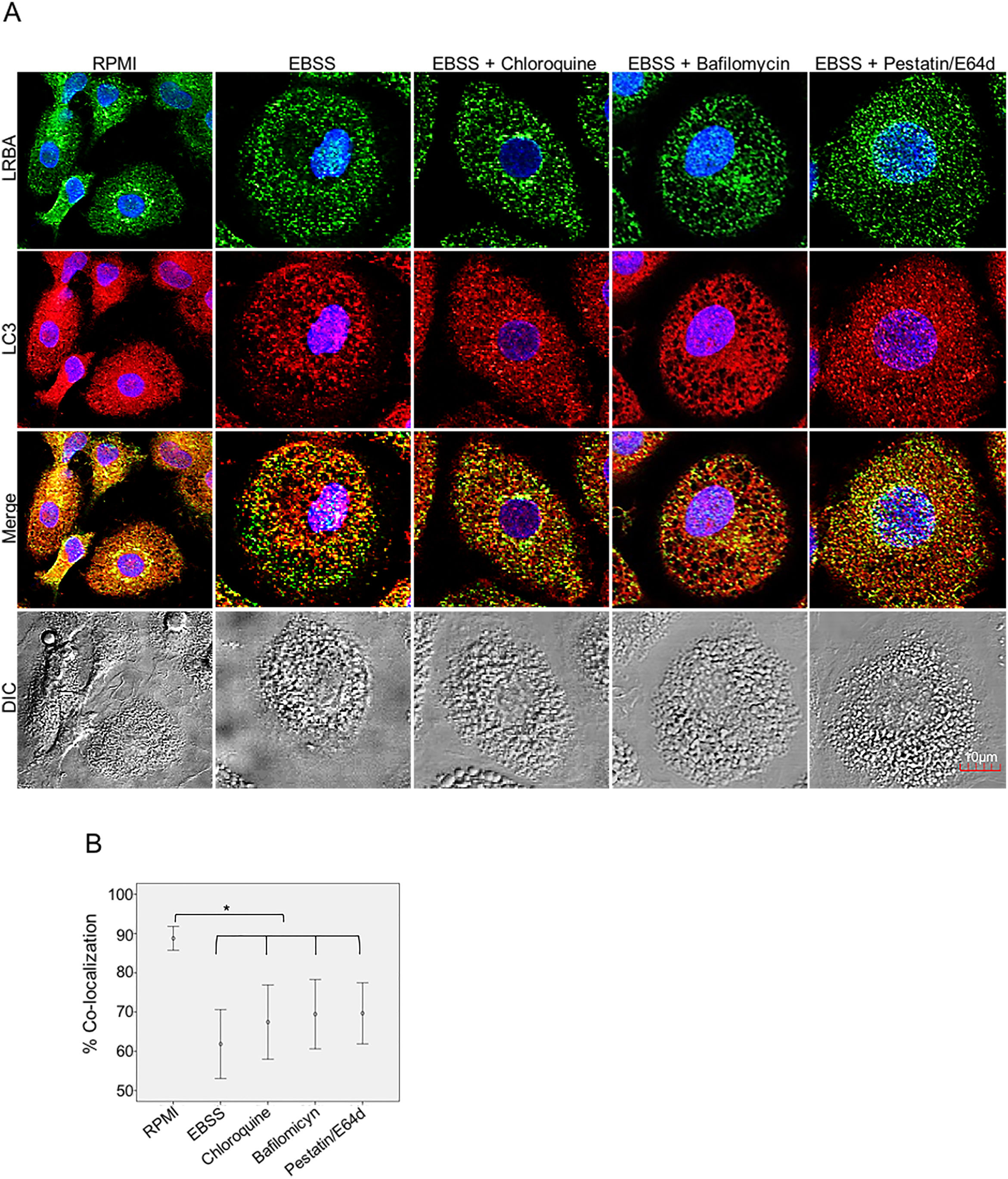
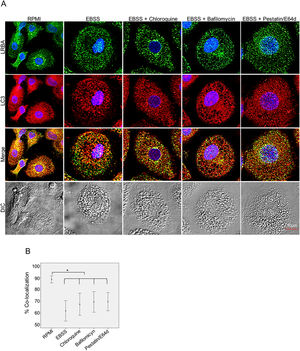
Inhibition of the LRBA/LC3 colocalization by using autophagy inhibitorsTo evaluate both possibilities, we inhibited the fusion of lysosomes with autophagosomes by using chloroquine, bafilomycin A1 and E64d/Pepstatin A in MP to cause accumulation of autophagosomes under starvation. This would impede the LRBA degradation in autophagolysosomes and in consequence observe an increase LRBA/LC3 colocalization. However, these agents did not alter the LRBA/LC3 colocalization observed after induction of the autophagy flux as shown in Fig. 5A. This result was confirmed by evaluating the colocalization percentages (Fig. 4B).
LRBA/LC3 colocalization in MP upon exposure to autophagy inhibitors in cells under autophagy flux. MP cells from healthy donors were incubated in medium alone (RPMI) or exposed to the autophagy inductor EBSS alone or together with autophagy inhibitors chloroquine (30μg/mL), pepstatin/E64d (10μg/mL) and bafilomycin (0,2μg/mL) for 1h. The intracellular staining of LRBA and LC3 by confocal microscopy (A) and the colocalization analysis LRBA/LC3 using the Pearson correlation (B) are shown. Results are representative from four experiments with MP obtained from different healthy controls (n=4). *p-value <0.05. Magnification: 63×.
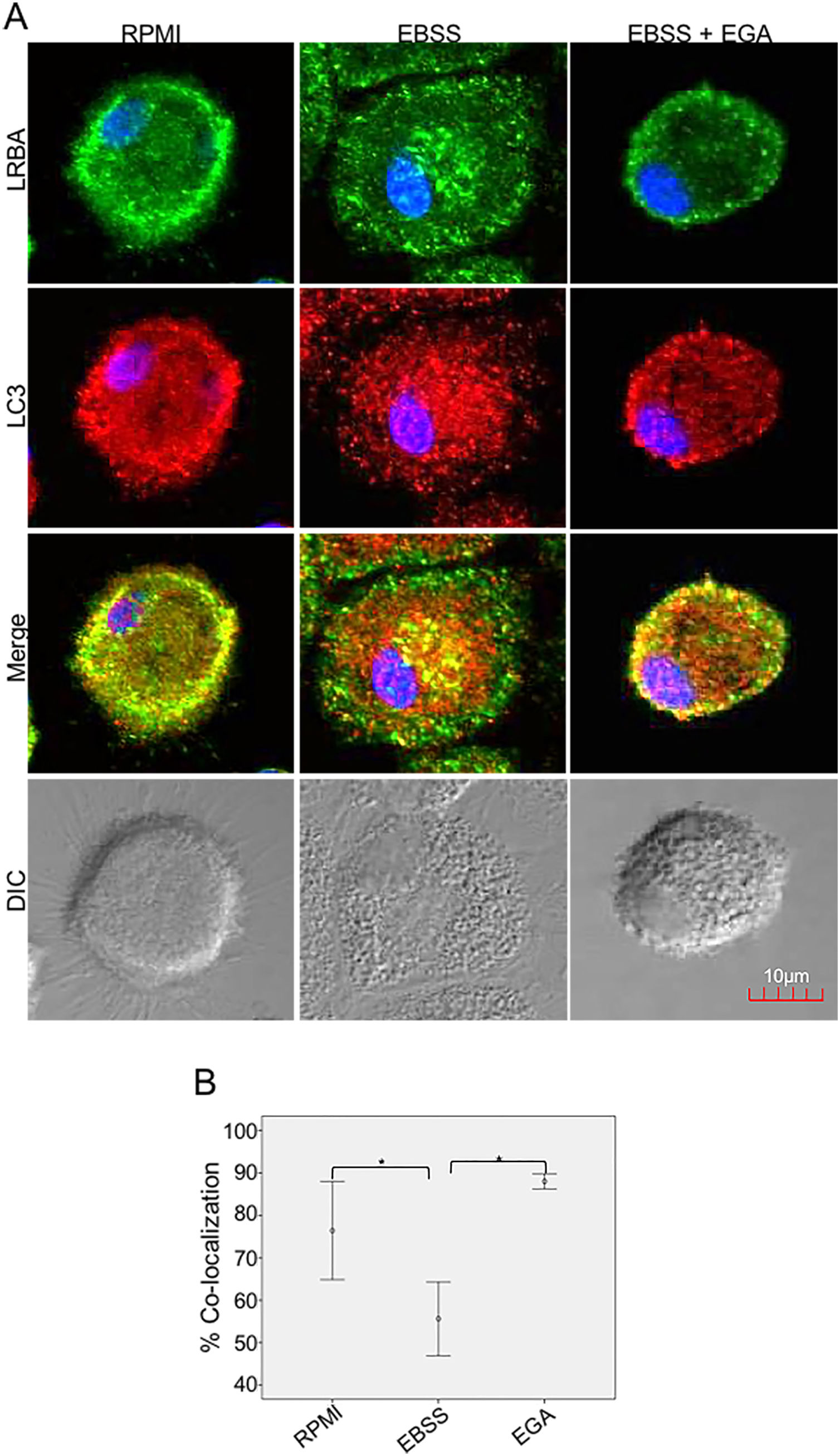
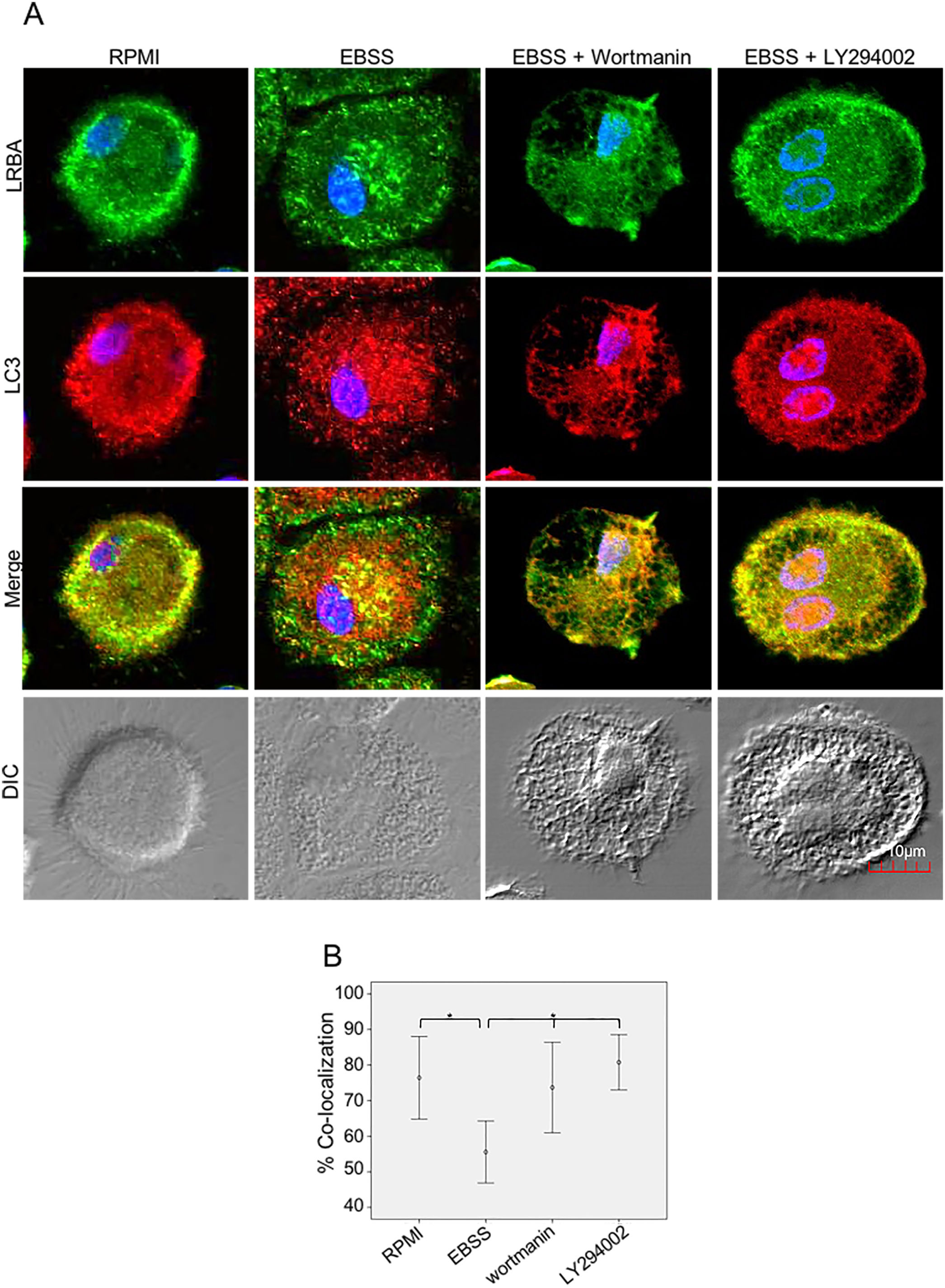
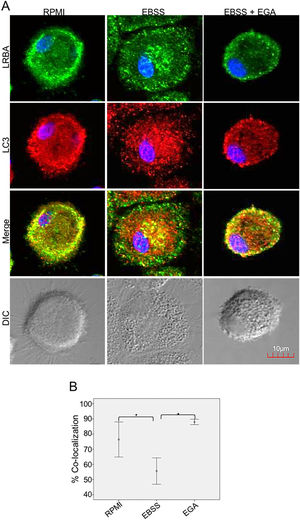
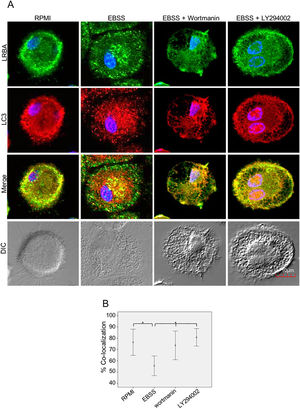
It is possible that LRBA is required either before formation or maturation of the autophagosome. Interestingly, Rab7 is required for the maturation of the autophagosome to form the amphisome8. To evaluate if the recruitment of LRBA to the autophagosome is dependent on the amphisome formation, we used EGA, an inhibitor of trafficking from early to late endosomes,15 in MP cells subjected or not to starvation. Notably, the LRBA punctuated pattern in the cells after the EGA treatment is no longer observed (Fig. 5A). This suggests that the traffic between early and late endosomes is important for the dynamics of LRBA in the cell. Moreover, EGA exposure in cells with the autophagy flux reverted the decrease in the LRBA/LC3 colocalization observed in cells cultured only with EBSS, levels similar to those observed in cells cultured with RPMI (Fig. 5B). Notably, the LRBA punctuated pattern in the cells after the EGA treatment is no longer observed (Fig. 5A). Our results suggest that LRBA is not recruited together with LC3 to the autophagosome upon triggering the autophagic and possible is required to maturation the autophagosome. But will LRBA also be necessary before the formation of the autophagosome? To answer this question, we subjected the autophagy-exposed cells to wortmannin and LY294002. Both reagents have been demonstrated to block the formation of autophagic vacuoles by inhibiting both class I and III PI3K complexes.16 After exposure to the PI3K inhibitors, we observed similar effects observed after treatment with EGA (Fig. 6A). Also, the colocalization of LRBA and LC3 is restored as compared to cells exposed to starvation (Fig. 6B). Taken together, our results suggest that LRBA is required in the early steps of autophagosome formation and maturation.
LRBA/LC3 colocalization in MP upon exposure to EGA in cells under autophagy flux. MP cells from healthy donors were incubated in medium alone (RPMI) or exposed to the autophagy inductor EBSS either alone or together with EGA (1mg/mL), for 1h. The intracellular staining of LRBA and LC3 by confocal microscopy (A) and the colocalization analysis LRBA/LC3 using the Pearson correlation (B) are shown. Results are representative from four experiments with MP obtained from different healthy controls (n=4). *p-value <0.05. Magnification: 63×.
LRBA/LC3 colocalization in MP upon exposure to the PI3K pathway in cells under autophagy flux.
MP cells from healthy donors were incubated in medium alone (RPMI) or exposed to the autophagy inductor EBSS alone or together with the PI3K inhibitors Wortmannin (1mg/mL) and LY294002 (1mg/mL), for 1h. The intracellular staining of LRBA and LC3 by confocal microscopy (A) and the colocalization analysis LRBA/LC3 using the Pearson correlation (B) are shown. Results are representative from four experiments with MP obtained from different healthy controls (n=4). *p-value <0.05. Magnification: 63×.
Supplementary Fig. S4 shows a representative example of the morphology effects of autophagy inducers and inhibitors. We showed either cells on a steady-state, exposed to EBSS w/o either a lysosome and autophagosome fusion inhibitor (chloroquine), an early to late endosome trafficking inhibitor (EGA) or a PI3K inhibitor (wortmannin). In steady-state, we observed small cells with few vacuoles; this morphology changes notoriously in cells under autophagic, since they became larger and contained many and big vacuoles. After EBSS+chloroquine exposure, cells became even larger and with bigger vacuoles, presumably due to the accumulation of autophagosomes. These effects were totally reverted in cells exposed to EBSS+EGA or EBSS+wortmannin.
DiscussionIn this study, we describe the localization and dynamics of LRBA in MP. We selected MP, which are cells that belong to the immune system, adherent and feasible to observe by confocal microscopy. In addition, its large cytoplasm allows us to study organelles, such as endosomes, lysosomes, endoplasmic reticulum, and cellular processes, such as phagocytosis and autophagy.
Endomembrane trafficking depends on a great variety of molecules including small ARF GTPases (Mead et al., 2005). The data presented here demonstrated that BFA-mediated ARF inhibition was sufficient to prevent the LRBA trafficking from the Golgi apparatus to cytoplasmic vesicles. Taking into account that LRBA has been demonstrated to interact with CTLA-44 and that BFA also prevents cell surface expression of CTLA-4,17 these results suggest a critical role for ARF in the movement of LRBA-vesicles containing from a perinuclear region to the plastic membrane.
We also demonstrated that LRBA significantly colocalized with early and late endosomes. However, despite reports that LRBA may be stored in secretory lysosomes,3 the amount of LRBA that colocalized with the lysosomal markers LAMP-1 and CD63 was less in comparison with endosomes markers, indicating that LRBA has a distinct trafficking itinerary. Rab GTPases are a family of small GTPases that control vesicular trafficking.18,19 Interestingly, Khodosh et al. in 2006 found a genetic interaction between bchs, a BEACH family member in Drosophila, and Rab11.20 These data further support the hypothesis that LRBA regulates vesicle trafficking via interaction with Rab GTPases. Therefore, studies that investigate the interaction between LRBA and Rab proteins are required, these organelles are part of the endomembrane system, which is necessary for internalization and recycling of the membrane receptor, not only of CTLA-421 but also of EGFR22 and Fas,23 receptors involved in cancer and autoimmunity in which LRBA plays an important role.24,25 Therefore, studies that determine the relationship of LRBA with these receptors are suggested.
On the other hand, our results demonstrate that LRBA colocalizes with LC3 before autophagy and this decreases due to the formation of autophagosomes. These data suggest that LRBA is not recruited together with LC3 during the autophagy flux but it is required in the first steps of the formation and maturation of the autophagosome, to continue with the normal progression of the autophagic flux. Previously it has been shown that other BEACH family members such as ALFY and WDR81are involved in the autophagy process. The BEACH domain from the WDR81 protein strongly interacts with LC3C and this interaction plays an essential role in eliminating ubiquitinated proteins through autophagy.26 Taking into account these observations further supports the hypothesis that LRBA is necessary for the formation and maturation of autophagosomes and to continue with the normal progression of the autophagic flux possibly by the interaction of LC3-I but not with LC3-II.
With respect to phagocytosis, we did not observe a difference in the LAMP1/LRBA colocalization before and after zymosan uptake. Moreover, we did not observe significant zymosan particles/LRBA colocalization in MP. Under these conditions, these results suggest that LRBA is not implicated in the phagocytosis uptake. However, phagocytosis is a multistep phenomenon that involves recognition of the target using surface receptors, membrane protrusion, phagolysosome formation and degradation of the cargo.27,28 Therefore, more studies are necessary to evaluate if the phagocytic cargo could be fully processed in LRBA-deficient phagocytic cells.
FundingThis work was supported by Departamento Administrativo de Ciencia, Tecnología e Innovación, COLCIENCIAS (Grant #111556934430); the fellowship COLCIENCIAS 727.
Declarations of interestNone.